Abstract
Angiotensin (ANGII) and secretin (SCT) share overlapping, interdependent osmoregulatory functions in brain, where SCT peptide/receptor function is required for ANGII action, yet the molecular basis is unknown. Since receptors for these peptides (AT1aR, SCTR) are coexpressed in osmoregulatory centers, a possible mechanism is formation of a cross-class receptor heterocomplex. Here, we demonstrate such a complex and its functional importance to modulate signaling. Association of AT1aR with SCTR reduced ability of SCT to stimulate cyclic adenosine monophosphate (cAMP), with signaling augmented in presence of ANGII or constitutively active AT1aR. Several transmembrane (TM) peptides of these receptors were able to affect their conformation within complexes, reducing receptor BRET signals. AT1aR TM1 affected only formation and activity of the heterocomplex, without effect on homomers of either receptor, and reduced SCT-stimulated cAMP responses in cells expressing both receptors. This peptide was active in vivo by injection into mouse lateral ventricle, thereby suppressing water-drinking behavior after hyperosmotic shock, similar to SCTR knockouts. This supports the interpretation that active conformation of AT1aR is a key modulator of cAMP responses induced by SCT stimulation of SCTR. The SCTR/AT1aR complex is physiologically important, providing differential signaling to SCT in settings of hyperosmolality or food intake, modulated by differences in levels of ANGII.—Lee, L. T. O., Ng, S. Y. L., Chu, J. Y. S., Sekar, R., Harikumar, K. G., Miller, L. J., Chow, B. K. C. Transmembrane peptides as unique tools to demonstrate the in vivo action of a cross-class GPCR heterocomplex.
Keywords: secretin, angiotensin II, in vivo analysis
G-protein-coupled receptors (GPCRs) are key to the communication of extracellular conditions to intracellular signaling events. As the largest receptor family, the mechanism of how GPCRs respond to the wealth of extracellular information in the regulation of intracellular signaling has been an important biological question. Our previous understanding of GPCRs was built based on the concept of one GPCR directly coupling to an individual effector (e.g., G-protein complex). However, the recent recognition of GPCR oligomerization has provided an alternative view, with fine-tuning of receptor function via changes in ligand binding, signaling, and receptor regulation (1). There is substantial in vitro evidence for formation of GPCR oligomers (2), but recognition of in vivo physiological roles has been quite limited, due to experimental limitations of current in vivo studies.
Based on the phenotypes of several luteinizing hormone receptor (LHR) transgenic mice, Vassart et al. (3) elegantly provided the first in vivo evidence for functional importance of the association of receptor molecules. In their study, homozygous LHR-knockout (KO) or heterozygous LHR-KO mice with either the LHR binding-deficient mutant (LHRLH) or the signaling-deficient mutant [LHR cyclic adenosine monophosphate (LHRcAMP)] exhibited hypogonadism and sterility. However, the presence of both LHRLH and LHRcAMP mutant genes in heterozygous mice was able to rescue gametogenesis and lead to the development of normal phenotypes. Further evidence was provided by Albizu et al. (4) where they showed dimerization of GPCRs in native tissues such as the mammary gland, brain, and muscle by fluorescence resonance energy transfer (FRET) measurements between 2 fluorescently tagged antagonists for oxytocin and vasopressin receptors.
ANGII is well studied for its actions in water/salt homeostasis in the brain and periphery, while there is emerging evidence supporting overlapping osmoregulatory functions of secretin (SCT) with ANGII in the brain (5). Intracerebroventricular (i.c.v.) administration of SCT or ANGII, as well as hyperosmotic treatment via saline drinking or peritoneal injection of high-salt solution, is able to stimulate water intake, hypothalamic expression, and pituitary release of vasopressin (Vp) and SCT (5, 6). The observation that remains highly elusive and hence controversial is that i.c.v. administration of ANGII in SCT−/− or SCTR−/− mice produced minimal or insignificant effects in stimulating water intake and Vp expression and release (6). These data, therefore, indicate that the presence of a functional SCT/SCT receptor (SCTR) axis in the brain is required for the central nervous system actions of ANGII. Since both angiotensin receptor type 1A (AT1aR; ref. 7) and SCTR (6) are expressed in brain osmoregulatory centers in circumventricular organs and hypothalamic paraventricular nucleus (PVN), a possible mechanism to explain these observations is the formation of cross-class heterocomplexes of the class B SCTR and the class A AT1aR, which could lead to modulation of receptor functions, and subsequently changes in physiological responses. In this report, by establishing that selected transmembrane (TM) peptides of SCTR and AT1aR were able to interact with their respective receptors alone as well as within complexes, and identifying one such peptide that selectively modulated only the heterocomplex, we demonstrated the in vivo function of heterocomplexes of these receptors in regulating water drinking behavior during hyperosmotic shock.
MATERIALS AND METHODS
For cAMP assay, Lance cAMP assay kits and white Opti-plates were purchased from Perkin Elmer (Wellesley, MA, USA). CHO-K1 cells and COS-1 cells were purchased from American Type Culture Collections (Manassas, VA, USA). Coelenterazine h in bioluminescence resonance energy transfer (BRET) assay was from Promega (Madison, WI, USA). The TM peptides were synthesized by Genscript (Piscataway, NJ, USA) or Anaspec (Fremont, CA, USA) with >90% purity. The sequences of the TM peptides are listed in Supplemental Table S2. Other reagents, including Versene, HBSS, MEM, and FBS, were purchased from Invitrogen (Carlsbad, CA, USA).
Animals
Procedures of animal care and handling were in accordance with the protocols approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong. All experiments were carried out using adult mice (20–25 g) of ≥N10 generation. The construction of SCTR−/− mice was described previously (8).
Receptor constructs
Mouse secretin receptor tagged with Renilla luciferase (Rlu) at the carboxyl-terminus was ligated into the pcDNA3.1 expression vector. This construct was the donor in the BRET studies. AT1aR, AT1bR, and AT2R were tagged with the yellow fluorescent protein (YFP) at the carboxyl terminus as acceptors by cloning of the cDNA into pcDNA3.1. AT1aR mutants, AT1aR-CAM and AT1aR-K199A, were constructed by Infusion cloning kit (Clontech, Palo Alto, CA, USA) with specific mutation primers. For FRET assay, SCTR, AT1aR, and AT2R were tagged with green fluorescent protein (GFP) and red fluorescent protein (RFP) in pTaqGFP2-N and pTaqRFP-N vector (Evrogen, Iowa City, IA, USA) respectively. Other constructs are described in previous publications (9, 10).
Cell culture and transfection
CHO-K1 cells and COS cells were cultured in MEM supplemented with 4.5 mg/ml d-glucose, 10% FBS, 100 U/ml penicillin G, and 100 μg/ml streptomycin at 37°C with 5% CO2. For transfection, cells were seeded onto 6-well plates (35 mm/well; Costar, San Diego, CA, USA) at a density of 2.0 × 105 cells/well. After 2 d, transient transfection was carried out using the X-tremeGene HP DNA transfection reagent (Roche Diagnostics, Shanghai, China) according to the manufacturer's instructions. For saturation BRET assay, SCTR-Rlu (1 μg/well) was cotransfected with different amounts of AT1aR-YFP, AT1bR-YFP, AT2R-YFP, or MOP-YFP DNA (ranging from 0.1 to 4.0 μg/well). An appropriate amount of the pcDNA3.1 was added so that the total amount of DNA used per well was the same.
BRET assay
BRET measurements were performed 48 h after transfection. Cells were detached with Versene and washed with HBSS. The cells were resuspended in HBSS buffer, counted (Luna; Logos Biosystems, Annandale, VA, USA), and seeded in 96-well Opti-plates (PerkinElmer) at a density of 100,000 cells/well. The cell-permeable Rlu substrate, Coelenterazine h (Promega), was added at a final concentration of 5 μM. BRET ratio was calculated based on the ratio of long (530 nm)/short (485 nm) emission signals (10) using the Victor X or 2103 Envision multiplate reader (PerkinElmer). In saturation BRET experiments, CHO cells were cotransfected with a fixed amount of Rlu-tagged receptor (1.0 μg/well in 6-well plate) and with increasing amounts of YFP-tagged receptors (0.1 to 4 μg/well). After 48 h, the transfected cells were harvested for BRET assays. The saturation curves were calculated using Prism 5.0 (GraphPad Software, San Diego, CA, USA). For TM peptide treatment, the transfected CHO cells were incubated with 40 μg/ml of TM peptides for 2 h at 4°C before the BRET assays.
Morphological FRET assay
Transfected CHO-K1 cells 24 h posttransfection were seeded at a density of 1.0 × 106 cells/ml in μ-Slide VI0.4 (Ibidi, Martinsried, Germany). After overnight incubation, the cells were rinsed twice with 1× PBS and fixed with ice-cold 3.7% paraformaldehyde for 10 min at room temperature. After fixation, the cells were rinsed twice with 1× PBS. Confocal images of (12 bit) were acquired using the Zeiss LSM 710 computerized image analysis system for multitrack channels (Carl Zeiss, Thornwood, NY, USA) with the following configurations: GFP channel (donor excitation/donor emission: 488 nm/506 nm), RFP channel (555 nm/584 nm), and FRET channel (488 nm/584 nm: FRETex/em). The LSM images were analyzed based on a previous study (11) using ZEN2012 (Carl Zeiss). In brief, the total gray value intensities in the 3 channels, IGFP (488 nm/506 nm), IRFP (555 nm/584 nm), and IFRET (488 nm/584 nm), were measured in the same regions of interest (ROIs). The net FRET (InFRET) was obtained by correcting contributions from spectral bleed-through (SBT) of the donor and acceptor in the FRET channel (InFRET = IFRET − IRFP × a − IGFP × b), where a and b are the coefficients due to SBT of acceptor and donor signal to the FRET channel, respectively. The SBTs were determined with CHO-K1 cells expressing pTagRFP-N (a=FRETex/em/RFPex/em) or pTagGFP2-N (b=FRETex/em/GFPex/em) only. To make the InFRET values comparable among different cells and ROIs, InFRET was normalized with IGFP.
cAMP assay
Briefly, the receptor-transfected cells were diluted in the stimulation buffer in the Lance cAMP assay kit (10,000 cells/5 μl) with the Alexa-647-labeled anti-cAMP antibody. The cells were incubated with the peptide hormones (SCT and/or ANGII) for 45 min. After that, a detection mix containing Eu-streptavidin and biotin-cAMP was added and incubated for 1 h. The TRF signals were detected in Victor X4 (PerkinElmer). For TM peptide treatment, the cells were preincubated with a TM peptide (40 μg/ml) for 2 h at 4°C before assay.
Receptor internalization studies
Fluorescently tagged receptors (ATR1aR-YFP and SCTR-GFP2) transiently expressed on HEK293 cells were tracked morphologically after stimulating the cells with agonists, following the methods previously reported (12). Cells grown on sterile coverslips were transfected with either of the receptor constructs alone or in combination (0.8 μg of total DNA/coverslip) using polyethyleneimine. At 48 h after transfection, the slides were washed with PBS (pH 7.4) and incubated with 100 nM of ligand as indicated (ANGII or SCT) at 4°C for 90 min. The cells were then washed with ice-cold PBS and incubated further with prewarmed PBS at 37°C for either 5 or 37 min, as indicated. The cells were fixed at each time point with 2% paraformaldehyde, washed with PBS, and mounted on slides. Receptor fluorescence was tracked morphologically using a Zeiss LSM510 confocal microscope (pinhole diameter of 210 μm with a plan-apochromat ×63 1.3 numerical aperture oil objective) configured to capture either GFP2 (λex 405 nm, λem 480–520 nm band-pass filter) or YFP (λex 488 nm, λem 500–550 nm band-pass filter) fluorescence. Background-subtracted images were assembled using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA, USA). Cells in which both receptors were expressed were stimulated with the natural agonist of the fluorescently tagged receptor as a positive control and were stimulated with the nonfluorescent receptor with its natural agonist to evaluate whether the two receptors in a heteroreceptor complex might internalize together. A negative control was provided by expressing only the fluorescently tagged receptor and stimulating those cells with the natural agonist of the opposite receptor. Time-courses were evaluated and documented morphologically.
I.c.v. incannulation and drug administration
C57 mice were anesthetized (10% chloral hydrate; i.p.) and positioned in a stereotactic apparatus (SR-6N; Narishige, Tokyo, Japan). The coordinates of cannula implantation were determined according to the mouse brain atlas of Paxinos and Franklin (13). The cannula (11 mm long, 21 gauge) was placed (Bregma: 0.5 mm, lateral: 1.0 mm, depth: 2.0 mm) and secured using Vetbond (3M Animal Care Products, St Paul, MN, USA) and dental cement (GC America, Alsip, IL, USA). The animals were left to recover for 3 d before experiments. To study the effect of TM peptides on hyperosmolality-induced drinking, mice received i.p. injections with 1 M NaCl (2% vol/BW). After 15 min, TM peptide (4 μg in 5 μl) or PBS was injected centrally through the i.c.v. cannula. The mice were housed in metabolic cages for another 15 min before water was supplied via a plastic burette (0.1 ml graduation) attached to a spout as described previously (14), and water intake was read directly from the burette at various time points. To study the dipsogenic effects of SCT and/or ANGII, PBS, SCT (AnaSpec), ANGII (Phoenix Pharmaceuticals, Burlingame, CA, USA), or both peptides in 5 μl was injected. Water intake was monitored 30 min after injection as described earlier.
Statistical analysis
All data are presented as means ± se. Deviations between groups were analyzed using Prism 5.01 software (GraphPad). Data obtained from cAMP assays were fitted by a 4-parameter logistic function yielding parameter values for a ligand's potency (EC50), maximum (Emax), and basal effect.
RESULTS
SCTR and AT1aR associate to form heterocomplexes that reduce cAMP responses to SCT
In a previous report (6), we have already shown colocalization of SCTR and AT1aR in magnocellular neurons of the PVN. This provided an anatomical basis for a possible interaction of these receptors in an in vivo context. Using receptor BRET assays, we were able to show in vitro the presence of heterocomplexes including mouse SCTR and AT1aR (Fig. 1A, B; also observed for human SCTR and AT1R; Supplemental Fig. S1). The positive static BRET assays were confirmed in saturation BRET assays, also demonstrating that these complexes form with very low quantities of receptor present. This process is receptor specific, since SCTR can form homomers and heteromers with AT1aR but did not associate with AT2R. The SCTR-AT1bR cotransfected cells also produced a significant BRET signal and a positive saturation curve, but with a substantially lower maximal signal, indicating that this structurally related isoform of AT1aR was also able to interact with SCTR. In addition to BRET, we were also able to use the FRET assay to further demonstrate the locality of these heterocomplexes within transiently transfected CHO-K1 cells. Figure 1C shows a series of confocal images that were used for FRET analysis. The GFP-RFP fusion construct was used as a positive control, showing robust FRET signals globally. A second positive control included the use of GFP- and RFP-tagged SCTR constructs, with homomers detected exclusively at the plasma membrane and is consistent with a previous report (9). The ability for SCTR and AT1aR to form heterocomplexes was also substantiated, with FRET localization of the heteromers in the plasma membrane but at a lesser degree than SCTR homomers as demonstrated by the reduced signal intensities both visually as well as quantitatively (Fig. 1D). This association is specific, since the coexpression of SCTR and AT2R was unable to generate any significant FRET signal.
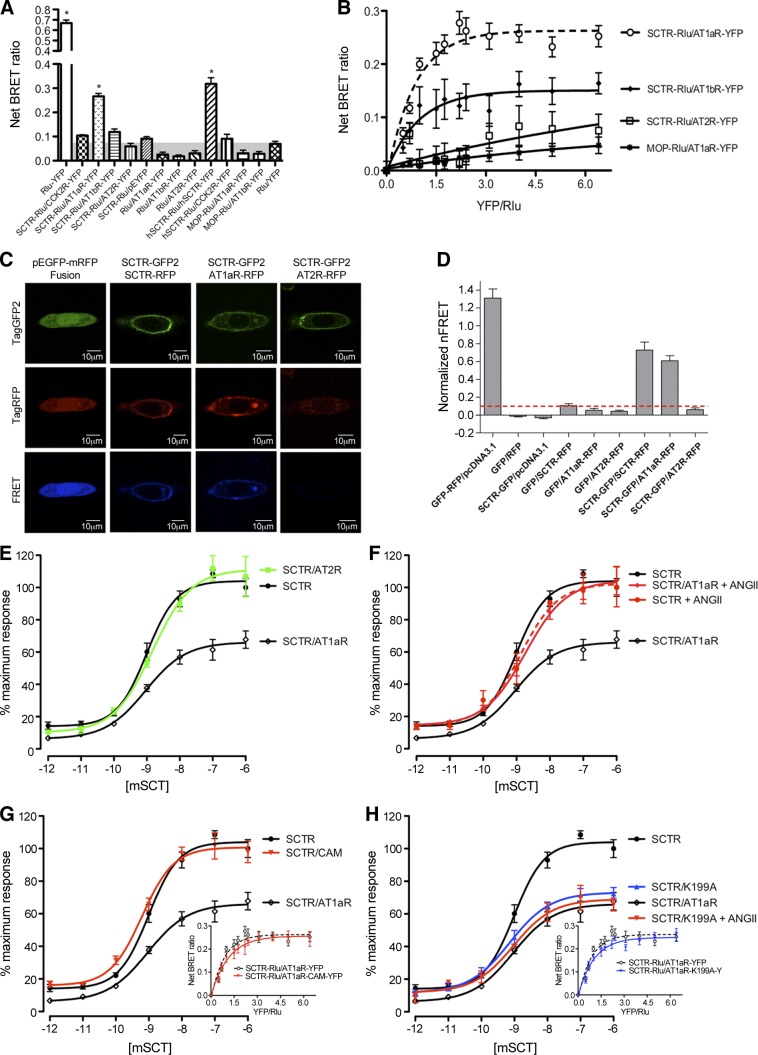
Figure 1.
SCTR forms heteromers with AT1aR and changes its cAMP response to SCT. A) BRET ratios in cells transfected with combinations of Rlu- and YFP-tagged GPCR constructs. *P < 0.001 vs. background BRET signal. B) BRET saturation curves plotted as a ratio of YFP- and Rlu-tagged constructs using a fixed amount of donor DNA (mouse SCTR-Rlu) and increasing amounts of acceptor DNA (YFP-tagged mouse receptors) that were cotransfected into cells. The SCTR-Rlu and AT1aR-YFP or AT1bR-YFP cotransfected cells produced exponential curves consistent with a saturable BRET signal. No saturation curves were observed in the cotransfections of SCTR-Rlu with AT2R-YFP or MOP-Rlu with AT1aR-YFP. C) Morphological FRET analyses of SCTR and AT1aR complex. Representative images of paraformaldehyde-fixed CHO-K1 cells expressing various combinations of GFP- and RFP- tagged receptors. Images in the top, middle, and bottom rows represent GFP, RFP, and FRET (normalized) signals, respectively. Images of cells coexpressing (from left to right) GFP-RFP fusion and pcDNA3.1; SCTR-GFP and SCTR-RFP; SCTR-GFP and AT1aR-RFP; and SCTR-GFP and AT2R-RFP. FRET signals were detected strongly throughout the entire cell when expressing the GFP-RFP fusion. Coexpression of SCTR-GFP with SCTR-RFP or AT1aR-RFP but not mAT2R resulted in FRET signals detected at the plasma membrane. D) SCT and ANGII receptor heterodimerization assessed by quantitative normalized FRET analysis. nFRET values taken of an average of ROIs chosen at the plasma membrane of CHO-K1 cells expressing combinations of GFP- and RFP-tagged constructs, inclusive of important controls. Data are expressed as means ± se of ≥4 independent experiments inclusive of 30–40 ROIs. E) The coexpression of AT1aR, but not AT2R, with mouse SCTR significantly reduced the maximal and basal cAMP responses of SCTR stimulated with graded concentrations of mouse SCT peptide. The maximum cAMP level stimulated by SCT in SCTR-transfected cells was set at 100%. F) This reduction in cAMP response was rescued in the presence of 100 nM ANGII. G) Coexpression of SCTR with an AT1aR mutant in a constitutively active conformation (CAM) also rescued the cAMP response of SCTR. H) Coexpression of SCTR with another AT1aR mutant (K199A), defective in its ability to bind ANGII, in the absence or presence of 100 nM ANGII, produced cAMP responses similar to cells cotransfected with SCTR/AT1aR in the absence of ANGII. These data are consistent in showing that the interaction of ANGII with AT1aR is important for the normal function of the SCTR when it is coexpressed in the same cell. Data are presented as means ± se of data from ≥4 independent experiments performed in triplicate. The small figures in G and H show the BRET saturation curves of mouse SCTR-Rlu with increasing amounts of YFP-tagged AT1aR mutant (AT1aR-CAM-YFP or AT1aR-K199A-YFP). Both mutants produced exponential curves consistent with a saturable BRET signal with SCTR-Rlu, similar to that of the wild-type AT1aR.
The effect of the mouse SCTR/AT1aR heterocomplex to modify intracellular signaling was investigated by comparing this with SCTR control. The cAMP levels in SCTR/AT1aR cotransfected cells when stimulated with a low concentration of SCT peptide (e.g., 1 pM) dropped from 14.0 to 6.2%, and the Emax was reduced to 81.2 and 66.1% using 1 and 2 μg of cotransfecting AT1aR DNA (Fig. 1E and Supplemental Fig. S2), while there were no significant differences in EC50 values (SCTR: 0.98 nM; AT1aR/SCTR: 0.92 nM; Supplemental Table S1). Again, the functional effect of this complex was specific, since coexpression of SCTR with AT2R, which does not associate with SCTR, had no effect, and the complex did not affect the Ca2+ signaling through AT1aR (Supplemental Fig. S3). Regarding the lowered basal response, GPCRs are known to have intrinsic activity by spontaneous formation of active states that are part of the normal sampling of tertiary structures. Our data, therefore, suggest that the presence of AT1aR in the system stabilizes/favors an inactive conformation of SCTR. Of note, these negative effects on cAMP responses could be rescued by the coincubation of ANGII together with SCT (Fig. 1F). In the presence of 100 nM ANGII, the maximal and basal responses of AT1aR/SCTR cells were 104.5 and 14.4%, respectively, which are similar to those in cells transfected with SCTR alone. Since AT1aR cannot trigger a cAMP response when stimulated with ANGII (Supplemental Fig. S4), this recovery of the cAMP response was not a direct downstream signaling event of AT1aR but was likely mediated through ANGII binding to the AT1aR affecting the conformation of SCTR, changing it to its active state. To further test this hypothesis, we have used 2 well-characterized AT1aR mutants, AT1aR-CAM and AT1aR-K199A (Fig. 1G, H). The AT1aR-CAM is a constitutively active mutant (CAM) of AT1aR in which Asn111 is changed to Gly (15). Coexpression of this mutant alone, without ANGII, was sufficient to rescue the normal cAMP response of the SCTR (Fig. 1G). As shown in BRET assays, both mutant receptors were capable of interacting with SCTR, similar to that of the wild-type AT1aR (Fig. 1G, H). These data therefore indicate that when AT1aR is active, either by mutation or by binding ANGII, the active conformation of the SCTR was favored. In contrast, cotransfecting the binding-deficient mutant AT1aR-K199A (15) with SCTR, even in the presence of ANGII, was unable to restore the cAMP response of SCTR (Fig. 1H).
A possible explanation for the recovery of cAMP responses of SCTR in the presence of AT1aR/ANGII could be a result of ANGII-induced internalization of AT1aR, leading to a diminished negative influence of AT1aR on SCTR. To study this, we performed BRET assays in the presence of 100 nM ANGII or SCT peptide (Supplemental Fig. S8). Our data indicate that there were no changes in heteroreceptor complex formation after ligand-induced internationalization, despite possible internalization of one or both receptors.
Receptor internalization studies
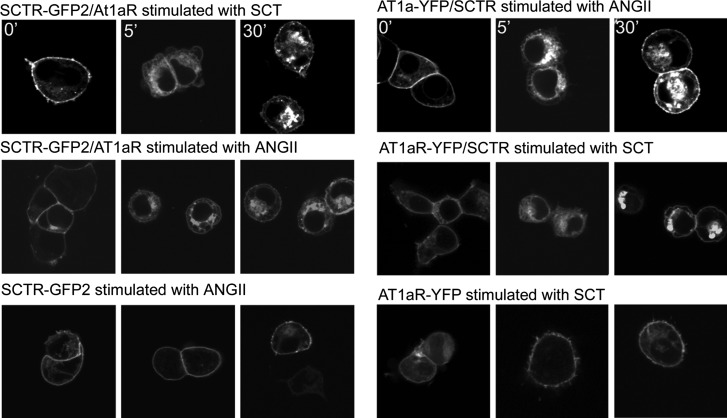
HEK293 cells expressing either AT1aR or SCTR or both receptors were used to directly study the impact of agonists on receptor internalization. Both AT1aR and SCTR have previously been reported to be internalized in response to occupation with their own natural agonist ligands (14). Also, there is precedent for receptors in a dimeric complex, including AT1aR, to internalize together (16). When HEK293 cells expressing both AT1aR-YFP and unlabeled SCTR were stimulated with 0.1 μM SCT, the fluorescently tagged AT1aR was observed to internalize promptly (Fig. 2). This time course was similar to that of the positive control in which the same cells were stimulated with ANGII. In contrast, when only AT1aR-YFP was expressed on the same cells in the absence of SCTR, SCT had no effect on the internalization of ATR1aR. Similarly, when cells expressed both SCTR-GFP2 and ATR1aR were stimulated with 0.1 μM ANGII, the fluorescently tagged SCTR was observed to internalize promptly (Fig. 2). Here, too, this time course was similar to the positive control in which the same cells were stimulated with SCT. In contrast, when only SCTR-GFP2 was expressed on the same cells in the absence of ATR1aR, ANGII had no effect of the internalization of SCTR.
Figure 2.
Internalization of SCTR and AT1aR in response to natural agonist ligands. Fluorescence images of HEK293 cells expressing the noted receptor or receptors that were stimulated with the noted agonist peptide. Each panel includes representative images of cells expressing each fluorescent receptor construct at 0, 5, and 30 min after exposure to the peptide ligand. Top panels: positive control in which the fluorescent receptor is internalized in response to its own agonist ligand. Middle panels: effect of stimulating the coexpressing cells with the natural ligand of the nonfluorescent receptor. Bottom panels: negative control to show that the opposite ligand has no effect to stimulate internalization of the other receptor when the 2 receptors are not coexpressed on the cells.
Use of transmembrane peptides from SCTR and AT1aR
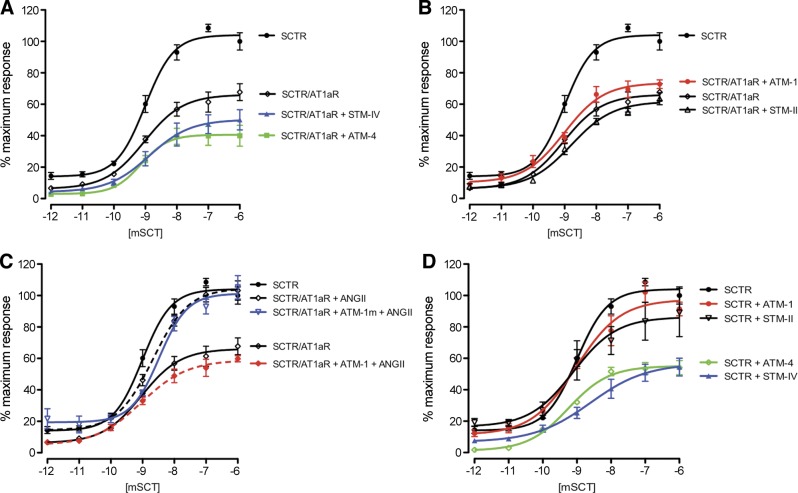
As shown by our previous studies (10), the lipid-exposed face of SCTR TM4 is a major determinant for its homodimerization. This event can be fully disrupted by competition with this peptide, as supported by peptide competition for static receptor BRET studies and saturation BRET studies. Further, this interface was confirmed by formation of symmetrical covalent dimers through disulfide bonding at the external face of TM-4 (17). This has been consistent for several members of the class B GPCR family (18). TM-4 of the class A GPCR, AT1R, is also believed to represent an important contributor to the natural ligand binding pocket (19). In this report, synthetic peptides corresponding to all 7 TM segments of SCTR and AT1aR were used in competitive BRET assays to further understand the role of these regions in the formation of the SCTR and AT1R heterocomplex. The peptides corresponding to the sequences of SCTR TM2 (STM-II) and TM-4 (STM-IV) (Fig. 3A, C), as well as AT1aR TM-1 (ATM-1) and TM-4 (ATM-4) significantly inhibited receptor BRET (Fig. 3B, D). To show the specificity of these effects, STM-IV, ATM-1, and ATM-4 with substitutions at their lipid-exposed faces (as shown in the helical wheel diagrams: STM-IVm, ATM-1m, and ATM-4m; Fig. 3E) as well as a TM peptide from AT2R (AT2R-TM4; Supplemental Fig. S5) were used, and all these peptides had no effect in reducing the BRET signal (Fig. 3C, D). Our data, therefore, indicate that peptides that include the highly hydrophobic, lipid-exposed faces of SCTR TM-4 and AT1aR TM-1 and TM-4 are able to interact with and inhibit the formation and cAMP signaling of the SecR-AT1aR heterocomplex.
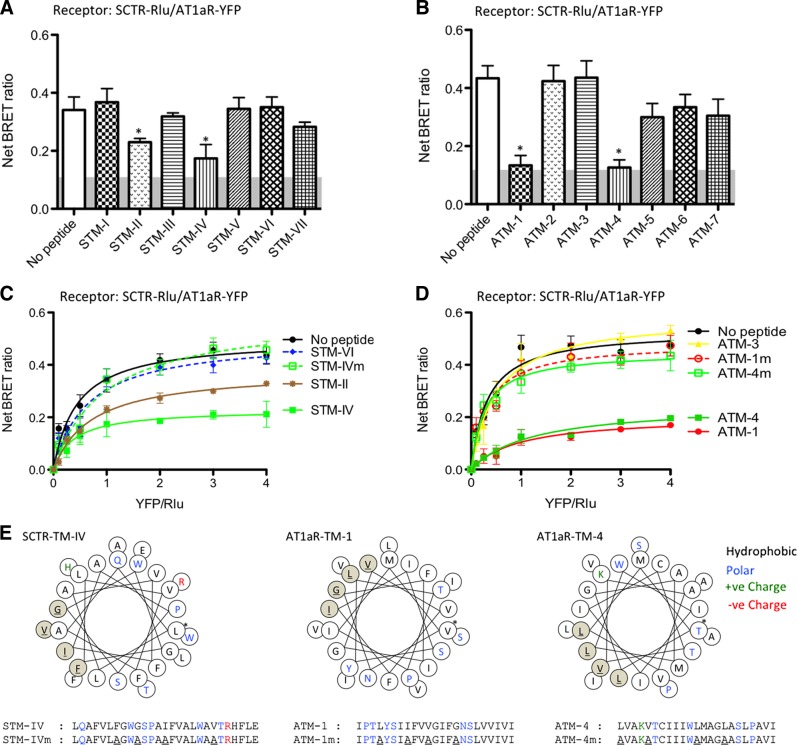
Figure 3.
Effects of TM peptides on formation of SCTR-AT1aR heterocomplexes. A, B) SCTR-Rlu- and AT1aR-YFP-cotransfected cells were treated with different SCTR TM peptides (STM-I to STM-VII; A) and AT1aR TM peptides (ATM-1 to ATM-7; B). The net BRET ratios were significantly reduced by incubation with STM-II, STM-IV, ATM1, or ATM4. C, D) Effects of TM peptides on heteromer formation were confirmed by saturation BRET studies. C) Saturation BRET signals were significantly reduced by STM-II and STM-IV, but not by the mutant forms of these peptides nor STM-VI. D) Similarly, ATM-1 and ATM-4 peptides, but neither their mutant forms nor ATM-3, could reduce BRET signals in the saturation curves. Data are presented as means ± se of data from ≥4 independent experiments performed in duplicate. *P < 0.01 vs. control (no peptide). E) Predicted helical wheels and the peptide sequences of the TM peptides used. Sites of amino acid substitutions of the mutant peptides are highlighted in red. Asterisk indicates the first residue in the helix. Hydrophobic, polar, and positively charged residues are in black, blue, and green, respectively.
The TM segment peptides had the ability to differentially interact with and modulate the BRET signal from their respective receptors when present alone or when coexpressed with the opposite receptor of the pair. When SCTR was expressed alone and homomer formation was determined by SCTR-SCTR BRET, only TM-4 of SCTR reduced the signal, and this was reduced to background levels (Fig. 4A). A linear signal on saturation BRET studies was compatible with the competitive disruption of the complex, consistent with the lipid face of TM-4 providing the interface between the protomers making up the dimeric complex (Fig. 4C). This was also consistent with only having a single interface and not being able to form higher order oligomers. When SCTR was coexpressed with AT1aR and the heteromer was assayed by SCTR-AT1aR BRET, SCTR TM-II, TM-IV, and TM-VII were all able to significantly reduce the BRET signal, however, not to the background level, and, indeed, saturation BRET continued to show a pattern of reaching an asymptote. This was consistent with interaction and change in conformation of the heterocomplex. It is likely that the TM-IV peptide affected the complex by disruption of the homodimer, while the other 2 SCTR peptides affected the complex by changing its conformation.
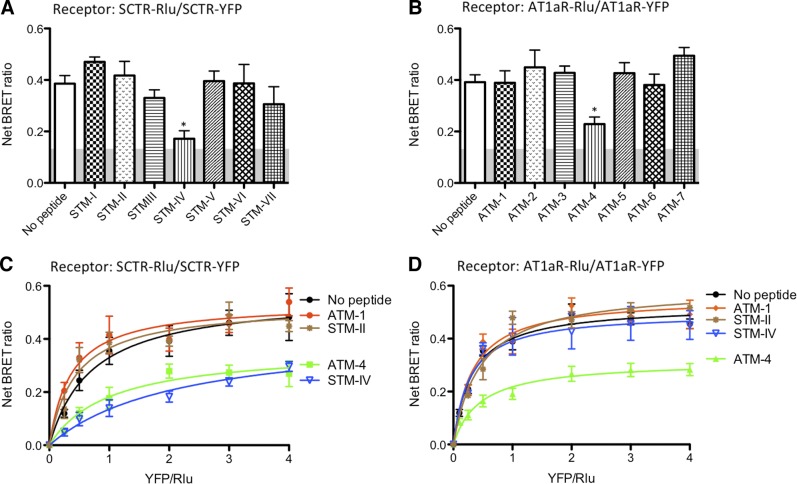
Figure 4.
Effects of TM peptides on SCTR and AT1aR homodimerization. A) SCTR-Rlu- and SCTR-YFP-cotransfected cells were treated with STM-I to STM-VII. STM-IV was able to significantly reduce BRET signals. B) Similarly, ATM-1 to ATM-7 were used to inhibit AT1aR homooligomerization. Although both ATM-1 and ATM-4 could inhibit SCTR/AT1aR heterooligomerization, only ATM-4 was able to significantly reduce the AT1aR-At1aR BRET signal reflecting homooligomerization. Results from A and B were confirmed by saturation studies. C) STM-IV was able to lower SCTR homooligomerization. Interestingly, AT1aR TM peptide ATM-4, but not ATM-1, was also capable of inhibiting homooligomerization D). Homooligomerization saturation studies of AT1aR indicate that only ATM4, but not ATM-1 or STM-IV, were able to disrupt AT1aR homodimerization. Data are presented as means ± se of data from ≥3 independent experiments performed in duplicate. *P < 0.01 vs. control (no peptide).
When AT1aR was expressed alone, and homomer formation was determined by AT1aR-AT1aR BRET, only TM-4 of AT1aR reduced the signal, although this was not to background levels (Fig. 4B). The saturation BRET assay also yielded a curve that reached an asymptote compatible with the residual presence of a homomeric complex (Fig. 4D). Because the AT1aR-AT1aR homomeric complex was not fully disrupted, it is not clear whether TM-4 was at the interface. When AT1aR was coexpressed with SCTR and the heteromer was assayed by SCTR-AT1aR BRET, both AT1aR TM-1 and TM-4 were able to reduce the BRET signal, but again not to the background level and the saturation BRET assay supported the residual existence of the mixed class heterocomplex.
The same TM peptides, except for replacing the lipid-facing residues with alanines, were also studied. In each situation, the modified peptides did not have the effect to reduce the relevant BRET assay that the analogous peptide with natural sequence did. This supports the prediction that the lipid face of these TM peptides is the site of interaction with the endogenous TM segment within the intact receptors. It is noteworthy that ATM1 and the STM-II peptides affected only the BRET signal from the heterocomplex (Fig. 3D), while not affecting either of the receptor homomeric complexes (Fig. 4C, D). Therefore, these peptides might provide a reagent that could distinguish the effects of the heterocomplex from those of either homomeric complex, and this reagent might be able to probe and affect the heterocomplex that forms in vivo.
TM peptides are functional modulators of cAMP signaling of the AT1aR/SCTR heterocomplex
The ability of TM peptides to modulate cAMP responses of SCTR with or without AT1aR were initially investigated in vitro. As shown above, the coexpression of both receptors reduced the efficacy of SCTR in cAMP production. Treatment of cells with STM-IV or ATM-4, but not its mutated counterparts (Supplemental Fig. S6), resulted in further reductions in the maximal and basal responses (Fig. 5A). These peptides also dramatically affected function of SCTR when expressed alone (Fig. 5D). This reveals disruption of the STM-IV/ATM-4 interface of the heterooligomer or the STM-IV/STM-IV interface of the SCTR homodimer affects cAMP signaling, indicating the involvement of TM-4 in G-protein-dependent responses, which is consistent with previous studies (10).
Figure 5.
Effect of TM peptides on SCTR cAMP signaling. Intracellular cAMP accumulation in response to graded concentrations of SCT in SCTR/AT1aR- or SCTR-transfected cells. A) Compared to no peptide treatment, ATM4 and STM-IV further reduced the efficacy of cAMP production through stimulation of the SCTR in SCTR/AT1aR-transfected cells. B) In contrast, there was no significant effect of ATM-1 or STM-II on cAMP production in SCTR/AT1aR-transfected cells. C) ANGII treatment was able to rescue the cAMP response to SCT in SCTR-AT1aR cells, but this effect was repressed by ATM1. D) In SCTR-transfected cells, ATM-4 and STM-IV significantly reduced receptor responses to SCT. ATM1 treatment did not alter the cAMP response of SCTR, whereas STM-II slightly reduced the Emax value. Data are presented as means ± se of data from ≥3 independent experiments performed in triplicate.
ATM-1 and STM-II, which reduced the BRET signal from the heterocomplex, but not from either homomeric complex, had no significant effect on cAMP signaling of the SCTR/AT1aR or SCTR/SCTR complexes (Fig. 5B, D and Supplemental Fig. S6). This echoes that disruption of the ATM-1/STM-II interface in the oligomer has minimal effect on signaling. The intriguing observation, however, is that after ATM1 treatment, ANGII was unable to rescue the cAMP response of SCTR/AT1aR as discussed in Fig. 2 (Fig. 5C). Our data show that the interaction of SCTR with AT1aR at the STM-II/ATM-1 interface is permissive to the activation of AT1aR, which is a prerequisite for the full activity of SCTR in the presence of AT1aR in response to SCT stimulation.
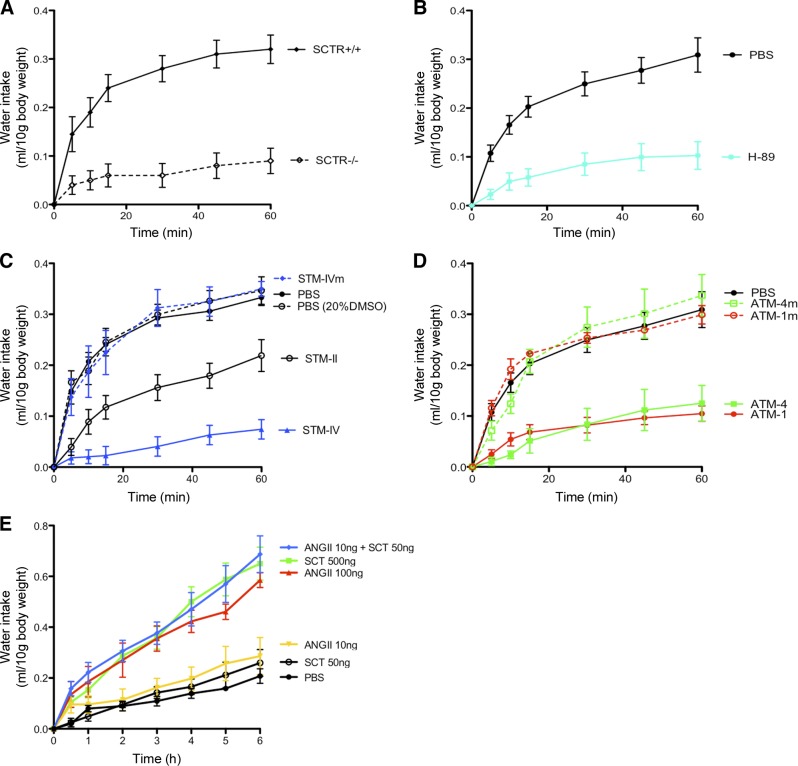
Hyperosmolality-induced drinking is suppressed by i.c.v. injection of ATM-1 peptide indicating an in vivo effect of the SCTR/AT1aR complex
Under hyperosmotic condition induced by i.p. hypertonic saline injection, significant increases in water intake in SCTR+/+ mice were found within the first 20 min, and this stimulated water intake was not observed in SCTR-KO mice (Fig. 6A). In addition, i.c.v.-H-89 injection produced similar effects as in SCTR KOs (Fig. 6B). These data collectively established that the hyperosmolality-induced drinking is mediated via the SCTR-cAMP-PKA pathway. To demonstrate in vivo effects of receptor oligomerization, STM-II, STM-IV, ATM-1, and ATM-4, as well as the mutated counterparts of these peptides, were injected into the lateral ventricles, and water intake was monitored (Fig. 6C, D). STM-IV and ATM-4 that inhibit both homo- and heterooligomer formation, as well as cAMP production by SCTR, were both able to strongly suppress water intake, while their mutated peptides had no effect. ATM-1 and STM-II, which affected only the BRET signal from the heterocomplex of AT1aR/SCTR, was still capable of strongly suppressing water intake (Fig. 6C, D). Consistent with this, STM-II was less effective in reducing BRET signal and was also less potent in inhibiting water intake when compared to STM-IV. To show receptor specificity of these TM peptides, AT2R-TM4 was used and was found to exhibit no effect in suppressing hyperosmolality-induced drinking (Supplemental Fig. S7). In summary, our data clearly show that the heterocomplex of AT1aR/SCTR plays a pivotal role in mediating water drinking behavior on hyperosmotic stress. This provides an explanation for our previous findings that without SCTR, central administration of ANGII is ineffective in stimulating water intake, as well as Vp expression and release (6), since it is the heterocomplex that mediates the functions of both neuropeptides. In addition, as shown earlier (Fig. 1), when both receptors are present on the same cell surface, the full activity of SCTR required that AT1aR be in its active conformation. Based on this observation, we predicted that the presence of ANGII can greatly enhance the physiological effects of SCTR in this experimental paradigm (Fig. 6E). As shown previously (6) and in this study, i.c.v. injection of high doses of SCT and ANGII (SCT 500 ng; ANGII 100 ng) could stimulate drinking in C57 mice. Cumulative water intake was significantly increased from 1 to 6 h postinjection. Lower dosage of SCT (50 ng) or ANGII (10 ng) was unable to induce significant increases in water intake when compared to PBS control, whereas when both peptides at low doses were co-i.c.v. injected into the lateral ventricle, a dramatic increase in water drinking was observed (Fig. 6E).
Figure 6.
TM peptides that disrupt SCTR-AT1aR heteromer formation significantly inhibit hyperosmolality-induced drinking in mice. A) Hyperosmolality was induced by i.p injection of 1M NaCl at 2% vol/BW in mice. At 30 min after injection, water was provided, and the amount of water intake in 1 h was monitored in metabolic cages. In wild-type mice, water intake was abruptly increased immediately. This drinking response was significantly reduced in SCTR−/− mice. B) When compared with the PBS control, the hyperosmolality-induced drinking was abolished by i.c.v. injection of a PKA inhibitor (H-89; 1 nmol, 2 μl). C) The effect of STM-II, STM-IV, and STM-IVm on hyperosmolality-induced drinking was studied by i.c.v. injection of these peptides (4 μg in 5 μl) vs. controls (5 μl PBS or vehicle control, 20% DMSO in PBS) into the lateral ventricle 15 min after hyperosmotic shock. Water was provided 15 min after peptide treatment. Significant reduction in water intake was observed in mice given i.c.v. injections with STM-II and STM-IV, but not STM-IV. D) Similar effects of AT1aR TM peptides were studied. I.c.v injection of ATM1 or ATM4 significantly suppressed hyperosmolality-induced drinking. No change in response was observed in ATM1m and ATM4m. E) Time course of dipsogenic effects of central injection of SCT or ANGII in mice (0.5, 1, 2, 4, and 6 h). Effects of i.c.v. injection (5 μl) of SCT (50 or 500 ng) or ANGII (10 or 100 ng), or coinjection of both peptides (50 and 10 ng, respectively) or control PBS into the lateral ventricle on cumulative water intake in C57 mice. Data are presented as means ± se of data from ≥3 independent experiments performed in duplicate.
DISCUSSION
Activation of GPCRs effected by extracellular stimulation is due to sequential conformational changes of the receptor and resultant molecular interactions and hierarchical intracellular signaling events (20). This activation process of GPCRs can be regulated by interacting with several allosteric protein modulators, including β-arrestin, receptor activity-modifying proteins (RAMPs), trimeric G-protein complex, as well as GPCRs themselves (21). One of the first studies that reopened the question regarding higher order GPCR complexes was the functional transcomplementation of the chimeras of α2-adrenergic/M3 muscarinic receptor (22). This indicated that the intermolecular interactions between inactive receptors could restore their functions. However, studies to show the functional cooperation of 2 receptors are difficult, since functional coupling between 2 receptors can be transmitted though many possible routes (23), other than direct physical interactions to form oligomers. Receptors can cooperate through the regulation of limiting signaling molecules and/or trafficking to the cell surface. If 2 receptors have similar downstream signaling pathways, it is difficult to establish the exact mechanisms responsible for the signaling changes. In this study, we focused on studying the possible association between the SCTR and the AT1aR, since they share overlapping physiological functions in osmoregulation, but stimulate different intracellular signaling pathways. On SCT activation, SCT robustly stimulates cAMP production, while ANGII is unable to elicit a cAMP response from either the AT1aR or the SCTR. As a consequence, modulatory actions of ANGII on cAMP production are necessarily mediated through the SCTR, thus providing an excellent model for studying the functional coupling of a class A GPCR, AT1aR, with a class B GPCR, SCTR.
In the past decade, there have been many reports showing that the signaling responses of a GPCR could be modified by its oligomerization. Interactions of GPCRs will change the overall conformational ensemble for a modification in ligand affinity and efficacy. In this regard, the AT1R is a particularly well-studied model. It has been shown that the interaction of AT1R with MAS receptor or AT2R will decrease the Gαq coupling (24–26), while dimerization with bradykinin B2 receptor could enhance the same event (27, 28). In the case of AT1aR/SCTR, the current study shows that heterocomplex formation resulting in a lower efficacy of SCT stimulation of cAMP through the SCTR when compared to its action on the SCTR homodimer. This drop of efficacy can be rescued by an active conformation of the AT1aR as shown in the CAM and K199A mutants, as well as the coapplication of ANGII with SCT. It has been shown that ANGII binding to the AT1aR can shift the receptor to an active status (14). Our data suggest that the AT1aR/SCTR heterocomplex will produces a biological effect, in which the activation of AT1aR is the key to regulate SCTR in mediating cAMP responses.
To be sure that this biological effect was not mediated by the preferential internalization of one receptor of this pair, we performed direct analysis of the agonist-stimulated internalization. As had previously been reported (15), each hormone stimulated the internalization of its own natural receptor. When both receptors were coexpressed on the same cell, each hormone stimulated the internalization of both its natural receptor as well as the associated receptor in the complex. This is similar to what had been reported for the complex of AT1aR and the associated bradykinin B2 receptor (16). We also directly established that the association between these receptors was stable, even after agonist treatment to stimulate internalization. Another important observation to emphasize was the included data that show that the cAMP response in this complex was affected, while the calcium response was not. Again, this emphasizes that this cannot be explained by selective internalization of one member of the pair of interacting receptors.
To validate an in vivo function of receptor association is extremely difficult since there have been no specific tools to inhibit such processes in living animals or patients. In this study, we demonstrate that TM peptides can be effective tools to investigate functions of specific GPCRs by competing with homomer- and/or heterooligomer formation in both in vitro and in vivo studies. As such, these GPCR-specific TM peptides are potentially powerful tools, and may even be considered as a new class of “drug” or “antagonist.” Our data also support the importance of several TM segments in the heteromeric complex between SCTR and AT1aR, there are 2 interacting interfaces involving TM1/2 and TM4/4 in the SCTR/AT1aR heterocomplex. This idea agreed with the recently published crystal structure of homooligomers of β1-adrenegic receptor (β1-AR) and μ-opioid receptor (29, 30), both of the oligomer structures also suggest more than one interface can be involved in the receptor association. In the β1-AR oligomer, interface 1 contains TM1 as well as some residues from the C-terminal helical domain H8, TM2, and exoloop 1, whereas interface 2 includes the TM4, TM5, and intracellular loop 2. In μ-opioid receptor, interface 1 is similar to β1-AR, but TM5 acts together with TM6 in the second interface. In our study, based on the use of TM peptides, we propose the interactions involving STM-IV/ATM-4 are responsible for both homo- and heterooligomerization, and STM-II/ATM1 is involved in the second interface that is only present in the heterocomplex. Similar to β1-AR, the interface involving STM-IV/ATM-4 is linked to Gαs-protein coupling as indicated in our data and is coherent with our previously model of SCTR homodimer; we found that the TM4-nondimerizing mutant of SCTR exhibited a lower efficiency in G-protein coupling (10). On the other hand, the interface concerning TM1/2 is insensitive in modulating Gαs-protein coupling, and again this is in line with studies in dopamine D2 receptor and serotonin 5HT2c receptor (31, 32).
As pleiotropic factors, ANGII and SCT have common and different roles in our body depending on the expression of various combinations of receptors in different cells. In SCTR- and AT1aR-coexpressing cells, SCTR is less sensitive to fluctuations of extracellular SCT unless AT1aR is active. Under hyperosmolality, both ANGII and SCT levels are increased both centrally and in the periphery to stimulate central mechanisms for water intake/Vp expression and release (6). However, SCT is also released from gastrointestinal S-cells postprandially to control exocrine secretion from the pancreas (33). Under this condition, SCT alone is inefficient in osmoregulatory functions due to the low concentrations of ANGII. SCTR/AT1aR oligomerization, therefore, plays a crucial physiological function in our body to distinguish elevated levels of SCT as a consequence of hyperosmolality or food intake. This idea is supported in this report by suppression of hyperosmotic stress-induced in vivo water intake by i.c.v. injection of ATM-1 peptides that disrupt only SCTR/AT1aR heterooligomerization but not SCTR or AT1aR homooligomerization. Our data clearly show that AT1aR/SCTR heterooligomer formation is critical in this pathway. A strong synergistic effect of SCT and ANGII on water intake at low doses of both peptides was observed, showing that AT1aR in response to graded concentrations of in vivo ANGII acts like a rheostat to sensitize/desensitize SCTR to bring about the fine-tuning of physiological consequences, in this case, water drinking. In fact, it had been proposed that the conformational dynamics of GPCRs make them behave more like rheostats than a simple on/off switch (34, 35). Different ligands can “dial in” and then regulate the outcome (36). As the conformations of the GPCRs could be modified by ligand binding, lipid environment and other allosteric modulators, this provides a great flexibility of GPCRs to shift their functions for fine-tuning of the cells in response to different external conditions.
As the most important gene family in bridging extracellular conditions with intracellular responses, GPCRs are the target of one-third of therapeutic drugs on the market. Our data show that TM peptides are potentially useful tools that can modulate GPCR functions by interaction and/or disruption of oligomeric complexes. These peptides are specific in their action, since they are structurally homologous to part of the target GPCRs. The functional specificity of each TM peptide has the potential to be applied in vivo to gain insights into whether these processes occur in that setting. In addition, these peptides, like the ATM-1 peptide, can be used to specifically inhibit physiological functions due to the formation of certain receptor complexes.
Supplementary Material
Acknowledgments
This study was supported by Hong Kong Government RGC grants 770212M (to L.T.O.L.) and CRFHKU6/CRF/11G, HKU 764812M and HKU 765011M (to B.K.C.C.), and U.S. National Institutes of Health grant DK046577 (to L.J.M.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- β1-AR
- β1-adrenegic receptor
- AT1aR
- angiotensin receptor type 1A
- BRET
- bioluminescence resonance energy transfer
- cAMP
- cyclic adenosine monophosphate
- FRET
- fluorescence resonance energy transfer
- GFP
- green fluorescent protein
- GPCR
- G-protein-coupled receptor
- i.c.v.
- intracerebroventricular
- KO
- knockout
- LHR
- luteinizing hormone receptor
- PVN
- paraventricular nucleus
- RFP
- red fluorescent protein
- Rlu
- Renilla luciferase
- ROI
- region of interest
- SBT
- spectral bleed-through
- SCT
- secretin
- SCTR
- secretin receptor
- TM
- transmembrane
- Vp
- vasopressin
- YFP
- yellow fluorescent protein
REFERENCES
- 1. Terrillon S., Barberis C., Bouvier M. (2004) Heterodimerization of V1a and V2 vasopressin receptors determines the interaction with beta-arrestin and their trafficking patterns. Proc. Natl. Acad. Sci. U.S.A. 101, 1548–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fanelli F., De Benedetti P. G. (2011) Update 1 of: computational modeling approaches to structure-function analysis of G protein-coupled receptors. Chem. Rev. 111, PR438–535 [DOI] [PubMed] [Google Scholar]
- 3. Rivero-Muller A., Chou Y. Y., Ji I., Lajic S., Hanyaloglu A. C., Jonas K., Rahman N., Ji T. H., Huhtaniemi I. (2010) Rescue of defective G protein-coupled receptor function in vivo by intermolecular cooperation. Proc. Natl. Acad. Sci. U.S.A. 107, 2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albizu L., Cottet M., Kralikova M., Stoev S., Seyer R., Brabet I., Roux T., Bazin H., Bourrier E., Lamarque L., Breton C., Rives M. L., Newman A., Javitch J., Trinquet E., Manning M., Pin J. P., Mouillac B., Durroux T. (2010) Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 6, 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu J. Y., Cheng C. Y., Lee V. H., Chan Y. S., Chow B. K. (2011) Secretin and body fluid homeostasis. Kidney Int. 79, 280–287 [DOI] [PubMed] [Google Scholar]
- 6. Lee V. H., Lee L. T., Chu J. Y., Lam I. P., Siu F. K., Vaudry H., Chow B. K. (2010) An indispensable role of secretin in mediating the osmoregulatory functions of angiotensin II. FASEB J. 24, 5024–5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez A. D., Wang G., Waters E. M., Gonzales K. L., Speth R. C., Van Kempen T. A., Marques-Lopes J., Young C. N., Butler S. D., Davisson R. L., Iadecola C., Pickel V. M., Pierce J. P., Milner T. A. (2012) Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 226, 489–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu J. Y., Chung S. C., Lam A. K., Tam S., Chung S. K., Chow B. K. (2007) Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol. Cell. Biol. 27, 2499–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lisenbee C. S., Miller L. J. (2006) Secretin receptor oligomers form intracellularly during maturation through receptor core domains. Biochemistry 45, 8216–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harikumar K. G., Pinon D. I., Miller L. J. (2007) Transmembrane segment IV contributes a functionally important interface for oligomerization of the class II G protein-coupled secretin receptor. J. Biol. Chem. 282, 30363–30372 [DOI] [PubMed] [Google Scholar]
- 11. Xia Z., Liu Y. (2001) Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys. J. 81, 2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harikumar K. G., Dong M., Cheng Z., Pinon D. I., Lybrand T. P., Miller L. J. (2006) Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry 45, 14706–14716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paxinos G., Franklin K. (2012) The Mouse Brain in Stereotaxic Coordinates, Elsevier/Academic Press, Waltham, MA, USA [Google Scholar]
- 14. Walker J. K., Premont R. T., Barak L. S., Caron M. G., Shetzline M. A. (1999) Properties of secretin receptor internalization differ from those of the beta(2)-adrenergic receptor. J. Biol. Chem. 274, 31515–31523 [DOI] [PubMed] [Google Scholar]
- 15. Nikiforovich G. V., Mihalik B., Catt K. J., Marshall G. R. (2005) Molecular mechanisms of constitutive activity: mutations at position 111 of the angiotensin AT1 receptor. J. Peptide Res. 66, 236–248 [DOI] [PubMed] [Google Scholar]
- 16. Quitterer U., Pohl A., Langer A., Koller S., Abdalla S. (2011) A cleavable signal peptide enhances cell surface delivery and heterodimerization of Cerulean-tagged angiotensin II AT1 and bradykinin B2 receptor. Biochem. Biophys. Res. Commun. 409, 544–549 [DOI] [PubMed] [Google Scholar]
- 17. Dong M., Pinon D. I., Bordner A. J., Miller L. J. (2010) Elucidation of the active conformation of the amino terminus of receptor-bound secretin using intramolecular disulfide bond constraints. Bioorg. Med. Chem. Lett. 20, 6040–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao F., Harikumar K. G., Dong M., Lam P. C., Sexton P. M., Christopoulos A., Bordner A., Abagyan R., Miller L. J. (2009) Functional importance of a structurally distinct homodimeric complex of the family B G protein-coupled secretin receptor. Mol. Pharmacol. 76, 264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan L., Holleran B. J., Lavigne P., Escher E., Guillemette G., Leduc R. (2010) Analysis of transmembrane domains 1 and 4 of the human angiotensin II AT1 receptor by cysteine-scanning mutagenesis. J. Biol. Chem. 285, 2284–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bock A., Merten N., Schrage R., Dallanoce C., Batz J., Klockner J., Schmitz J., Matera C., Simon K., Kebig A., Peters L., Muller A., Schrobang-Ley J., Trankle C., Hoffmann C., De Amici M., Holzgrabe U., Kostenis E., Mohr K. (2012) The allosteric vestibule of a seven transmembrane helical receptor controls G-protein coupling. Nat. Comm. 3, 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouvier M. (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2, 274–286 [DOI] [PubMed] [Google Scholar]
- 22. Maggio R., Vogel Z., Wess J. (1993) Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular “cross-talk” between G-protein-linked receptors. Proc. Natl. Acad. Sci. U.S.A. 90, 3103–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyngso C., Erikstrup N., Hansen J. L. (2009) Functional interactions between 7TM receptors in the renin-angiotensin system: dimerization or crosstalk? Mol. Cell. Endocrinol. 302, 203–212 [DOI] [PubMed] [Google Scholar]
- 24. AbdAlla S., Lother H., el Massiery A., Quitterer U. (2001) Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat. Med. 7, 1003–1009 [DOI] [PubMed] [Google Scholar]
- 25. Canals M., Jenkins L., Kellett E., Milligan G. (2006) Up-regulation of the angiotensin II type 1 receptor by the MAS proto-oncogene is due to constitutive activation of Gq/G11 by MAS. J. Biol. Chem. 281, 16757–16767 [DOI] [PubMed] [Google Scholar]
- 26. Kostenis E., Milligan G., Christopoulos A., Sanchez-Ferrer C. F., Heringer-Walther S., Sexton P. M., Gembardt F., Kellett E., Martini L., Vanderheyden P., Schultheiss H. P., Walther T. (2005) G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111, 1806–1813 [DOI] [PubMed] [Google Scholar]
- 27. AbdAlla S., Abdel-Baset A., Lother H., el Massiery A., Quitterer U. (2005) Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. J. Mol. Neurosci. 26, 185–192 [DOI] [PubMed] [Google Scholar]
- 28. AbdAlla S., Lother H., Quitterer U. (2000) AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 407, 94–98 [DOI] [PubMed] [Google Scholar]
- 29. Huang J., Chen S., Zhang J. J., Huang X. Y. (2013) Crystal structure of oligomeric beta1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat. Struc. Mol. Biol. 20, 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manglik A., Kruse A. C., Kobilka T. S., Thian F. S., Mathiesen J. M., Sunahara R. K., Pardo L., Weis W. I., Kobilka B. K., Granier S. (2012) Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 485, 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo W., Urizar E., Kralikova M., Mobarec J. C., Shi L., Filizola M., Javitch J. A. (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 27, 2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mancia F., Assur Z., Herman A. G., Siegel R., Hendrickson W. A. (2008) Ligand sensitivity in dimeric associations of the serotonin 5HT2c receptor. EMBO Rep. 9, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gray M. A., Greenwell J. R., Argent B. E. (1988) Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J. Membr. Biol. 105, 131–142 [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. (2009) The structure and function of G-protein-coupled receptors. Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O'Hayre M., Vazquez-Prado J., Kufareva I., Stawiski E. W., Handel T. M., Seshagiri S., Gutkind J. S. (2013) The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 13, 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kobilka B. K., Deupi X. (2007) Conformational complexity of G-protein-coupled receptors. Trends Pharmacol. Sci. 28, 397–406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.