Abstract
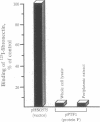
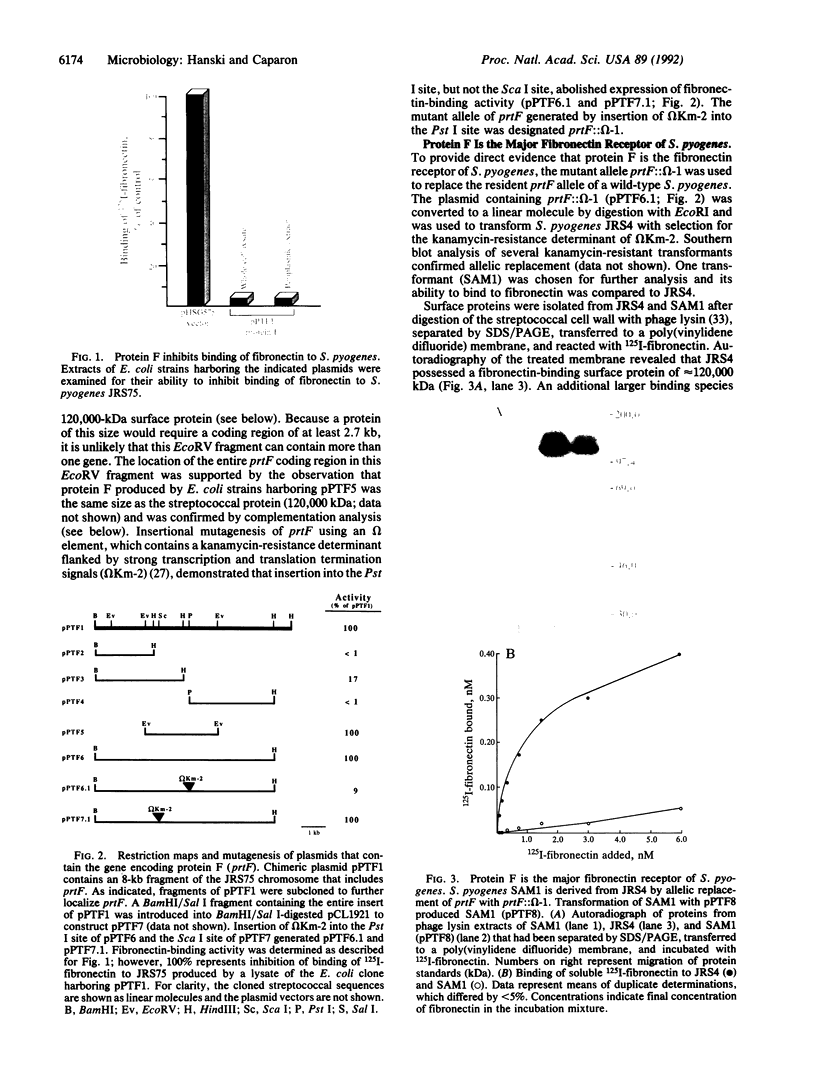
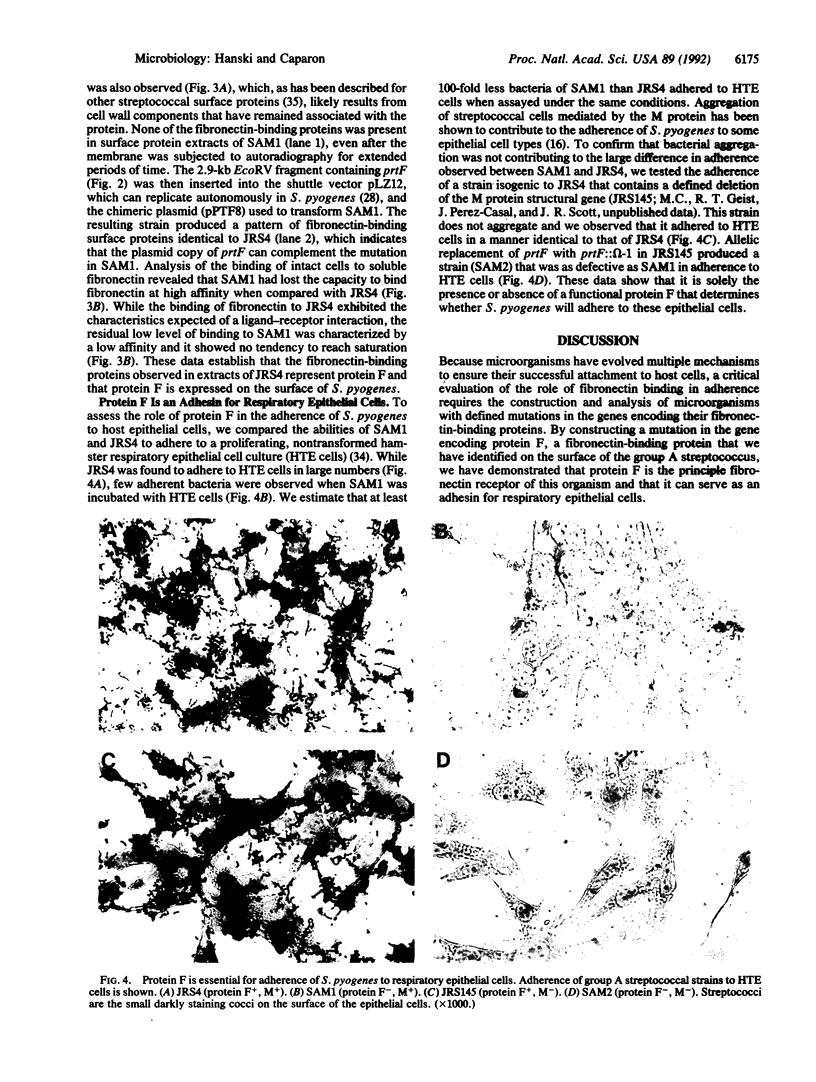
Binding to fibronectin has been suggested to play an important role in adherence of the group A streptococcus Streptococcus pyrogenes to host epithelial cells; however, the identity of the streptococcal fibronectin receptor has been elusive. Here we demonstrate that the fibronectin-binding property of S. pyogenes is mediated by protein F, a bacterial surface protein that binds fibronectin at high affinity. The gene encoding protein F (prtF) produced a functional fibronectin-binding protein in Escherichia coli. Insertional mutagenesis of the cloned gene generated a mutation that resulted in the loss of fibronectin-binding activity. When this mutation was introduced into the S. pyrogenes chromosome by homologous recombination with the wild-type allele, the resulting strains no longer produced protein F and lost their ability to bind fibronectin. The mutation could be complemented by prtF introduced on a plasmid. Mutants lacking protein F had a much lower capacity to adhere to respiratory epithelial cells. These results demonstrate that protein F is an important adhesin of S. pyogenes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Genetic manipulation of pathogenic streptococci. Methods Enzymol. 1991;204:556–586. doi: 10.1016/0076-6879(91)04028-m. [DOI] [PubMed] [Google Scholar]
- Caparon M. G., Stephens D. S., Olsén A., Scott J. R. Role of M protein in adherence of group A streptococci. Infect Immun. 1991 May;59(5):1811–1817. doi: 10.1128/iai.59.5.1811-1817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock J. I., Fröman G., Jönsson K., Guss B., Signäs C., Nilsson B., Raucci G., Hök M., Wadström T., Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987 Aug;6(8):2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Baseman J. B. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro. 1980 Apr;16(4):313–319. doi: 10.1007/BF02618337. [DOI] [PubMed] [Google Scholar]
- Hook M., McGavin M. J., Switalski L. M., Raja R., Raucci G., Lindgren P. E., Lindberg M., Signas C. Interactions of bacteria with extracellular matrix proteins. Cell Differ Dev. 1990 Dec 2;32(3):433–438. doi: 10.1016/0922-3371(90)90060-a. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Lerner C. G., Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990 Aug 11;18(15):4631–4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kuusela P. Binding of human fibronectin to group A, C, and G streptococci. Infect Immun. 1983 Apr;40(1):29–34. doi: 10.1128/iai.40.1.29-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Noisin E. L., Villalta F. Fibronectin increases Trypanosoma cruzi amastigote binding to and uptake by murine macrophages and human monocytes. Infect Immun. 1989 Apr;57(4):1030–1034. doi: 10.1128/iai.57.4.1030-1034.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Caparon M. G., Scott J. R. A method for allelic replacement that uses the conjugative transposon Tn916: deletion of the emm6.1 allele in Streptococcus pyogenes JRS4. Infect Immun. 1989 Dec;57(12):3846–3850. doi: 10.1128/iai.57.12.3846-3850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Simpson W. A., Beachey E. H. Formation of molecular complexes between a structurally defined M protein and acylated or deacylated lipoteichoic acid of Streptococcus pyogenes. J Bacteriol. 1982 Feb;149(2):426–433. doi: 10.1128/jb.149.2.426-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsén A., Jonsson A., Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989 Apr 20;338(6217):652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- Perez-Casal J., Caparon M. G., Scott J. R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991 Apr;173(8):2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Schneewind O., Jones K. F., Fischetti V. A. Sequence and structural characteristics of the trypsin-resistant T6 surface protein of group A streptococci. J Bacteriol. 1990 Jun;172(6):3310–3317. doi: 10.1128/jb.172.6.3310-3317.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. A turbid plaque-forming mutant of phage P1 that cannot lysogenize Escherichia coli. Virology. 1974 Dec;62(2):344–349. doi: 10.1016/0042-6822(74)90397-3. [DOI] [PubMed] [Google Scholar]
- Scott J. R., Guenthner P. C., Malone L. M., Fischetti V. A. Conversion of an M- group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J Exp Med. 1986 Nov 1;164(5):1641–1651. doi: 10.1084/jem.164.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signäs C., Raucci G., Jönsson K., Lindgren P. E., Anantharamaiah G. M., Hök M., Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(2):699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Courtney H. S., Ofek I. Interactions of fibronectin with streptococci: the role of fibronectin as a receptor for Streptococcus pyogenes. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S351–S359. doi: 10.1093/clinids/9.supplement_4.s351. [DOI] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Membrane lipoteichoic acid of Streptococcus pyogenes and its stabilized L-form and the effect of two antibiotics upon its cellular content. J Bacteriol. 1976 Aug;127(2):855–862. doi: 10.1128/jb.127.2.855-862.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S. Gonococcal and meningococcal pathogenesis as defined by human cell, cell culture, and organ culture assays. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S104–S111. doi: 10.1128/cmr.2.suppl.s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sting R., Schmitt M., Blobel H. Isolierung eines Fibronektin-Rezeptors von Streptococcus pyogenes durch Affinitätschromatographie und anschliessender Hochleistungflüssigkeitschromatographie. J Chromatogr. 1989 Dec 29;497:258–262. [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Talay S. R., Ehrenfeld E., Chhatwal G. S., Timmis K. N. Expression of the fibronectin-binding components of Streptococcus pyogenes in Escherichia coli demonstrates that they are proteins. Mol Microbiol. 1991 Jul;5(7):1727–1734. doi: 10.1111/j.1365-2958.1991.tb01921.x. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Enhanced levels of attachment of fibronectin-primed Treponema pallidum to extracellular matrix. Infect Immun. 1986 Jun;52(3):736–741. doi: 10.1128/iai.52.3.736-741.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W. Differences between streptococcal infections of the throat and of the skin. I. N Engl J Med. 1970 Jan 1;282(1):23–31. doi: 10.1056/NEJM197001012820106. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]