Substrate stiffness regulates chemotaxis, with speed and persistence regulated independently; directionality time is introduced as a novel metric that quantifies the global directedness of migration.
Keywords: integrins, migration, mechanosensing, biophysics
Abstract
A direct consequence of cellular movement and navigation, migration incorporates elements of speed, direction, and persistence of motion. Current techniques to parameterize the trajectory of a chemotaxing cell most commonly pair migration speed with some measure of persistence by calculating MSD, RMS speed, TAD, and/or CI. We address inherent limitations in TAD and CI for comparative analysis by introducing two new analytical tools to quantify persistence: directionality index and directionality time. With the use of these tools, we show that the mechanical properties of the underlying substrate contribute significantly to the regulation of human neutrophil chemotaxis toward fMLP on Fgn-, Col-, and Fn-coated gels of varying elasticity. The β1-integrin ligand Col demonstrated mechanosensitive speed. In contrast, β2-integrin ligand Fgn supported mechanosensitive persistence. Fn, recognized by β1 and β2 integrins, mechanoregulated speed and persistence. Blocking β2 integrins of cells migrating on Fn identified an underlying β2-integrin-directed modulation of persistence. These data demonstrate that individual components of the neutrophil chemotactic response show integrin dependence and are finely tunable with different ligand, mechanotactic, and chemotactic cues, underscoring the need for sensitive analytical methods.
Introduction
Neutrophils serve as the body's first line of defense against invading pathogens, transmigrating though vascular endothelium and chemotaxing through vicinal ECM to reach the target area of infection or injury [1]. Improper neutrophil activity has grave clinical consequences: insufficient activity results in recurrent life-threatening infections and impaired wound-healing, whereas excessive activity leads to an exaggerated immune response, resulting in tissue damage.
Mechanosensing of cells within tissues refers to the ability of the cells to perceive differences in the mechanical properties of its environment. The mechanical environment has been shown to influence gene expression, proliferation, cytoskeletal organization, and survival significantly in a number of cell types [2–7]. Neutrophil emigration into tissue is a multistep process, culminating with entry into notably different tissue microenvironments. In so doing, cells interpret a number of biochemical cues that hone their directed movement and regulate their host-defense functions within injured or infected tissues. The physical properties of the tissue microenvironment contribute significantly to the regulation of neutrophil migration speed and generation of traction [8, 9]. Relatively few host-defense functions of neutrophils take place in circulating or nonadherent cells nor does the physiological context in which they occur resemble rigid surfaces, such as glass or plastic. With the use of gels of physiologically relevant stiffnesses coated with the ECM protein Fn, Oakes et al. [8] found that human neutrophils migrating up a concentration gradient of the chemoattractant fMLP were faster but less directed on softer gels (Young's modulus of 10 kPa) than they were on stiffer gels (Young's modulus of 100 kPa). Fn is a canonical β1-integrin ligand, and α5β1 is a key mediator of migration on this substrate. β1 Integrins have been shown to play a role in the cellular mechanosensing apparatus in other cell systems [10, 11]. Fn, however, is also recognized by several other receptors, one of which is the β2 integrin, CR3 (macrophage 1 antigen; αMβ2).
To examine the contribution of CR3 in transducing mechanotactic cues, we analyzed the migration of human neutrophils up a concentration gradient of fMLP on polyacrylamide gels, ranging in stiffness from 10 to 100 kPa, coated with Fn or the CR3-restricted ligand, Fgn, which is known to be associated with the ECM under inflammatory conditions, forming the provisional fibrin matrix at virtually all sites of tissue damage [12]. We also extended our study of neutrophil migration to include gels coated with type IV Col, an ECM ligand recognized by β1 but not β2 integrins.
Analytical dissection of chemotaxis into its constituent parts was performed by pairing MSD with multiple measures of persistence. The root MSD divided by time gives RMS speed for a particular time interval, and persistence is traditionally characterized by interpreting the TAD and/or by calculating the CI, which is the ratio of total displacement to total migration path length [8, 9, 13]. TAD and CI implicitly depend on the time interval between the cell's centroid positions along its migration trajectory. Because of this dependence, these measures of persistence may skew from report to report. In this work, we introduce a new time interval-independent metric called the directionality index, from which we derive three parameters that quantify persistence more generally and precisely than does TAD or CI. The first parameter, termed the directionality time, is the minimum observation time required to assert that a cell is moving directionally. The second and third parameters, called micro- and macro-directionality index, respectively, quantify the degree of randomness in the migration path over short time intervals <Td and the degree of directedness in the migration path over long time intervals >Td. Our characterizations of MSD, TAD, CI, Td, DI<, and DI> across migration conditions identified ligand-specific mechanosensitivities for each chemotactic parameter that appears to be modulated independently. Furthermore, through integrin-blocking studies, we show a role for β2 integrins in modulating chemotaxis on compliant substrates by enhancing migration persistence. Td, derived from MSD exponent fitting, represents a technical advance in quantifying cell chemotaxis by providing a robust, sensitive metric that describes more globally the directedness of migration paths.
MATERIALS AND METHODS
Reagents
Anti-Fgn (85D4) and anti-Fn (FN-15) antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA); anti-type IV Col pAb and anti-integrin β1-blocking antibody (P5D2) were purchased from Millipore (Billerica, MA, USA); and anti-integrin β2-blocking antibody (TS1/18) was purchased from Pierce Biotechnology (Rockford, IL, USA). L-15 and HBSS were purchased from Invitrogen (Carlsbad, CA, USA), and polymyxin B and sulfo-SANPAH were purchased from Pierce Biotechnology. Human Fn isolated from plasma (>95% purity) and human type IV Col from human placenta (>95% purity) were purchased from Sigma-Aldrich. Human Fgn isolated from plasma (>95% purity) was purchased from Molecular Innovations (Detroit, MI, USA), and IRDye800-conjugated goat anti-mouse IgG was obtained from Rockland Immunohistochemicals (Gilbertsville, PA, USA).
Cell preparation
Under the approval and guidelines of the Rhode Island Hospital Institutional Review Board, neutrophils were isolated from healthy human volunteers by collection into EDTA-containing Vacutainer tubes (BD Biosciences, San Jose, CA, USA). Histopaque 1077 (Ficoll Histopaque) was used for the initial cell separation, followed by gravity sedimentation through 3% dextran (average 400–500 kDa MW). Contaminating erythrocytes were removed by hypotonic lysis, yielding a neutrophil purity of >95%. Neutrophils were suspended in HBSS (without Ca++/Mg++) on ice until use in the experiments. All reagents contained <0.1 pg/ml endotoxin, as determined by Limulus amoebocyte lysate screening (BioWhittaker, Walkersville, MD, USA).
Chemotactic migration
For chemotactic migration assays, ∼1 × 106 neutrophils were resuspended at 37°C in 2 ml L-15-glu. Neutrophils were allowed to settle for approximately 2 min. A femptotip (Eppendorf North America, New York, NY, USA) was filled with 5 μl of a 1-mM concentration of bacterial fMLP and placed with the tip at the migration surface within the field of view. When indicated, neutrophils were pretreated on ice for 30 min with 10 μg/ml β2-blocking antibody (TS1/18), β1-blocking antibody (P5D2), or isotype control in L-15-glu, which was maintained for the duration of the experiment.
Substrate preparation
Migration experiments were carried out in heatable glass-bottom DeltaT dishes (Bioptechs, Butler, PA, USA) on 10 kPa, 50 kPa, or 100 kPa gels, prepared following a method described by Pelham and Wang [2]. Briefly, a solution of acrylamide and bisacrylamide was polymerized using tetramethylethylenediamine and ammonium persulfate. Gels were made in DeltaT dishes using AbGene frames (AbGene, Epsom, UK) as molds. The gels were allowed to polymerize at room temperature. Once polymerized, gels were soaked overnight in PBS, allowing unpolymerized acrylamide to diffuse out. The final size of the gels was ∼1 cm × 1 cm × 300 μm.
Gel stiffness was regulated by varying the percentage of bisacrylamide in relation to the percentage of acrylamide in the initial mixture [14], and elasticity was confirmed with an AR2000 oscillating plate rheometer (TA Instruments, New Castle, DE, USA) [15]. The gels were coated with Fgn, Col, or Fn using the chemical cross-linker sulfo-SANPAH, which was allowed to bond covalently to the acrylamide gel for ∼1 h at room temperature. Gels were washed three times with 50 mM HEPES (pH 7) and then incubated in 200 μl 20 μg/ml Fgn, Col, or Fn, all diluted in 50 mM HEPES (pH 7), while exposed to UV using a Foto/Prep I transilluminator (Fotodyne, Hartland, WI, USA), equipped with 15 W, 312 nm bulbs with an energy output of ∼4000 μW/cm2 for 12 min at room temperature, washing three times with PBS before use.
The elasticity of the gels has been shown to be unaffected by the protein-coating procedure, and the density of protein on the surface of the gel is unaffected by the elasticity of the gels [8, 16]. The uniformity of the protein coating on the acrylamide substrate was confirmed by immunofluorescence. Gels were cast on activated glass slides and coated with Fgn, Col, or Fn, as described above or 90:10 and 10:90 mixtures by volume of 20 μg/ml Fgn and 20 μg/ml Fn. Gels were blocked with 200 μl 2% BSA at room temperature for 30 min before being incubated with a 100-μg/ml solution of mouse anti-human Fgn, Col, or Fn antibody as appropriate in 2% BSA for 1 h at room temperature. The gels were then washed three times with PBS. Next, gels incubated with 1:10,000 IRDye800-conjugated anti-mouse IgG in 2% BSA for 1 h at room temperature. After incubation, the gels were washed three times with PBS and scanned using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA). Each gel was divided into four, 5-mm2 regions of interest. Fluorescence intensity from regions of interest was obtained for six gels from two to four independent experiments/condition.
Microscopy
A Nikon TE-2000U inverted microscope (Nikon, Melville, NY, USA) coupled to a CoolSNAP HQ CCD camera (Roper Scientific, Martinsried, Germany) or an iXonEM + 897E back-illuminated Electron Multiplying CCD camera (Andor, Belfast, UK), outfitted with a Bioptechs (Butler, PA, USA) stage heater and a 20× Nikon Plan apochromat objective, was used for all experiments. DIC images were captured over 30 min on a 10-s interval using the Elements program (Nikon). All data were analyzed using Excel (Microsoft, Redmond, WA, USA), ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA), and MATLAB (MathWorks, Natick, MA, USA) computational software.
Migration analysis
The neutrophils chosen for analysis exhibited movement for at least 300 s. Under the conditions analyzed in this study, 25–30% of cells migrated to the fMLP point source in a given 30-min observation period. Cells were tracked frame-by-frame using custom MATLAB software [8]. Individual cell boundaries were determined through thresholding of the DIC images with respect to a median image, calculated by taking the median intensity of all images in a sequence. Each cell centroid was determined through a center of mass calculation, based on the cell border. Finally, cell migration trajectories, r⃗(t), were assembled from the time-course of all centroid positions. With the use of these trajectories, the MSD was calculated as a function of time interval, Δt, by
where r⃗ is the position of the cell centroid, t is the time at which the cell's centroid was measured, and 〈〉t brackets represent an average over all time, t. The MSD follows the proportionality, d2 ∝ Δtβ. The MSD, as discussed in terms of the exponent, β = β(Δt), characterizes the directionality of motion and depends on time interval Δt. A value of β(Δt) = 1 indicates that migration appears diffusive (random) when observed over a period of time Δt. A value of β(Δt) = 2 is the theoretical limit, indicating ballistic (directed) motion over time period Δt. Other values represent subdiffusive (0<β<1) or superdiffusive (1<β< 2) motion. As the persistence of the motion increases, β increases from one to two. When MSD is plotted against time interval in log–log coordinates, the slope is an approximate measure for β. We call this slope the DI, which is interpreted just as β(Δt) is above.
The persistence of cells in this study is also characterized by three quantities derived from DI. The first quantity, Td, characterizes the time scale at which migration transitions from random to directed. The second and third quantities are DI< and DI>. DI< quantifies the degree of randomness in the migration path over short time intervals <Td. In comparison, DI> quantifies the degree of directedness in the migration path over long intervals >Td. To measure these quantities, DI(Δt) is fit using Levenberg-Marquardt least square fitting [17] to the three-parameter fit function,
where DI∞, DIΔ, and Td are fit parameters. DI< and DI> are mathematically defined by the time averages over the corresponding experimental time domain, DI< = 〈DIfit〉Δt<Td, and DI> = 〈DIfit〉Δt>Td, respectively. The values of Td, DI<, and DI> reported in this work are averages over all cell trajectories.
The RMSS was calculated by taking the square root of MSD at time interval, Δt, and dividing by the corresponding time interval. TADs were calculated as the distribution of angles between sequential displacement vectors r⃗(t + Δt) − r⃗(t), binned in 10° increments. Time increment, Δt = 60 s, was used to calculate turning angles in this work. Positive angles indicate counter-clockwise turns, whereas negative angles indicate clockwise turns. CI was calculated as the fraction: end-to-end displacement of the migration path divided by total migration path length [18]. Like TAD, CI depends on time interval Δt and is routinely reported for time interval Δt = 10 s, as we do here.
Statistics
Data were pooled from a minimum of three independent experiments representing three to six different donors, with an n equal to 15–40 cells for each condition. ANOVA with Newman-Keuls post hoc analysis, as appropriate, was performed using MATLAB (MathWorks) or Excel (Microsoft) running the statistiXL data package (Needlands, Western Australia). The null hypothesis was rejected if P < 0.05.
Online Supplemental material
Supplemental Fig. 1 shows that protein coating is unaffected by gel stiffness, and mixtures of Fn and Fgn are cross-linked to the gel in proportion to their percentage in the applied protein mixture. In Supplemental Fig. 2, neutrophils migrating on Col-coated substrates toward fMLP show no difference in MSD, Td, DI<, or DI> upon blocking of β2 integrins. Supplemental Fig. 3 shows that blocking β1 integrins do not change MSD or transition significantly from random to directed motion in cells migrating on Fgn-coated gels toward fMLP. Video 1 shows neutrophils migrating on Fgn-coated gels of 10 kPa and 100 kPa stiffness toward a fMLP point source; Video 2, neutrophils migrating on Col-coated gels of 10 kPa and 100 kPa stiffness toward a fMLP point source; Video 3, neutrophils migrating on Fn-coated gels of 10 kPa and 100 kPa stiffness toward a fMLP point source; and Video 4, neutrophils migrating on Fn-coated gels of 10 kPa + β2-integrin block and 100 kPa + β2-integrin block stiffness toward a fMLP point source.
RESULTS AND DISCUSSION
In this work, we demonstrate that the process of human neutrophil chemotaxis toward a fMLP point source is ligand-directed and is underpinned additionally by distinct constituent processes directing cellular speed, direction, and persistence of motion that can be modulated independently by the tissue microenvironment and integrin engagement. A novel feature of this work is the development of biophysical tools, some introduced for the first time here, which allow fine dissection of the mechanisms that lead to the mechanosensitive differences in neutrophil chemotaxis.
Previous work from our lab [8] found that the mechanical properties of a Fn-coated substrate strongly affected neutrophil morphology, with cells spreading over a larger area and more quickly on substrates of greater stiffness. In addition, they also observed that neutrophil function was affected on stiffer substrates, resulting in slower migration, but with an increase in directedness, such that a greater net distance was traveled over time. Their finding that the elastic properties of the substrate dictate the ability and efficiency of the neutrophils to adhere and migrate, taken in the context that the mechanical properties of cells and tissues can be altered dramatically in states of disease or inflammation [19], suggests that studies of cell adhesion and migration on flexible substrates represent a more physiologically appropriate and biologically relevant approach than similar studies on plastic or glass. Physiologically, these findings also suggest that neutrophil function may be subject to the relative tonicity of the tissue in which the response is taking place.
We were interested in investigating if these findings were restricted to Fn or if they could be extended to matrices more reflective of an inflammatory site and recognized by other integrin families. To that end, polyacrylamide gels of 10 kPa, 50 kPa, and 100 kPa stiffness were coated with Fgn, Col, or Fn using the photoactivatable cross-linker, sulfo-SANPAH. In these experiments, ligand density was held constant across conditions to allow the isolation of substrate stiffness as an independent variable. Immunological detection was used to confirm that the protein coating was uniform and proportional and that the density of protein was independent of substrate stiffness (Supplemental Fig. 1). Neutrophils do not migrate through a matrix-coated polyacrylamide gel, and therefore, they provide us an experimental system that allows for the interrogation of cellular mechanosensing, based purely on the mechanical properties of the substrate at constant ligand density.
Neutrophil morphology is dependent on substrate stiffness and independent of ligand coating
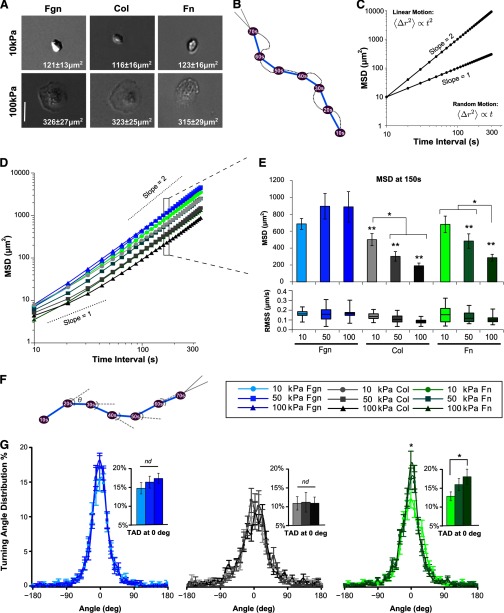
Consistent with cell morphology on Fn, neutrophils adhered to coated substrates show distinct changes in morphology that are dependent on substrate stiffness and independent of ligand coating. A dramatic increase in spreading was observed on 100 kPa gels compared with that on 10 kPa gels, regardless of ligand Fgn, Col, or Fn at 120 s after initial cell adhesion, a time when cells typically reach their stable spread area [8] (Fig. 1A). Strikingly, the spread area of cells on 100 kPa substrates was more than double that of cells on 10 kPa substrates (Fig. 1A).
Figure 1. Mobility and TAD of neutrophil chemotaxis toward fMLP on surfaces of varying stiffness depend on ligand coating.
(A) Human neutrophils adhered to Fgn-, Col-, or Fn-coated gels show distinct changes in morphology that are dependent on substrate stiffness. Inset on each micrograph is the average spread area of migrating neutrophils at 120 s after adhesion (20× bright-field magnification; bar=50 μ). (B) For a migrating cell, trajectory plots were generated at 10-s increments between cell centroids. (C) Idealized MSD plots in log–log coordinates. The slope characterizes the type of motion. A constant value of one indicates diffusive motion, whereas a constant value of two indicates ballistic motion. (D) MSD plotted, based on the average migration trajectories of human primary neutrophils migrating on Fgn-, Col-, and Fn-coated gels of 10 kPa, 50 kPa, or 100 kPa stiffness toward a fMLP point source. Migration was tracked over a 30-min period by time-lapse DIC images acquired every 10 s (Supplemental Videos 1–3). Error bars represent sem. (E) The average MSD at a time interval of 150 s and corresponding RMS speed (denoted RMSS) for each condition. Error bars on MSD represent sem, whereas RMS speed data are plotted in quartiles by Box and Whisker Plot. *P < 0.05, and **P < 0.05 versus Fgn-coated surfaces of all stiffnesses. (F) Turning angles during cell migration are defined by the angles between subsequent displacement vectors that make up the migration trajectory. The TAD implicitly depends on the time interval used to determine the displacement vectors. (G) TADs at a 60-s time interval of cells migrating on Fgn-coated substrates (left), Col-coated substrates (middle), and Fn-coated substrates (right) are shown. (Insets) The percentage of turning angles between ±5° for each coating, denoted TAD at 0°. Error bars represent sem. *P < 0.05, 10 kPa Fn-versus 100 kPa Fn-coated surfaces. nd, No difference.
The MSD mechanosensitivity of the neutrophil chemotaxis toward fMLP is ligand-dependent
Unlike the ligand-independent effect in cell morphology, the migration dynamics on these single substrates were found to have three distinct patterns of behavior in response to mechanotactic cues. Neutrophils were allowed to migrate on Fgn-, Col-, or Fn-coated substrates toward a fMLP point source, and migrating neutrophils were tracked over a 30-min period using time-lapse DIC images acquired every 10 s (Supplemental Videos 1–3). With the use of these images, we were able to track individual cells, calculate their centroid, and generate cell-migration trajectories (Fig. 1B). We used these trajectories to quantify migration dynamics.
The MSD of tracked cells is a measure of the net distance that an average cell will travel during a particular interval of time. The dependence of the MSD on the time interval portrays the type of underlying motion: for a simple random walk, the MSD is linear with time, whereas for directed motion along a straight line, the MSD increases quadratically with time. Generally, MSD is proportional to Δtβ, where exponent β characterizes the directionality of motion. When MSD is plotted against time interval Δt in log–log coordinates, the exponent β can be estimated by the slope of the curve (Fig. 1C). A value of β = 1 indicates motion that appears random. A value of β = 2 is the theoretical upper limit and indicates purely directed motion at that given time scale (Fig. 1C). At long-time intervals, where the displacement of the migrating cell approximates a straight line from the starting position to the fMLP point source, the slope of the MSD curve approaches two, whereas at shorter time intervals, which reveal the contours of the natural path wiggle of the cell migration, the slope tends toward one. Overall, β increases from one to two as the time interval increases.
The MSDs for cells migrating on Fgn-, Col-, or Fn-coated substrates are plotted in Fig. 1D. The corresponding MSDs and RMS speed at a time interval of 150 s are shown in Fig. 1E. This time interval corresponds to constant speed ballistic motion under all conditions studied and is appropriate for comparing the overall relative motion of cells migrating toward the chemotactic source. Cells migrating on Fn- and Col-coated substrates have mechanosensitive changes in MSD, with cells showing greater displacements on softer substrates. Cells migrating on Fgn-coated surfaces show the largest displacements, which are independent of substrate stiffness.
The TAD mechanosensitivity of neutrophil chemotaxis toward fMLP is ligand-dependent
Prior studies from this lab demonstrated mechanosensitive differences in TAD of neutrophils migrating on 10 kPa and 100 kPa Fn-coated substrates [8]. The TAD during cell migration is determined by measuring the angle of displacement with respect to the previous step at a given time interval (shown in Fig. 1F for a 10-s time interval). The TAD for cells migrating as a function of ligand and substrate stiffness at a time interval of 60 s is shown in Fig. 1G. When analyzing TAD, a comparison of histogram height at 0°, which represents the percentage of turning angles that fall between −5° and +5°, enables a measure of migration persistence relative to treatment conditions (Fig. 1G, inset bar graphs; denoted TAD at 0°). Consistent with our previous data [8], cells migrating on Fn-coated gels demonstrate mechanosensitive TAD, with cells migrating on 100 kPa substrates showing a significantly greater persistence than cells migrating on 10 kPa substrates. In contrast, cells migrating on Fgn-coated surfaces show strongly directed motion with no significant dependence on substrate stiffness by this measure. In contrast, cells migrating on Col-coated substrates demonstrated a TAD independent of substrate stiffness and migration trajectories significantly less directed than all but cells migrating on the softest Fn-coated substrate.
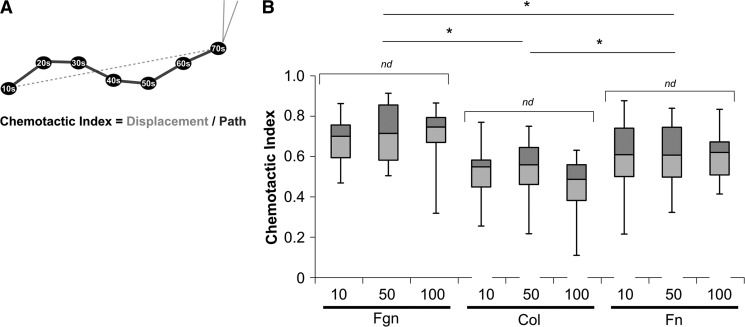
The CI of neutrophil chemotaxis toward fMLP is ligand-dependent and independent of substrate stiffness
Another measure of migration persistence, CI, which is calculated as a ratio of the net distance a cell migrates toward the chemotactic point source to the total migration path length, is used to quantify the twistedness of the migration path [18] (Fig. 2A). With the use of a time interval of 10 s to calculate the total migration path, CI values for neutrophils migrating on Fgn-, Col-, or Fn-coated gels showed no mechanosensitive variation (Fig. 2B). The value of CI depended on ligand coating and varied significantly between ligands, with cells on Fgn-coated substrates migrating with the highest CI, followed by cells migrating on Fn-coated substrates, and with cells on Col-coated substrates migrating with the lowest CI. This differs from our TAD results. We attribute these inconsistencies in persistence to time-interval dependence of TAD and CI and an incomplete representation of the data based on using a single time interval for the characterization of persistence. TAD and CI depend on the time interval, as the turning angles and total migration path length, respectively, depend on time interval. The subjectivity of choosing a single time interval introduces inconsistency, making comparisons across current persistence measurements potentially inaccurate. This variability led us to develop a novel, time interval-independent metric, called DI, to quantify persistence without subjective user input. DI is derived as the slope of MSD, plotted in log–log coordinates. We fit the DI to a three-parameter exponential to calculate three parameters (Td, DI<, and DI>) that describe persistence, based on data about all time intervals. Directionality time, which corresponds to the decay time of the exponential function, describes the minimum observation time necessary to determine that chemotactic motion up the fMLP gradient is directional. All things equal, a larger value for Td indicates more inherent “wiggle” to a cell's migration path. DI< and DI> are averages of DI at time intervals <Td and >Td, respectively. These parameters give a measure for the amount of randomness (values near one) of the migration path at short time scales compared with the amount of directedness (values near two) in the migration path at large time scales (see Materials and Methods).
Figure 2. CI of neutrophil chemotaxis toward fMLP is ligand-dependent but independent of substrate stiffness.
(A) CI is calculated as the ratio of total displacement (dotted gray line) to total path (solid black line), as shown in the schematic, and represents the overall directionality of the entire migration pathway. CI depends on time interval associated with the displacement vectors that make up the path. (B) The average CI for neutrophils migrating on Fgn-, Col-, and Fn-coated gels toward a fMLP point source is shown as a Box and Whisker Plot, showing mean and quartile data. CI did not vary significantly by substrate stiffness among gels of a given protein coating. CI did vary significantly by protein coating with cells on Fgn-coated gels migrating most directedly, followed by Fn-coated gels, and Col-coated gels migrating the least directedly. *P < 0.05 versus all other protein coatings.
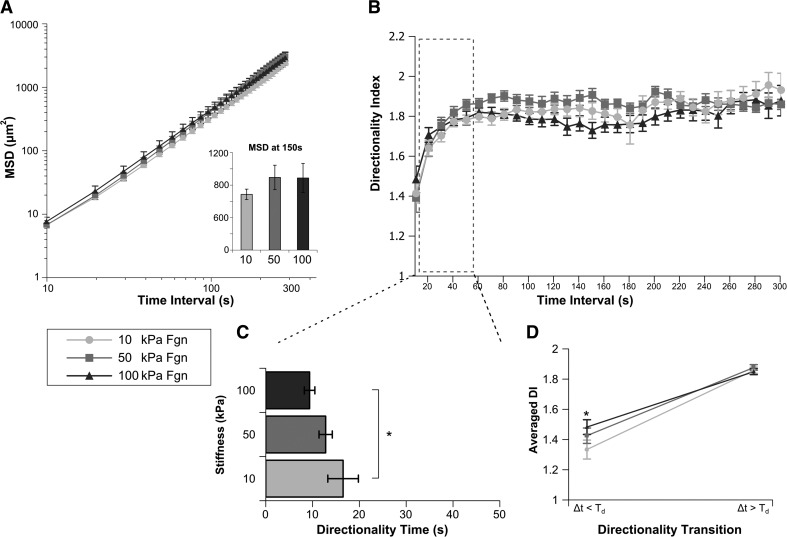
Neutrophils migrating on Fgn-coated substrates toward fMLP show mechanosensitive changes in Td and DI<
Cells migrating on Fgn-coated substrates toward a fMLP point source show no mechanosensitive difference in MSD (Fig. 3A and Supplemental Video 1) or similarly, RMS speed (Fig. 1E). Values of Td, DI<, and DI> were derived from the DI over time-interval curves (Fig. 3B). For cells migrating on Fgn-coated gels, we have identified a mechanosensitive shift in Td, with cells migrating on 100 kPa substrates showing significantly more persistent behavior than cells migrating on 10 kPa substrates (Fig. 3C). Values of DI< and DI> are represented in Fig. 3D as the directionality transition. As we increase the time interval of observation beyond Td, the essential directedness of migration becomes apparent for all conditions that we have studied, with the common DI> of approximately 1.8. When looking at time intervals smaller than Td, we see a measure of how closely the migrating cell stays fixed on its path. This value shows significant differences between conditions studied and may represent the interplay between a “homing” and a “surveying” phenotype. Cells on Fgn-coated substrates have significantly more persistent migration on 100 kPa substrates at time intervals shorter than Td than do cells migrating on 10 kPa substrates (Fig. 3D).
Figure 3. Cells migrating on Fgn-coated gels toward fMLP have similar MSD and RMS speed but show a mechanosensitive change in Td and DI<.
Human primary neutrophils migrating on Fgn-coated gels of 10 kPa, 50 kPa, or 100 kPa stiffness toward a fMLP point source were tracked over a 30-min period (Supplemental Video 1). (A) The MSD of neutrophil migration paths is plotted as a function of time between steps. (Inset) The average MSD at time interval of 150 s for each condition. MSD and RMS speed for cells migrating on Fgn-coated gels are independent of substrate stiffness. (B) The average DI of each condition is plotted over time interval. (C) For each cell, index DI(Δt) was fit to an exponential DI fit function, yielding mean values of Td that represents the time interval at which migration transitions from random to directed. Cells migrating on Fgn-coated gels show a stiffness-dependent change in Td, with cells migrating on 100 kPa gels transitioning to directed motion at a significantly shorter time interval than those on 10 kPa gels. (D) The fit of DI(Δt) was also used to generate values for DI< and DI>, which quantify the degree of randomness in the migration path over short time intervals <Td and the degree of directedness in the migration path over long time intervals >Td, respectively. These data are plotted as the directionality transition. Cells migrating on 100 kPa gels have significantly more directed DI< than cells on 10 kPa. DI> of cells on Fgn-coated gels is independent of substrate stiffness. Error bars represent sem. *P < 0.05, 10 kPa versus 100 kPa.
Neutrophils migrating on Col-coated substrates toward fMLP show a Td independent of substrate stiffness
Cells migrating on Col-coated substrates toward a fMLP point source show a mechanosensitive difference in MSD at 150 s, with cells migrating on softer substrates covering a greater distance per time interval (Fig. 4A and Supplemental Video 2). Measurements of Td, DI<, and DI>, derived from the DI(Δt) curves (Fig. 4B), plotted in Fig. 4C and D, show that migration persistence is independent of substrate stiffness. Cells migrating on Col-coated substrates show Td values significantly larger than cells migrating on Fgn-coated substrates, indicating that cells show more locally diffusive motion.
Figure 4. Neutrophils migrating on Col-coated substrates toward fMLP show Td, DI<, and DI>, independent of substrate stiffness.
Human primary neutrophils migrating on Col-coated gels of 10 kPa, 50 kPa, or 100 kPa stiffness toward a fMLP point source were tracked over a 30-min period (Supplemental Video 2). (A) The MSD of neutrophil migration paths is plotted as a function of time between steps. (Inset) The average MSD at a 150-s time interval for each condition. Cells migrating on 10 kPa Col-coated gels show a significant increase in RMS speed over cells on 50 kPa or 100 kPa gels. *P < 0.05, 10 kPa versus 50 kPa and 100 kPa. (B) The average DI of each condition is plotted over time interval. (C) For each cell, index DI(Δt) was fit to an exponential DI fit function, yielding mean values of Td. The Td of cells migrating on Col-coated gels is independent of substrate stiffness. (D) The fit of DI(Δt) was also used to generate values for DI< and DI>. These data are plotted as the directionality transition. Cells migrating on Col-coated gels have no significant differences in DI< or DI>. Error bars represent sem.
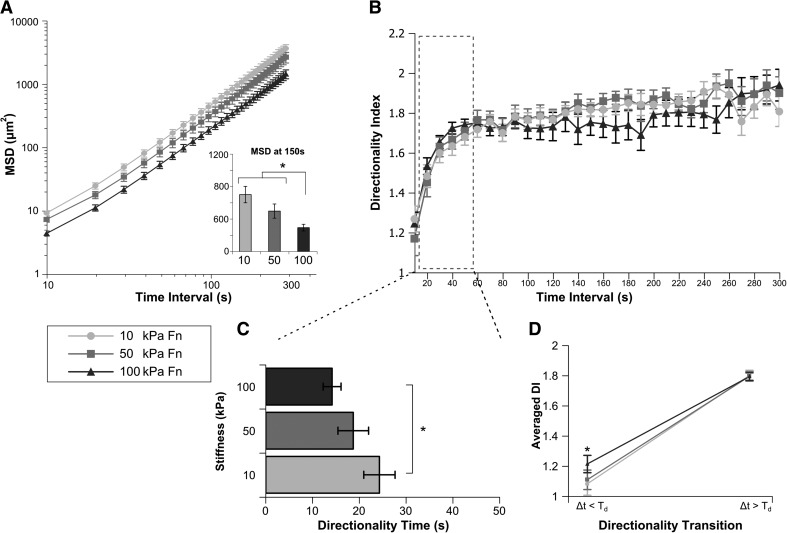
Neutrophils migrating on Fn-coated substrates toward fMLP show mechanosensitive differences in Td and DI<
Cells migrating on Fn-coated substrates toward a fMLP point source show a mechanosensitive difference in MSD at 150 s, with cells migrating on stiff substrates covering the least distance per time interval (Fig. 5A and Supplemental Video 3). Again, index DI(Δt) was fit for each cell (averages plotted in Fig. 5B) to calculate Td (Fig. 5C), and both DI< and DI> (Fig. 5D). Cells migrating on 100 kPa Fn-coated substrates show significantly smaller Td compared with those migrating on 10 kPa substrates (Fig. 5C). Cells migrating on 100 kPa substrates also showed significantly larger DI<. Taken together, cells on stiffer Fn-coated gels migrate more persistently.
Figure 5. Neutrophils migrating on Fn-coated substrates toward fMLP show mechanosensitive differences in MSD, Td, and DI<.
Human primary glneutrophils migrating on Fn-coated gels of 10 kPa, 50 kPa, or 100 kPa stiffness toward a fMLP point source were tracked over a 30-min period (Supplemental Video 3). (A) The MSD of neutrophil migration paths is plotted as a function of time between steps. (Inset) The average MSD at a 150-s time interval for each condition. Cells migrating on 100 kPa Fn-coated gels show a significant decrease in MSD when compared with cells on 50 kPa or 10 kPa gels. *P < 0.05 100 kPa versus 50 kPa or 100 kPa. (B) The average DI of each condition is plotted over time interval. (C) For each cell, index DI(Δt) was fit to an exponential DI fit function, yielding mean values of Td. Cells migrating on Fn-coated gels show a stiffness-dependent change in Td, with cells migrating on 100 kPa gels transitioning at significantly shorter time scales to directed motion than those on 10 kPa gels. (D) The fit of DI(Δt) was also used to generate values for DI< and DI>. These data are plotted as the directionality transition. The DI< of cells migrating on 100 kPa gels is significantly more directed than cells on 10 kPa. DI> of cells on Fn-coated gels is independent of substrate stiffness. Error bars represent sem. *P < 0.05, 10 kPa versus 100 kPa.
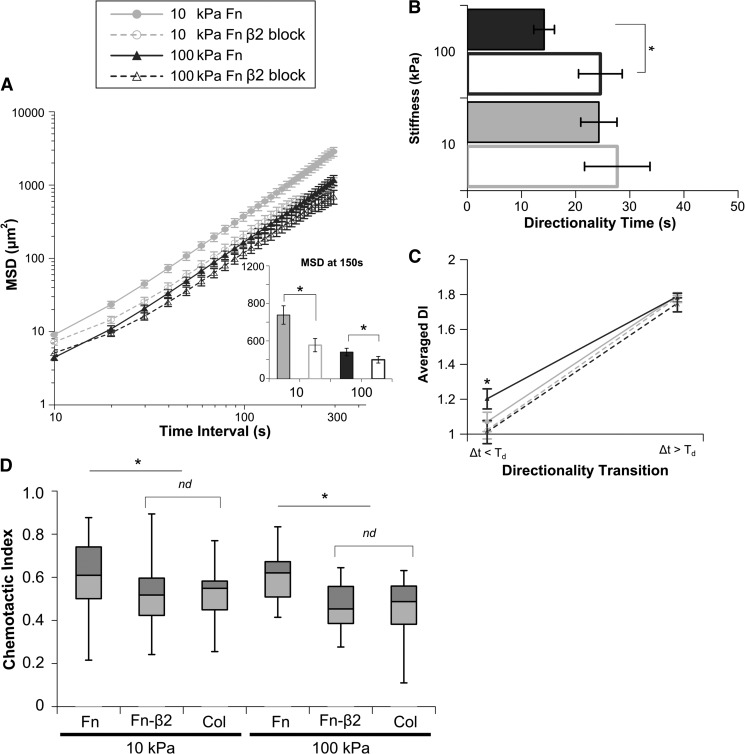
The mechanosensitive component of persistence for neutrophils migrating on Fn-coated substrates toward fMLP is dependent on β2 integrins
For cells migrating on Fn-coated surfaces that engage β1 and β2 integrins, we were able to gain some insight into a β2-integrin-dependent modulation of chemotactic parameters under our experimental conditions. Neutrophils were pretreated on ice for 30 min with 10 μg/ml β2-blocking antibody (TS1/18, which binds the I-domain and blocks the function of all β2 integrins) or isotype control (data not shown), which was maintained for the duration of the experiment. Cells migrating on Fn-coated substrates toward a fMLP point source after β2-integrin blocking show significant differences in MSD (Fig. 6A), with cells on both stiffnesses showing slower relative motion. Persistence measures, Td (Fig. 6B and Supplemental Videos 3 and 4) and DI< (Fig. 6C), demonstrate significantly less persistent migration after β2-integrin blocking that is statistically indistinguishable from that of untreated cells migrating on Col-coated substrates. This shift in directedness also holds for our other measures of persistence, CI (Fig. 6D) and TAD (data not shown). Cells migrating on Col-coated substrates toward a fMLP point source after β2-integrin blocking show no difference in relative motion or persistence measures (Supplemental Fig. 2). Additionally, blocking β2 integrins of cells on Fn-coated gels did not significantly change their spread areas, 120 s after cell adhesion (data not shown). Cells pretreated with the β1-blocking antibody (P5D2) and allowed to migrate on Fgn-coated surfaces showed no significant changes in MSD (Supplemental Fig. 3A), Td (Supplemental Fig. 3B), or DI (Supplemental Fig. 3C). Cells pretreated with the β1-blocking antibody (P5D2) neither adhere nor migrate on Col- or Fn-coated surfaces (data not shown). These data suggest that for human neutrophils on compliant surfaces, β1 integrins are driving adhesion and migration on Fn, whereas β2-integrin engagement is directing a mechanosensitive enhancement of migration persistence. Neutrophils do not regularly encounter ECM ligands in isolation. By necessity, cells are incorporating input of varying ligand ratios and substrate compliance. The interplay of β1-integrin mechanotactic shifts in RMS speed and β2-integrin-dependent modulation of persistence suggests a mechanism by which the cellular mechanosensing apparatus can be tuned to fine, incremental changes in the microenvironment through which the cell is migrating.
Figure 6. The mechanosensitive component of persistence for neutrophils migrating on Fn-coated substrates toward fMLP is dependent on β2 integrins.
Human primary neutrophils migrating on Fn-coated gels of 10 kPa or 100 kPa stiffness toward a fMLP point source were treated with β2-blocking antibody and tracked over a 30-min period (Supplemental Videos 3 and 4). (A) The MSD of neutrophil migration paths is plotted as a function of time between steps. (Inset) The average MSD at a 150-s time interval for each condition. Blocking β2 integrins significantly decreased MSD and RMS speed on Fn-coated gels of 10 kPa and 100 kPa. (B) For each cell, index DI(Δt) was fit to an exponential DI fit function, yielding mean values of Td that represent the time interval at which migration transitions from random to directed. Blocking β2 integrins increased Td on 100 kPa Fn-coated gels. β2-Integrin-blocked cells migrating on Fn-coated gels have a Td equivalent to untreated cells migrating on Col-coated gels. (C) The fit of DI(Δt) was also used to generate values for DI< and DI>. These data are plotted as the directionality transition. Blocking β2 integrins significantly changes DI< and alters the directionality transition profile to one indistinguishable from untreated cells on Col-coated gels. (D) Cells migrating on Fn-coated gels after β2-integrin blocking have significantly lower CIs that are statistically equivalent to those of cells migrating on Col-coated gels. The average CI for neutrophils migrating on Fn- and Col-coated gels toward a fMLP point source is shown as a Box and Whisker Plot, showing mean and quartile data. Error bars represent sem. *P < 0.05 untreated versus β2-integrin block on Fn.
These different patterns of substrate-dependent mechanotactic sensitivity are particularly interesting in that they point to the fine interpretation of extracellular signals required to regulate a neutrophil during chemotaxis: integrating mechanical properties of the substrata, nature, and concentration of chemotactic signals, as well as the composition of the ECM and ligand density. For example, regulation of the migratory response may be more subject to mechanical cues under conditions of suboptimal chemokine concentrations rather than under conditions of saturating chemokine concentrations. Alternatively, an examination of different chemotactic signals, such as IL-8 or C5a, might yield more pronounced differences in migration between the stiffnesses of Fgn-coated substrates examined here. Perhaps Fgn, as a nondiffusible cue of an inflamed matrix, and the chemotactic stimulus of the fMLP gradient may coordinate to increase efficiency of neutrophil targeting to a degree that overwhelms mechanotactic input. Mechanotactic shifts in RMS speed may prove to be dependent on β1-integrin engagement.
Our work reported here, using the predominantly β1-integrin ligands Fn and Col and the β2-restricted ligand Fgn, identifies an extraordinary level of complexity in the mechanical regulation of neutrophil migration dependent on the nature of the matrix ligand (Table 1). The RMS speed and persistence of neutrophil migration are both affected by substrate stiffness on Fn. Persistence alone is affected by surface stiffness on the β2-restricted ligand Fgn, whereas only the RMS speed alone is altered by surface stiffness on the β1-restricted ligand Col. Therefore, under the conditions studied, differences between the parameters of migration dynamics demonstrate a selectivity among integrin ligands that allows for differential regulation of MSD and persistence in response to mechanotactic cues. Additionally, the metrics that we derived to examine the time scale of the transition from diffusive to directed migration reveal a previously unrecognized intricacy in the persistence of neutrophil migration that can be mechanodirected by β2-integrin engagement, underscoring the need for sensitive quantitative measures of chemotaxis.
Table 1. Mechanosensitive Parameters of Human Neutrophil Chemotaxis toward fMLP.
| Mechanosensitive migration parameters | |||||
|---|---|---|---|---|---|
| Characteristic measured | Metric | Fgn | Col | Fn | Fn + β2 block |
| Morphology | Spread area | ✓ | ✓ | ✓ | ✓ |
| Relative motion | MSD or RMSS | ✓ | ✓ | ✓ | |
| Current measures of path wiggle | TAD | ✓ | |||
| CI | |||||
| New measures of path wiggle (this study) | Td | ✓ | ✓ | ||
| DI (t<Td) | ✓ | ✓ | |||
| DI (t>Td) | |||||
Chemotactic parameters found to be mechanosensitive for different coating and treatment conditions. A check mark represents a parameter found to vary significantly by stiffness under the condition indicated.
Other investigations into mechanosensing focus on the lower range of stiffness, 0.3–3 kPa [9], which fits well with reported measures of endothelial cell stiffness. The 10- and 100-kPa substrates used in these studies represent the low and high end of physiological stiffness reported in diverse tissue types [14, 20, 21]. In particular, we are interested in discovering how mechanotactic changes in tissue, such as in formation of some tumors, combine with ligand composition and chemotactic influences to progress or support a pathologic or diseased tissue environment. This is likely a key step in mediating chronic inflammation and fibrosis, where the inflammatory response itself changes the tissue environment. In the context of a resolving site of inflammation, where matrix deposition is dense, the local environment is also stiffened. It is plausible that increased stiffness may affect matrix remodeling, such as through increased matrix metalloproteinase release, but this hypothesis may be confounded by the inability to dissociate the effect of matrix stiffness from ligand density. Moreover, this may vary among tissues, where the response to injury may result in deposition of different matrix components. Under conditions where an injury response includes progressive tissue stiffening, such as Acute Respiratory Distress Syndrome, tumor growth, or fibrosis, the changes in rigidity would be temporally regulated. The mechanosensitive response of the extravasated neutrophil would be expected to vary according to the time in the host response that the cell entered the site of injury. The synchronicity of β1- and β2-integrin engagement may additionally afford a transition between surveying and homing phenotypes in chemotaxing cells. β1-Integrin engagement supports decreased cell motility on stiffer substrates responding to the tonicity of a tissue as a marker of increased injury or inflammation, while maintaining the local diffusivity of a surveying phenotype. With β1- and β2-integrin engagement, we see both modulation of RMS speed and persistence, with β2-integrin engagement supporting a homing phenotype of enhanced cell motility and increasing persistence. Our data suggest that Fgn may be serving as a biologically significant CR3 ligand in this context by acting as a nondiffusible cue of the inflamed matrix that aids neutrophil targeting by increasing β2-integrin engagement and by extension, migration persistence up a fMLP gradient. These data combine to show that neutrophil mechanosensitivity is not only ligand-dependent but also that mechanosensitive shifts in migration dynamics are not binary. Even at this simple level, the parameters of neutrophil response are finely tunable with environmental cues. For example, it may be that cells entering a site of progressive stiffness, resulting from a wound, are mechanosensitive for Col, whereas cells encountering Fn and/or Fgn deposition are likely to be associated with an acute injury before scarring.
Current techniques to parameterize the trajectory of a chemotaxing cell most commonly pair migration speed with some measure of persistence [8, 9, 13]. The root MSD, divided by time, gives RMS speed over a particular time interval, whereas persistence is typically characterized by measuring the TAD and/or CI. TAD and CI implicitly depend on an arbitrarily chosen time interval, causing such measures to skew potentially from report to report. Furthermore, a particular time interval used to calculate TAD or CI for one experiment may not be applicable to other experiments if image-acquisition frequency is constrained. The distinction of true randomness in the migration path from randomness caused by uncertainty in determining the centroid positions is challenging. As our data represent here, migration dynamics can vary significantly by ligand coating, and it follows that the optimal time interval by which to analyze and compare migration conditions may also vary. To address this concern, we introduce directionality time, a time-interval invariant measure of persistence motivated by MSD fitting that incorporates trajectory characteristics for all time intervals. Directionality time characterizes the time scale at which migration transitions from random to directed. Conceptually, it measures the time it takes for a cell that has veered off of its directed course to reorient itself to its chemotactic path. A larger value for Td suggests that there is more inherent wiggle to a cell's migration path evocative of a surveying phenotype. Conversely, small Td suggests a more directed homing phenotype. All else equal, migration trajectories are more persistent when Td decreases. As a result of its incorporation of data across all time intervals, Td enables a global, less subjective characterization of migration persistence that can uncover subtle shifts in migration dynamics not reflected accurately using TAD or CI.
In conclusion, to migrate effectively, neutrophils must integrate many divergent signals, including chemotactic, mechanotactic, and substrate context, to initiate an appropriate cellular response to injury or inflammation. This migration necessarily incorporates elements of speed, direction, and persistence of motion. Neutrophils are mechanosensitive but may acquire mechanosensitivity by different mechanisms while migrating on different matrices. The understanding of the underlying mechanisms that regulate directed neutrophil migration dynamics, the teasing apart of the constituent contributions, and the knowledge of how they interact can provide insight into comprehending how subtle shifts in migration dynamics and overall phenotype caused by environmental cues lead to significant shifts in cellular behavior and clinical outcome. Sensitive analytical methods introduced in this work that are capable of capturing these fine changes in cellular behavior may be key to the identification of novel nodes for clinical intervention and immune modulation, which can be targeted, not just to cell type but also to the relevant tissue microenvironment.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the U.S. National Institutes of Health (GM-066194 and AI-079582 to J.S.R.); Grant Assistance in Areas of National Need from the U.S. Department of Education (X.M.O.); Natural Sciences and Engineering Research Council of Canada Postgraduate Fellowship (A.J.L.); and allocations to the Department of Surgery by Rhode Island Hospital.
The authors acknowledge Dr. Nicole Morin and Dr. Patrick Oakes for early technical support, Angel Byrd for assistance with the Odyssey Scanner, and Dr. Meredith Crane for critical reading of the manuscript.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- CCD
- charge-coupled device
- CI
- chemotactic index
- Col
- collagen
- CR3
- CD11b/CD18
- DI
- directionality index
- DI<
- microdirectionality index
- DI>
- macrodirectionality index
- Fgn
- fibrinogen
- Fn
- fibronectin
- L-15
- Liebovitz's L-15 medium
- L-15-glu
- Liebovitz's L-15 medium plus 2 mg/ml D-glucose
- MSD
- mean squared displacement
- RMS
- root mean squared
- RMSS
- root mean squared speed
- sulfo-SANPAH
- sulfosuccinimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate
- TAD
- turning angle distribution
- Td
- directionality time
AUTHORSHIP
X.M.O., A.J.L., K.E.O., J.X.T., and J.S.R. designed and planned the study. X.M.O. and K.E.O. performed the experiments. X.M.O., A.J.L., and K.E.O. analyzed the data and wrote the manuscript. X.M.O. prepared the figures.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Mayadas T. N., Cullere X. (2005) Neutrophil β2 integrins: moderators of life or death decisions. Trends Immunol. 26, 388–395 [DOI] [PubMed] [Google Scholar]
- 2. Pelham R. J., Wang Y. L. (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94, 13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghosh K., Pan Z., Guan E., Ge S., Liu Y., Nakamura T., Ren X-D., Rafailovich M., Clark R. A. F. (2007) Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 28, 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flanagan L. A., Ju Y-E., Marg B., Osterfield M., Janmey P. A. (2002) Neurite branching on deformable substrates. Neuroreport 13, 2411–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engler A. J., Griffin M. A., Sen S., Bönnemann C. G., Sweeney H. L., Discher D. E. (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, 877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engler A., Bacakova L., Newman C., Hategan A., Griffin M., Discher D. (2004) Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 86, 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 [DOI] [PubMed] [Google Scholar]
- 8. Oakes P. W., Patel D. C., Morin N. A., Zitterbart D. P., Fabry B., Reichner J. S., Tang J. X. (2009) Neutrophil morphology and migration are affected by substrate elasticity. Blood 114, 1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jannat R. A., Dembo M., Hammer D. A. (2010) Neutrophil adhesion and chemotaxis depend on substrate mechanics. J. Phys. Condens. Matter 22, 194117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Litzenberger J. B., Kim J-B., Tummala P., Jacobs C. R. (2010) β1 Integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif. Tissue Int. 86, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gehler S., Baldassarre M., Lad Y., Leight J. L., Wozniak M. A., Riching K. M., Eliceiri K. W., Weaver V. M., Calderwood D. A., Keely P. J. (2009) Filamin A-β1 integrin complex tunes epithelial cell response to matrix tension. Mol. Biol. Cell 20, 3224–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altieri D. C., Bader R., Mannucci P. M., Edgington T. S. (1988) Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J. Cell Biol. 107, 1893–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin F., Nguyen C. M-C., Wang S-J., Saadi W., Gross S. P., Jeon N. L. (2004) Effective neutrophil chemotaxis is strongly influenced by mean IL-8 concentration. Biochem. Biophys. Res. Commun. 319, 576–581 [DOI] [PubMed] [Google Scholar]
- 14. Yeung T., Georges P. C., Flanagan L. A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W., Weaver V., Janmey P. A. (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell. Motil. Cytoskeleton 60, 24–34 [DOI] [PubMed] [Google Scholar]
- 15. Mueller O., Gaub H. E., Baermann M., Sackmann E. (1991) Viscoelastic moduli of sterically and chemically cross-linked actin networks in the dilute to semidilute regime: measurements by oscillating disk rheometer. Macromolecules 24, 3111–3120 [Google Scholar]
- 16. Lo C. M., Wang H. B., Dembo M., Wang Y. L. (2000) Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marquardt D. W. (1963) An algorithm for least-squares estimation of nonlinear parametrics. J. Soc. Ind. Appl. Math. 11, 431–441 [Google Scholar]
- 18. McCutcheon M. (1946) Chemotaxis in leukocytes. Physiol. Rev. 26, 319–336 [DOI] [PubMed] [Google Scholar]
- 19. Discher D. E., Janmey P., Wang Y-L. (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto T., Abe H., Ohashi T., Kato Y., Sato M. (2002) Local elastic modulus of atherosclerotic lesions of rabbit thoracic aortas measured by pipette aspiration method. Physiol. Meas. 23, 635–648 [DOI] [PubMed] [Google Scholar]
- 21. McKnight A. L., Kugel J. L., Rossman P. J., Manduca A., Hartmann L. C., Ehman R. L. (2002) MR elastography of breast cancer: preliminary results. Am. J. Roentgenol. 178, 1411–1417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.