Abstract
Resveratrol has been proposed as a potential therapeutic to improve metabolic health during pregnancy, yet little is known about the fetal effects of this maternal dietary supplement. We hypothesized that when administered to pregnant nonhuman primates (NHPs), resveratrol would increase uterine blood flow and mitigate the harmful consequences of maternal Western-style diet (WSD) consumption. NHPs were fed a WSD (36% fat) supplemented with 0.37% resveratrol throughout pregnancy. Outcomes were compared with cohorts fed WSD alone and control chow (14% fat) to distinguish between WSD and resveratrol-specific effects in these animals. In the early third trimester, uterine blood flow was measured by Doppler ultrasound before fetal delivery and tissue collection. Resveratrol resulted in 30% maternal weight loss and improved glucose tolerance, increased uterine artery volume blood flow, and decreased placental inflammation and liver triglyceride deposition. In addition, fetal pancreatic mass was enlarged by 42%, with a 12-fold increase in proliferation by Ki67 immunohistochemistry. These results demonstrate that resveratrol use during pregnancy yields improvements in maternal and placental phenotype with beneficial effects in the fetal liver but an unexplained and concerning alteration in fetal pancreatic development, which strongly cautions against the use of resveratrol by pregnant women.—Roberts, V. H. J., Pound, L. D., Thorn, S. R., Gillingham, M. B., Thornburg, K. L., Friedman, J. E., Frias, A. E., Grove, K. L. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates.
Keywords: insulin resistance, pancreas, uterine blood flow, Western-style diet
The small polyphenol resveratrol has received widespread attention over the past decade as a potential therapy or preventive agent for metabolic diseases. Numerous studies have suggested that resveratrol has anti-inflammatory and antioxidant properties and that it mimics aspects of calorie restriction (reviewed in refs. 1, 2). Outcomes from both rodent and human studies demonstrate conflicting effects of resveratrol on body weight but consistent improvements in metabolic parameters, including liver lipid accumulation and islet function (3–7). In addition, resveratrol has been demonstrated as a vasodilator in human studies of cerebral artery blood flow (8), in brachial flow-mediated dilation (9), and in thyroid artery vascular reactivity studies from patients with hypertension and dyslipidemia (10).
Meanwhile, obesity rates are rising dramatically in the developed world as the availability of calorie-rich, high-fat foods increases. Although 20% of women are obese (body mass index >30 kg/m2) at the start of pregnancy (11), a larger percentage of the population is consuming a Western-style diet (WSD), which in itself is a causal factor for long-term offspring risk of obesity and metabolic disease (reviewed in ref. 12). Indeed, according to recent studies, 17% of children and, of greatest concern, 10% of infants are above the 95th percentile for body weight (13), paralleling the recent change in maternal dietary trends. The complex relationship between maternal overnutrition and obesity with offspring outcomes are not fully understood but likely involve developmental programming resulting in persistent effects postnatally (reviewed in ref. 14). What is clear is that in the metabolic environment of the obese mother, the fetoplacental unit develops under conditions of both excess nutrients and inflammation. Importantly, we have previously demonstrated in our nonhuman primate (NHP) model that consumption of a WSD during pregnancy reduces uterine blood flow, exacerbates placental inflammation, and increases the risk of stillbirth (15). The excess dietary triglycerides from a WSD may be contributing to endothelial dysfunction in the placental vasculature leading to an increase in vascular resistance and an overall reduction in blood supply to the developing fetus. Furthermore, in the NHP model, in utero WSD exposure alters the normal development of key metabolic fetal organs in the early third trimester independent of maternal obesity. Specifically, offspring born to mothers fed a WSD display an increase in the accumulation of liver triglycerides and increased hepatic oxidative stress and apoptosis (16, 17). Within the fetal pancreatic islet, in utero exposure to a WSD results in reduced α-cell mass and an increase in the β:α-cell ratio, which may result in impaired intraislet communication (18). In combination, these adverse outcomes significantly increase the risk for metabolic complications later in life.
As resveratrol is a readily available dietary supplement receiving growing public attention due to its apparent metabolic health benefits, its use during pregnancy may be an appealing option to obese women and those consuming a WSD. However, despite its therapeutic potential, the safety of resveratrol use during the developmental period and the longer term risks of in utero exposure are largely unknown. One prior rodent study demonstrated that resveratrol doses up to 750 mg/kg/d from gestational day 5 (G5) to G20 were well tolerated in pregnant female Sprague-Dawley rats, with no adverse effects on litter size or on fetal or placental weights (19). More recently, a study of resveratrol use in pregnant murine models of reduced uterine blood supply showed vasodilatory effects of resveratrol on the uterine artery with no adverse effects on fetal or placental growth (20). Indeed, use of resveratrol as a therapeutic agent for human pregnancies complicated by diabetes has been postulated (21) based on rodent observations, but before resveratrol can be deemed a safe and effective supplement in this vulnerable population, animal studies in a relevant and translatable model are warranted. Here we present maternal and fetal outcomes from a diet intervention study in a NHP model consuming a WSD supplemented with resveratrol prior to and throughout pregnancy. Since resveratrol improves inflammation and enhances blood flow in other organ systems (1, 8, 10), we sought to test the hypothesis that when administered to NHPs throughout pregnancy, resveratrol intervention would increase uterine blood flow, enhance placental function and mitigate the complications of a WSD in both the mother and the developing fetus.
MATERIALS AND METHODS
Experimental design
Japanese macaques were maintained on a WSD (36% calories from fat; Purina Mills, Inc., St. Louis, MO, USA) supplemented with calorie-dense treats for 4–7 yr. The composition of this WSD represents a typical WSD in regard to the saturated fat content. Ctr animals were fed standard chow (14% calories from fat). Resveratrol (high-purity trans-resveratrol, Resvida; DSM Nutritional Products, Inc., Parsippany, NJ, USA) was incorporated in the resveratrol-supplemented WSD (WSD/Resv) chow by the manufacturers (Purina Mills) at the time of production to a final concentration of 0.37%. Resveratrol supplementation was initiated 3 mo before the breeding season. All diets are sufficient in vitamin, mineral, and protein content for normal growth.
Animals were socially housed in indoor/outdoor pens with 1–2 males and 3–11 females per diet-determined cohort and had ad libitum access to food and water. Animals were allowed to breed naturally, and pregnancies were identified by routine ultrasound. Gestational age dating was achieved by key fetal measures. Metabolic parameters [body weight and composition, fasting glucose, insulin, triglycerides, nonesterified fatty acids (NEFAs), and glucose tolerance] were assessed in the dams at regular intervals (Fig. 2A).
Figure 2.
Resveratrol supplementation yields significant weight loss in the WSD-fed dam. A) Schematic overview of the study design. B, C) Maternal (B) and fetal (C) resveratrol levels at G130 (NonResv, non-resveratrol-supplemented cohort comprised of Ctr and WSD animals, n=8; WSD/Resv, n=6). D) Maternal body mass in the WSD/Resv cohort (n=6). E) Maternal body mass at prepregnancy and third trimester. Significant weight gain within diet cohorts is indicated. **P < 0.01. F) Mean food intake in WSD and WSD/Resv animals. G, H) Change in fat mass (G) and lean mass (H) in the WSD/Resv cohort. I) Leptin levels in maternal plasma measured prepregnancy and in the early third trimester. Maternal Ctr: n = 29; WSD: n = 33; WSD/Resv: n = 6. Data are expressed as means ± se. Bars with different letters differ significantly (P<0.05).
Ultrasound
On G123, after overnight food withdrawal, sedation was induced with a 5- to 15-mg/kg i.m. injection of ketamine. Subjects were positioned in dorsal recumbency, and physiological vital signs were monitored throughout the procedure. Image-directed pulsed and color Doppler equipment (GE Voluson 730 Expert; Kretztechnik, Zipf, Austria) with a 5- to 9-MHz sector probe was used for ultrasonographic data collection by an ultrasonographer (A.E.F.). The lowest high-pass filter level was used (100 Hz), and an angle of 15° or less between the vessel and Doppler beam was deemed acceptable. Blood flow velocity waveforms were obtained from the proximal portion of the uterine artery as described previously (22, 23). The cross-sectional area (CSA) of the vessel was calculated as CSA = π(d/2)2, where d is diameter. The calculated uterine artery volume blood flow (cQuta) was calculated as velocity time integral × CSA × heart rate. For the quantitative estimation of blood flow on the fetal side of the placenta, the umbilical venous volume blood flow (cQuv) was calculated as mean velocity × CSA × 60, where the mean velocity was calculated as 0.5 of the maximum velocity, as described previously (15).
Acute resveratrol infusion
Acute resveratrol intravenous infusion studies were conducted in a subset of 4 animals; 3 WSD-fed Japanese macaques and 1 Ctr chow-fed rhesus macaque. A 100 mM stock of resveratrol (Sigma-Aldrich, St. Louis, MO, USA) was prepared in 70% ethanol. Doses of resveratrol were then prepared in sterile saline at a final concentration of 0.2, 0.5, and 1.0 mg in 5 ml total volume. During a 2-h experiment, animals were given a continuous intravenous infusion of lactated Ringers (5 ml/kg/h) with 5-ml boluses of vehicle Ctr and resveratrol doses at 30-min intervals. For the Ctr animal, vehicle Ctr corresponding to the final concentration of ethanol (range: 0.07 to 0.35%) for the appropriate resveratrol dose was given at each bolus. Doppler ultrasound measurements were made at 5-min intervals until 90 min and then at 15-min intervals for the remainder of the 120 min experiment for calculation of Quta as described above. Blood samples (3 ml) were collected in heparinized tubes at baseline and immediately before each bolus infusion. Plasma samples were stored at −80°C for later assay of resveratrol content.
Resveratrol assay
Maternal and fetal plasma samples were assayed for resveratrol levels at the Bioanalytical Pharmacokinetics Core at Oregon Health and Science University (OHSU). Plasma was collected, and the total (free plus the conjugated resveratrol) was determined by incubating a 100-μl aliquot of plasma with β-glucuronidase (250 U, type H-2; Sigma) at 37°C for 5 h. After incubation, the sample was treated with 500 μl of acetonitrile containing the internal standard daidzein (2 ng/ml). The samples were vortexed, and precipitated protein was removed by centrifugation at 12,000 g for 5 min at 5°C. The supernatant was transferred to a glass tube and dried under reduced pressure. The residue was dissolved in 100 μl of 1:1 methanol/water and filtered through a Durapore-PVDF 0.22-μm spin filter (Millipore, Billerica, MA, USA), and 10 μl was used for LC-MS/MS analysis.
The HPLC system for the analysis (Shimadzu, Columbia, MD, USA) was an SIL-20AC XR autosampler, a CBM-20A system controller, 2 LC-20AD XR LC pumps, a DGU-20 A5 inline solvent degasser, and a CTO-20A column oven. Resveratrol and the internal standard were separated using a Thermo Hypersil Gold column (100×2.1 mm, 5 μm; Thermo Scientific, Rockford, IL, USA) with a 10-mm guard of the same packing. The solvent system consisted of solvent A: 0.1% formic acid and solvent B: methanol with 0.1% formic acid. The analytes were eluted with a linear gradient that consisted of 40 to 98% solvent B over 5 min and held at that concentration for 2 min and then returned to start conditions of 40% solvent B and equilibrated for 3 min. The temperature was maintained at 30°C and the flow rate was 0.3 ml/min. The retention time of resveratrol was 2.9 min and 3.59 for the internal standard. The eluant was interfaced to an Applied Biosystems/MDS Sciex 5500 QTrap triple-quadrupole hybrid linear ion-trap mass spectrometer (Applied Biosystems, Foster City, CA, USA) and was used in triple-quadrupole mode with multiple reaction monitoring (MRM). It was equipped with a TurboIonSpray electrospray ionization (ESI) source operated in the negative mode with the following settings: source voltage −3.5 kV, nebulizer gas (GS1) 50 psi, heater gas (GS2) 50 psi, curtain gas (CUR) 25 psi, source temperature (TEM) 550°C, and collision associated dissociate gas (CAD) high. The MRM transitions monitored were m/z 227.0 → 143 for resveratrol and m/z 252.9 → 132.0 for diadzein. Optimal parameters for the MRM transitions for resveratrol and diadzein were as follows: collision energy (CE; V), −8 and −52, respectively; declustering potential (DP; V), −95 and −115, respectively; collision cell exit potential (CXP; V), −11 and −9, respectively; and exit potential (EP), −10 V. Dwell times were 150 ms, and Q1 and Q3 were operated at unit resolution. Standards were prepared in by spiking naive plasma with 5 different concentrations of resveratrol (0.5–500 ng/ml) processed as above. Area ratios of resveratrol to the internal standard were used to generate standard curves for quantification for each set of samples. Instrument control and data analysis were performed with Analyst 1.5 (AB Sciex, Framingham, MA, USA).
Food intake assessment
Food intake from group-housed animals was assessed at varying time points throughout the study. Animal runs were cleaned and a known amount of food was added to the run twice daily. Before food replenishment, uneaten chow pellets were carefully collected and weighed. Measurements were taken over 2–3 consecutive days, and food intake was determined based on amount eaten and the number of animals per run.
Dual-energy X-ray absorptiometry (DEXA) measurements
Total body fat for each animal was determined by conducting DEXA scans (Hologic QDR Discovery A; Hologic, Inc., Bedford, MA, USA). Animals were sedated with 3–5 mg/kg Telazol and positioned supine on the bed of the scanner. Total body scans (core; collar bones through to hip bones and periphery; limbs) were performed on each animal. QDR software (Hologic) was used to calculate body composition.
Intravenous glucose tolerance tests (IVGTTs)
IVGTTs were performed on dams after overnight food withdrawal at 1 mo before resveratrol administration and at 1 wk before delivery by Cesarean section (C section). Ctr and WSD group dams received GTTs in the fall, at the onset of the breeding season, and at 1 wk before C section to establish normal glucose clearance and glucose-stimulated insulin secretion (GSIS) during pregnancy. GTTs were performed as described previously (18). Glucose area under the curve (AUC) was calculated from T = 0 baseline. GSIS was calculated as AUC from baseline insulin values at T = 0.
Measurement of maternal plasma hormones
Leptin and insulin measurement in monkey plasma was performed by the Endocrine Technology and Support Core (ETSC) Laboratory at the Oregon National Primate Research Center (ONPRC). Leptin was measured using a primate leptin radioimmunoassay (RIA) kit assay (Millipore-Linco, Billerica, MA, USA). Insulin was measured using a chemiluminescence-based automatic clinical platform (Cobas e411; Roche Diagnostics, Indianapolis, IN, USA). This platform has been validated for several hormones, including insulin, in serum of NHPs (24).
Measurement of fetal glucose, insulin, and glucagon
Blood was obtained by fetal heel stick at C section and measured immediately using a OneTouch Ultra blood glucose monitor (LifeScan, Milpitas, CA, USA). For the measurement of islet hormones, fetal blood was collected at necropsy from the abdominal aorta in heparinized tubes. Plasma was isolated by centrifugation and stored at −80°C until assayed. Plasma insulin concentrations were assayed by the ETSC Laboratory as described above. Glucagon in monkey serum or plasma was performed by the ETSC Laboratory using a primate glucagon RIA kit assay (Millipore-Linco).
Measurement of NEFA and triglyceride levels
Blood was obtained from the femoral artery of dams following overnight food withdrawal. Plasma and serum were isolated by centrifugation. Triglycerides were measured in plasma using the Serum Triglyceride Determination Kit (Sigma-Aldrich), while NEFAs were measured in serum using the NEFA-HR kit (Wako Diagnostics, Richmond, VA, USA) according to the manufacturers' instructions.
Fatty acid profiles
Maternal and fetal plasma collected at G130 was analyzed by the methods first described by Lagerstedt et al. (25) with modifications reported in Grant et al. (17).
Tissue collection
At G130, fetuses were delivered by C section in the Surgical Services Unit (ONPRC) and immediately taken to the Pathology Unit (ONPRC) for full necropsy. Fetal organs were dissected by the veterinary pathologist, weights and measures were documented, and individual tissues were processed according to protocol requirements. Placental tissue biopsies were flash-frozen in liquid nitrogen for later protein extraction. Pieces of both liver lobes were fixed in formalin and analyzed with hematoxylin and eosin (H&E) or flash-frozen in liquid nitrogen for triglyceride extraction. The pancreas was isolated and divided into sections from head to tail to be fixed in zinc formalin for immunohistochemical analysis or flash-frozen in liquid nitrogen for RNA isolation as described previously (18).
Placental inflammation
Protein was extracted from placental villous tissue following homogenization in lysis buffer containing 20 mM Tris (pH 7.5), 1 mM EDTA, 1 mM EGTA, 20 mM sodium fluoride, 0.15 M sodium chloride, 0.5% Nonidet P-40, 0.5% Triton X-100, 200 μM sodium orthovanadate, 2 μM leupeptin, 5.8 μM pepstatin, 200 μM 4-(2-aminoethyl) benezenesulfonyl fluoride hydrochloride, and 5 μM N-tosyl-l-lysine chloromethyl ketone. Homogenate was centrifuged for 5 min at 20,000 g to remove cell debris from the placental samples. Inflammation in placental protein was assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kits [regulated on activation, normal T-cell expressed and secreted (RANTES), Thermo Scientific; IL-1 β, Invitrogen Life Technologies, Grand Island, NY, USA; Serpine-1, Abnova, Walnut, CA, USA; and migration inhibitory factor (MIF), R&D Systems, Minneapolis, MN, USA]. ELISA assays were performed according to the manufacturers' instructions and expression levels derived from known standards. All reported data were corrected for protein content determined using a BCA assay (Thermo Scientific).
Liver triglycerides
Liver triglyceride content was measured in liver tissue using Infinity TG reagent (Thermo Scientific) following lipid extraction with methanol, chloroform, and water (1:2:0.8 ratio) as described previously (16).
Pancreas RNA isolation and quantitative RT-PCR
Total RNA isolation, reverse transcription, and quantitative PCR were performed and analyzed as described previously (18). RNA polymerase II was used to normalize real-time expression, and data were analyzed using the Pfaffl method as described previously (26).
Islet immunohistochemistry and quantitative analysis
Paraffin-embedded 5-μm sections from the pancreatic tail were deparaffinized, and standard immunohistochemical methods were used for subsequent analyses (18). Exocrine cell proliferation was assessed by Ki67 staining (1:25; Dako, Carpinteria, CA, USA) in DAPI-positive (+), islet hormone-negative (−) area. Primary antibodies against insulin (1:2500; Dako) and glucagon (1:100; Cell Signaling, Danvers, MA, USA) were applied for 48 h at 4°C and were used to identify and quantify β and α cells, respectively. Islet cell proliferation was assessed by Ki67 staining in islet hormone+ area, while neogenesis was measured by islet hormone colocalization with CK7 (1:50; Abcam, Cambridge, MA, USA). Secondary antibodies were applied for 1 h at room temperature (1:1500; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA).
Images were acquired with the Marianas imaging workstation (Intelligent Imaging Innovations, Denver, CO, USA) as described previously (18). Cell proliferation and neogenesis were assessed using the stereology module within the tail region of each pancreata. Proliferation is expressed as percentage of Ki67+ cells per islet area. Neogenesis is expressed as islet hormone+ area per ductal area. Representative images were acquired with the Leica SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) using the ×40 objective at 1024- × 1024-pixel resolution. Focal planes were 1 μM apart.
Data analysis
Plasma resveratrol measurements were analyzed by Student's t test. Maternal body weight in the WSD/Resv cohort was analyzed using repeated-measures 1-way ANOVA with Tukey's multiple comparisons post hoc analysis. Body composition was analyzed by paired Student's t test. For maternal metabolic parameters, differences between third trimester and baseline were calculated, and 1-way ANOVAs were performed between the 3 cohorts with Tukey's multiple comparison post hoc analysis. All other data were analyzed using a 1-way ANOVA with Tukey's multiple comparison post hoc analysis (Ctr vs. WSD vs. WSD/Resv).
Study approval
All animal procedures were conducted in accordance with the guidelines of the institutional Animal Care and use Committee of the ONPRC and OHSU. The ONPRC abides by the Animal Welfare Act and regulations enforced by the U.S. Department of Agriculture, the Public Health Service Policy on Humane Care and Use of Laboratory Animals, in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
RESULTS
Acute vasodilator effects of resveratrol
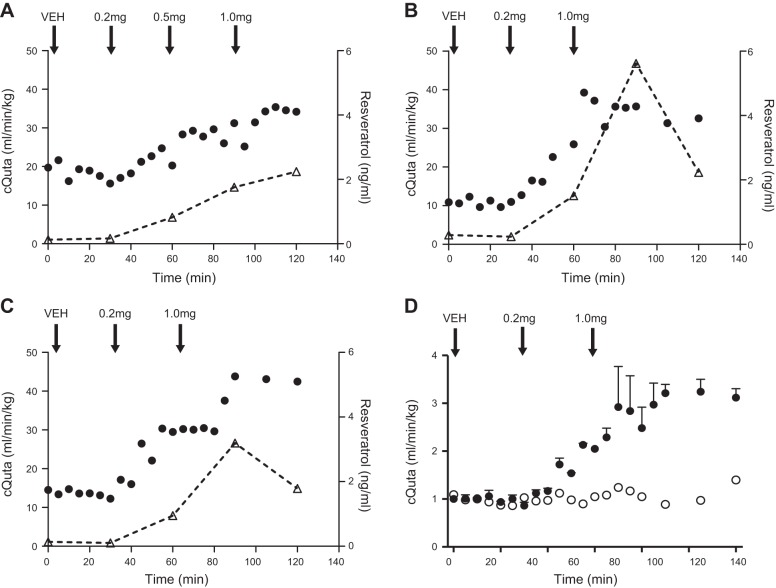
As resveratrol is known to improve vascular function, we hypothesized that administration during pregnancy would alleviate the impaired cQuta demonstrated in WSD-fed animals (15). Thus, we first measured cQuta by Doppler ultrasound in response to an acute intravenous administration of resveratrol in a subset of WSD-fed pregnant female macaques. We observed a dramatic increase in uterine artery vessel diameter accompanied by a rise in the blood flow velocity time integral, resulting in a 2.5-fold increase in cQuta following intravenous infusion of 1.0 mg resveratrol (Fig. 1). This infusion dose yielded a maternal plasma resveratrol range of 2.24 to 5.62 ng/ml. Notably, at maternal levels as low as 1 ng/ml of resveratrol, we demonstrate a marked increase in cQuta (Fig. 1). Circulating resveratrol levels began to fall 1 h after administration of the maximum bolus supporting prior evidence that resveratrol is readily metabolized and indicating pronounced but short-lived effects of this intravenous administration.
Figure 1.
Acute resveratrol intravenous infusion significantly increases uterine blood flow. A–C) cQuta in 3 individually represented WSD-fed animals that received a vehicle infusion bolus (Veh) followed by increasing doses of resveratrol (0.2, 0.5, and 1.0 mg as indicated). Right axis shows maternal plasma levels of resveratrol (open triangles). D) cQuta calculated as the fold change from baseline in 1 Ctr animal (open circles) and mean data from the 2 WSD-fed animals represented in panels B and C that received the same resveratrol dosing as indicated (solid circles).
Metabolic outcomes of chronic dietary supplementation with resveratrol
Considering the improved vascular function following acute resveratrol administration, we sought to investigate whether chronic supplementation in the NHP could ameliorate the adverse effects of WSD consumption during pregnancy previously observed in this model. A cohort of chronic WSD-fed animals were switched onto WSD/Resv 3 mo before the breeding season (Fig. 2A). Maternal fasting plasma levels of resveratrol were in the range of 0.21 to 1.01 ng/ml (Fig. 2B) at the third trimester. However, we have found that postprandial levels in animals consuming the WSD/Resv can reach as high as 70 ng/ml. Significantly, the maternal fasting resveratrol levels we report are comparable to the acute study where a circulating amount of 1 ng/ml produces a physiologically relevant effect on cQuta (Fig. 1). Detection of fetal plasma levels in the range of 0.19 to 1.93 ng/ml at the time of C-section following maternal overnight food withdrawal demonstrates that resveratrol crosses the placenta and enters the fetal circulation in physiologically relevant concentrations (Fig. 2C).
After 9 mo of resveratrol supplementation, dams displayed a significant reduction in body mass with the majority of weight loss occurring during the first 3 mo (Fig. 2D) prior to the breeding season. Notably, this decreased weight was maintained in the third trimester of pregnancy at a time when Ctr and WSD dams have typically gained 0.7–1.0 kg (Fig. 2E). Importantly, there was no difference in food intake between WSD/Resv and WSD cohorts assessed at varying time points throughout the study (Fig. 2F). Weight loss can be attributed largely to the loss of fat mass and, to a lesser extent, some loss of lean mass (Fig. 2G, H). Despite the prepregnancy maternal weight loss, leptin levels increased significantly from prepregnancy to the third trimester in the WSD/Resv cohort (Fig. 2I), indicative of a healthy pregnant status (27).
Chronic resveratrol supplementation did not significantly alter maternal fasting blood glucose concentrations (Fig. 3A) but improved fasting plasma insulin levels in the third trimester (Fig. 3B). The improvement in glucose clearance during pregnancy was greater in WSD/Resv compared with Ctr dams (Fig. 3C) and was not accompanied by an increase in insulin secretion (Fig. 3D). Glucose and GSIS curves for the resveratrol-supplemented dams in their current and prior (WSD-alone) pregnancies are shown in Supplemental Fig. S1. Consistent with normal maternal fasting glucose and glucose AUC in the third trimester, fetal glucose and insulin levels are unchanged between groups (Supplemental Table S1).
Figure 3.
Resveratrol supplementation improves glucose tolerance during pregnancy in the WSD-fed dam. A–D) Maternal fasting glucose (A), insulin (B), maternal glucose AUC (C), and glucose-stimulated insulin secretion AUC (D) following an IVGTT challenge performed at prepregnancy and in the early third trimester. AUC is calculated from fasting baseline values. E, F) Maternal circulating triglyceride (E) and NEFA content (F) in plasma samples at prepregnancy and in the early third trimester. Prepregnancy values correspond to 3 mo on WSD/Resv. Ctr: n = 29; WSD: n = 33; WSD/Resv: n = 6. Data are expressed as means ± se. Bars with different letters differ significantly (P<0.05).
In our model, resveratrol significantly improved circulating triglycerides during the treatment period (Fig. 3E); however, this reduction resulted in no significant difference between the 3 cohorts at the third trimester, as WSD/Resv dams had significantly higher baseline triglyceride levels. Consistent with this observation, fetal triglyceride levels at G130 were unaltered (Supplemental Table S1). In addition, resveratrol had no effect on circulating NEFA levels in the dam (Fig. 3F) or the fetal offspring (Supplemental Table S1). We have previously demonstrated a shift in the n-6:n-3 ratio toward a proinflammatory state with the WSD compared with Ctr cohort (17). Here we demonstrate no significant effect of resveratrol on the fatty acid profiles, where the fetus mirrors the maternal diet-derived profile (Table 1), as previously reported (17). Specifically, neither resveratrol-supplemented dams nor fetuses demonstrate a significant alteration in fatty acid profiles compared with WSD animals. Overall, resveratrol supplementation has beneficial effects on maternal weight loss and glucose clearance but appears to have no impact on fetal insulin or triglyceride levels or fatty acid profiles.
Table 1.
Fatty acid profiles
| Fatty acids | Ctr | WSD | WSD/Resv |
|---|---|---|---|
| Maternal | |||
| n | 10 | 11 | 6 |
| SFAs | 2987 ± 727 | 3329 ± 460 | 2973 ± 828 |
| MUFAs | 1041 ± 510 | 1330 ± 366 | 1486 ± 616 |
| PUFAs | 2436 ± 746 | 2173 ± 512 | 2199 ± 622 |
| n-6 | 2141 ± 684 | 2034 ± 452 | 2059 ± 593 |
| n-3 | 295 ± 98 | 139 ± 74** | 140 ± 52** |
| n-6:n-3 | 8.14 ± 4.53 | 17.26 ± 6.88** | 15.49 ± 3.72* |
| Fetal | |||
| n | 8 | 13 | 6 |
| SFAs | 1465 ± 161 | 1577 ± 255 | 1385 ± 253 |
| MUFAs | 317 ± 84 | 723 ± 285** | 729 ± 205** |
| PUFAs | 1047 ± 221 | 1304 ± 624 | 1221 ± 468 |
| n-6 | 863 ± 161 | 1177 ± 571 | 1084 ± 405 |
| n-3 | 183 ± 64 | 127 ± 71 | 137 ± 70 |
| n-6:n-3 | 4.92 ± 0.83 | 10.62 ± 4.52 | 8.63 ± 2.33** |
Data are means ± se. Maternal and fetal fatty acid profiles in plasma samples collected at G130 delivery from CTR, WSD, and WSD/RESV animals. SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
P < 0.05,
P < 0.01 vs. Ctr.
Resveratrol effect on placental blood flow and inflammation
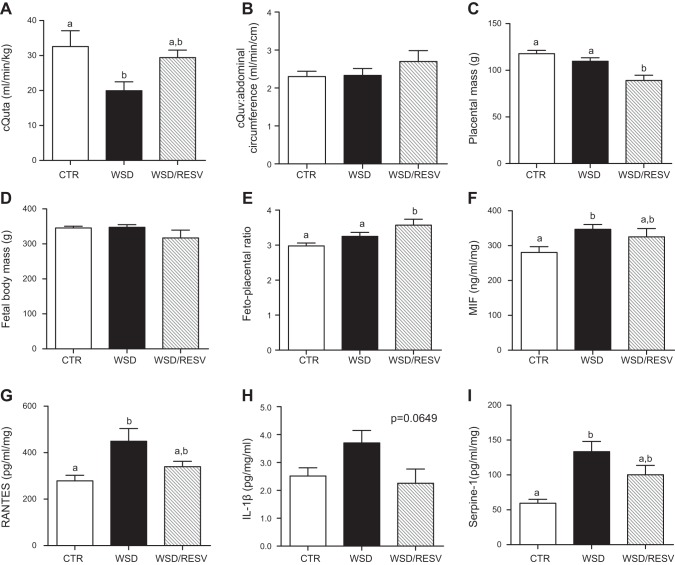
Our group has previously reported that WSD exposure during pregnancy reduces cQuta (15). In our chronic WSD/Resv-fed cohort, this decrease in cQuta is mitigated by resveratrol supplementation throughout pregnancy, as assessed by Doppler ultrasound in early third trimester (Fig. 4A). In addition, cQuv in the WSD/Resv dams is maintained at levels comparable to Ctr and WSD dams (Fig. 4B).
Figure 4.
Resveratrol restores uterine blood flow and improves placental inflammation. A, B) cQuta normalized to maternal body weight (A) and cQuv (B) expressed as blood flow in ml/min normalized to fetal abdominal circumference calculated from Doppler ultrasound measurements at G123. Ctr: n = 4; WSD: n = 8; WSD/Resv: n = 6. C–E) Placental mass (C), fetal body mass (D), and fetoplacental ratio (E) at G130. Ctr: n = 25; WSD: n = 29; WSD/Resv: n = 6. F–I) Placental expression of MIF (F), the chemokine RANTES (G), interleukin-1β (H), and Serpine-1 (I). Ctr: n = 10; WSD: n = 14; WSD/Resv: n = 6. Data are means ± se. Bars with different letters differ significantly (P<0.05).
Interestingly, placental weight was significantly decreased in the WSD/Resv cohort (Fig. 4C) although fetal body weight was unchanged between the three experimental groups (Fig. 4D), resulting in an increased fetoplacental ratio (Fig. 4E). Furthermore, placental inflammation is upregulated in the WSD cohort, and resveratrol mitigates these effects, showing no significant difference compared with Ctr expression levels of the inflammatory cytokines MIF, RANTES, interleukin-1β, and Serpine-1, an inhibitor of fibrinolysis (Fig. 4F–I). Taken together, these data demonstrate that resveratrol improves uteroplacental blood flow, reduces WSD-induced placental inflammation, and preserves fetal growth.
Effects of maternal resveratrol and WSD on fetal development
In keeping with our previously published data (16), liver triglyceride content was 3-fold higher in the WSD compared with Ctr fetuses in this cohort, while resveratrol supplementation improved liver triglyceride content (Fig. 5A). Histological analysis confirmed the presence of lipid accumulation in the WSD fetal liver and reduction in WSD/Resv fetal livers (Fig. 5B).
Figure 5.

Resveratrol reduces lipid deposition in the fetal liver. A) Fetal liver triglyceride levels. Ctr: n = 16; WSD: n = 26; WSD/Resv: n = 6. Data are means ± se. Bars with different letters differ significantly (P<0.05). B) Representative H&E staining of fetal liver. Black arrowheads indicate points of lipid deposition.
Although exposure to resveratrol in utero does not affect total fetal body mass or the mass of other key fetal organs, it did lead to a 42% increase in pancreatic mass (Table 2). Consistent with this, we observed an increase in the proliferation marker Ki67 throughout the pancreas (Fig. 6A). Specifically, fetal offspring born to WSD/Resv dams displayed a ∼12-fold increase in Ki67 staining in the exocrine compartment of the pancreas, compared with WSD offspring, in the early third trimester (Fig. 6A, C). Similarly, gene expression of BCL2, an antiapoptotic marker, was significantly elevated in total pancreatic samples from WSD/Resv offspring as was the BCL2:BAX ratio, an index indicative of proliferative state (Supplemental Table S2). Resveratrol supplementation during pregnancy did not significantly impact fetal islet mass (data not shown) or β-cell mass (Fig. 6A, D). Surprisingly, in utero resveratrol intervention not only failed to restore α-cell mass following WSD exposure but rather exacerbated this effect (Fig. 6A, D). However, despite the reduction in α-cell mass, glucagon gene expression did not differ between groups (Supplemental Table S2). In fact, when corrected for α-cell mass, glucagon expression was increased in the WSD/Resv offspring on a per-cell basis, compared with Ctr (Supplemental Table S2).
Table 2.
Fetal body and key organ weights
| Parameter | Ctr |
WSD |
WSD/Resv |
|||
|---|---|---|---|---|---|---|
| Value | n | Value | n | Value | n | |
| Body mass (g) | 345.5 ± 5.1 | 29 | 347.6 ± 7.4 | 34 | 317.2 ± 22.3 | 6 |
| Brain mass (g) | 45.85 ± 1.05 | 16 | 46.56 ± 0.81 | 21 | 43.38 ± 1.55 | 6 |
| Heart mass (g) | 2.09 ± 0.08 | 15 | 2.11 ± 0.07 | 24 | 2.05 ± 0.20 | 6 |
| Liver mass (g) | 9.43 ± 0.19 | 26 | 9.99 ± 0.21 | 29 | 9.47 ± 0.60 | 6 |
| Pancreas mass(mg) | 249.4 ± 11.80 | 27 | 246.5 ± 11.95 | 31 | 349.3 ± 20.14**,## | 6 |
| Pancreas:body mass (mg/g) | 0.72 ± 0.04 | 27 | 0.72 ± 0.04 | 31 | 1.11 ± 0.06***,#### | 6 |
Data are means ± se. Measurements were made at G130 necropsy.
P < 0.01,
P < 0.001 vs. Ctr;
P < 0.01,
P < 0.0001 vs. WSD.
Figure 6.
Fetal pancreas islet morphology, neogenesis, and proliferation are altered by maternal resveratrol. A, B) Representative IHC images of islet morphology and proliferation (A) and neogenesis (B). White arrowheads indicate Ki67+ cells (A) and insulin+ or glucagon+ ductal cells (B). C–F) Quantification of exocrine proliferation (C), β-cell and α-cell mass (D), β-cell and α-cell neogenesis (E), and β-cell and α-cell proliferation (F) at G130. Ctr: n = 7; WSD: n = 6; WSD/Resv: n = 6. Data represent means ± se. Bars with different letters differ significantly (P<0.05).
Despite the overall decrease in fetal α-cell mass, there was a significant increase in the presence of glucagon+ ductal cells, indicating increased α-cell neogenesis in the WSD/Resv offspring (Fig. 6B, E) with no change in the proliferation marker Ki67 (Fig. 6A, F). In contrast to the α cell, there was no significant change in the presence of insulin+ ductal cells in the fetal pancreas (Fig. 6B, E) but there was a significant increase in Ki67 staining in the WSD/Resv offspring in the β cell (Fig. 6A, F). Consistent with the enhanced proliferation in this cell type, WSD/Resv offspring did display a trend toward increased β-cell mass (Fig. 6D). Interestingly, however, insulin gene expression was significantly reduced on a per-cell basis (Supplemental Table S2).
DISCUSSION
Despite widespread interest in resveratrol as a dietary supplement, this is the first study to examine its effects in a WSD model during pregnancy. Our novel data demonstrate that resveratrol supplementation in pregnant NHPs results in maternal weight loss and improved insulin action, restored uterine volume blood flow, reduced placental inflammation, improved lipid deposition in the fetal liver, and an unexpected enlargement of the fetal pancreas without a concomitant increase in islet mass. The question remains as to whether the fetal outcomes observed in this study are a result of a direct action of resveratrol on the placental and fetal tissues or are secondary to an improved maternal metabolic milieu. Undoubtedly the improved maternal phenotype could contribute to some of the outcomes reported; however, several key pieces of evidence in this study suggest a direct effect of resveratrol action on the placenta and fetal organs. First, resveratrol crosses the placenta and enters into the fetal circulation. Second, our acute intravenous administration data demonstrate a direct and potent effect of resveratrol on vascular function, which is mimicked in the chronic study by the restored uterine volume blood flow and further supported by the well maintained cQuv; all indicative of improved placental vascular function. Third, fetal liver triglyceride content is improved by resveratrol despite maternal and fetal circulating lipid levels comparable to the WSD-fed animals. Finally, we would not anticipate the dramatic increase in pancreas mass and proliferation as a result of maternal weight loss or improved glucose clearance. Specifically, in a diet reversal study in our NHP model where WSD dams were switched back onto a chow diet during pregnancy, we observed normal pancreatic mass with no significant increase in exocrine Ki67 staining (unpublished data) in the fetal offspring in contrast to the WSD/Resv offspring in the current study. Thus, our data are consistent with both an effect on the mother to improve glucose clearance and a direct role of maternal resveratrol consumption on fetal pancreatic development.
The improvements in maternal metabolic parameters that we report in our NHP model are largely consistent with published rodent studies and human clinical trials. However, weight loss following resveratrol consumption remains controversial. To date, weight loss has been demonstrated in obese rodent studies but has not been reported in short-term human clinical trials of resveratrol supplementation (6, 28). Importantly, it should be noted that human subjects on resveratrol have typically been mildly obese but otherwise healthy. Studies in metabolically challenged individuals may yield significant weight loss outcomes. Our study demonstrated improvements in maternal glucose clearance that were not accompanied by increased insulin secretion (Fig. 3D). Notably, the improvement in glucose clearance is consistent with a number of studies on the effect of resveratrol in both rodents and humans (29–31) and may reflect the loss of adiposity in these animals.
We observed a significant reduction in placental weight in the WSD/Resv cohort without adverse changes in fetal body weight. The resulting increase in the fetoplacental ratio suggests improved placental efficiency in these animals. It is plausible that the increased cQuta enhances placental nutrient transfer, as this smaller placental mass appears not to compromise fetal growth. However, the primate placenta does contain a significant reserve capacity to conserve the fetal growth trajectory (32, 33), and thus a 20–25% reduction in placental weight can be well tolerated by the developing fetus. Alterations in placental weight following resveratrol supplementation in a rodent model have been recently reported; resveratrol use in hypoxic rats increased placental weight by 25%, but when administered to normoxic females, resveratrol resulted in an 11% reduction in placental weight (34) that is similar to our findings. Conversely, in a study utilizing two murine knockout models of reduced uterine blood supply, resveratrol did not alter placental weight from wild-type Ctr mice (20). Notably, there appears to be no detriment of reduced placental size on fetal growth in our NHP model; the mechanisms underlying the altered growth and any possible impact on function remain to be investigated but are likely due, in part, to improved placental blood flow. In a previous human study, it was demonstrated that resveratrol acts via AMPK-activated phosphorylation to increase endothelial nitric oxide synthase (eNOS) activity and thus NO production (10), and similarly in a rat model of diabetes, resveratrol was shown to act via eNOS- and neuronal NOS (nNOS)-mediated pathways (35). It seems plausible that the vascular changes observed following resveratrol administration in the current study may be driven by an eNOS-dependent mechanism (36) although endothelium-independent mechanisms of resveratrol induced relaxation of the uterine artery have also been reported (36).
Surprisingly, our data indicate that resveratrol supplementation during pregnancy has contradictory outcomes in the fetal offspring, although the reasons for this are unknown. We demonstrate that resveratrol supplementation improved liver triglyceride accumulation in the fetal offspring despite the lack of effect on the circulating lipid profiles in either the dam (Fig. 3F) or the fetus (Supplemental Table S1). Interestingly, consistent with these findings, resveratrol has been previously shown in a rodent model to reduce intracellular lipids in the liver (37) but does not affect serum free fatty acids and triglycerides (38). Given that both WSD and WSD/Resv cohorts are consuming the same dietary content and amount, similar circulating lipid profiles are not entirely surprising; rather, the difference in triglyceride content in the liver suggests a direct mechanism of resveratrol action, which is the focus of our ongoing investigations. In stark contrast to the other seemingly beneficial effects to the placenta and developing fetus, we find a dramatic increase in fetal pancreatic mass and exocrine proliferation, independent of an increase in islet mass, following maternal resveratrol supplementation which is clinically concerning. We demonstrate a 42% increase in pancreatic mass and a 12-fold increase in exocrine proliferation in fetal offspring born to WSD/Resv dams in the early third trimester. The long-term consequences of this phenotype in the offspring is currently unknown but clearly requires further investigation before resveratrol can be deemed safe for use during pregnancy.
In addition, this study demonstrated a significant increase in the β:α-cell ratio following exposure to resveratrol in utero. Others have demonstrated that dissociated β cells, compared with intact islets, fail to appropriately secrete insulin in response to glucose, presumably due to the loss of signaling from the α cell or other islet cell types (39, 40). Thus, the additional loss of α-cell mass observed in WSD/Resv offspring in this study may cause a further impairment in paracrine signaling and overall islet function. The long-term effects of the increase in the β:α-cell ratio and whether α-cell plasticity is impaired later in life provide strong rationales for the need for follow up studies in the offspring of resveratrol supplemented mothers. Our group has previously demonstrated that in utero exposure to a WSD leads to reduced fetal islet α-cell mass with no change in β-cell mass, resulting in an increase in the β:α-cell ratio (18). Furthermore, at 1 yr of age, offspring born to WSD dams continue to display a significant reduction in α-cell area (18), indicating that an early insult to the development of the α-cell can persist in the juvenile period. However, the increase in α-cell neogenesis in WSD/Resv offspring may reflect a compensatory response by the fetus to overcome the decreased α-cell mass at this developmental time point and thus may allow the fetus to recover from the early developmental insult. Interestingly, however, glucagon expression was elevated on a per-cell basis in the WSD/Resv offspring which may explain how WSD/Resv offspring are able to maintain normal circulating glucagon levels despite impaired α-cell mass (Supplemental Table S1).
In our acute intravenous resveratrol administration studies, we demonstrate potent yet short-lived effects of resveratrol dosing, which are most likely explained by the rapid metabolism of systemic resveratrol. In a clinical study of 11 obese male subjects who were given 150 mg/d oral resveratrol for 30 d, the mean circulating level of resveratrol in nonfasting plasma samples was 231 ng/ml, and following an overnight fast, resveratrol levels were barely detectable (28), which is a similar observation to our study in which postprandial levels reached 70 ng/ml, while fasting levels of resveratrol were <1 ng/ml. Our chronically supplemented animal cohort consumed resveratrol throughout the day as it was incorporated into their diet rather than administered as an oral supplement. In one human trial, the half-life of resveratrol was determined to be 9.2 ± 0.6 h, and the absorption rate of oral resveratrol was demonstrated to be >70% but with low bioavailability and trace amounts of circulating resveratrol reported as <5 ng/ml following an oral dose of 25 mg/d (41). Thus our data, in agreement with a limited number of human studies, suggest that to achieve a physiologically relevant circulating threshold of resveratrol, frequent dosing would be required, and studies are needed to ascertain appropriate dosing levels. In addition to maternal dosing, investigation into the dose-dependent mechanisms of action and pharmacokinetics of resveratrol in fetal tissues is also warranted due to the contradictory impact of maternal resveratrol supplementation during pregnancy reported here. Follow-up studies in the offspring are necessary to reveal the longer-term consequences of maternal resveratrol consumption on offspring weight gain, insulin resistance, and pancreatic function. Thus, while it is clear that resveratrol lessens many of the immediate detrimental effects associated with obesity and WSD consumption, the drastic increase in fetal pancreas mass and proliferation strongly cautions against the clinical use of resveratrol in pregnant women at this time.
Supplementary Material
Acknowledgments
The authors thank Diana Takahashi, Karalee Baquero, Ashley Kostrba, India Tindale, Peter Blundell, Jessica Walker, and Tyler Dean (Oregon National Primate Research Center) for technical assistance and guidance with the animal studies; Dr. Dennis Koop (Pharmacokinetics Core, Oregon Health and Science University) for developing the resveratrol assay; Melissa Kirigiti for confocal images; Anda Cornea for microscopy assistance; Barbra Mason for histology; and Francis Pau and the Endocrine Technology Support Core Laboratory (Oregon Health and Science University) for performing hormone assay measurements.
This work was supported by U.S. National Institutes of Health grants R24-DK-090964 (to K.L.G., K.L.T., and J.E.F.), P51-OD-011092 (partial salary support for K.L.G. and A.E.F. and Marianas imaging use), K01-DK-090199 (S.T.), and S10-RR-024585 (Leica confocal imaging).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AUC
- area under the curve
- C section
- Caesarean section
- cQuta
- calculated uterine artery volume blood flow
- cQuv
- calculated umbilical venous volume blood flow
- CSA
- cross-sectional area
- Ctr
- control
- DEXA
- dual-energy X-ray absorptiometry
- eNOS
- endothelial nitric oxide synthase
- G
- gestational day
- GSIS
- glucose-stimulated insulin secretion
- H&E
- hematoxylin and eosin
- IVGTT
- intravenous glucose tolerance test
- MIF
- migration inhibitory factor
- MRM
- multiple reaction monitoring
- NEFA
- nonesterified fatty acid
- NHP
- nonhuman primate
- NO
- nitric oxide
- RANTES
- regulated on activation, normal T-cell expressed and secreted
- RIA
- radioimmunoassay
- WSD
- Western-style diet
- WSD/Resv
- resveratrol-supplemented Western-style diet
REFERENCES
- 1. Baur J. A., Sinclair D. A. (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506 [DOI] [PubMed] [Google Scholar]
- 2. Lam Y.Y., Peterson C. M., Ravussin E. (2013) Resveratrol vs. calorie restriction: data from rodents to humans. Exp. Gerontol. 48, 1018–1024 [DOI] [PubMed] [Google Scholar]
- 3. Ajmo J. M., Liang X., Rogers C. Q., Pennock B., You M. (2008) Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. You M., Cao Q., Liang X., Ajmo J. M., Ness G. C. (2008) Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J. Nutr. 138, 497–501 [DOI] [PubMed] [Google Scholar]
- 5. Szkudelski T., Szkudelska K. (2011) Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 1215, 34–39 [DOI] [PubMed] [Google Scholar]
- 6. Poulsen M. M., Vestergaard P. F., Clasen B. F., Radko Y., Christensen L. P., Stodkilde-Jorgensen H., Moller N., Jessen N., Pedersen S. B., Jorgensen J. O. (2013) High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 62, 1186–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vetterli L., Brun T., Giovannoni L., Bosco D., Maechler P. (2011) Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through a SIRT1-dependent mechanism. J. Biol. Chem. 286, 6049–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kennedy D. O., Wightman E. L., Reay J. L., Lietz G., Okello E. J., Wilde A., Haskell C. F. (2010) Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 91, 1590–1597 [DOI] [PubMed] [Google Scholar]
- 9. Wong R. H., Berry N. M., Coates A. M., Buckley J. D., Bryan J., Kunz I., Howe P. R. (2013) Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 31, 1819–1827 [DOI] [PubMed] [Google Scholar]
- 10. Carrizzo A., Puca A., Damato A., Marino M., Franco E., Pompeo F., Traficante A., Civitillo F., Santini L., Trimarco V., Vecchione C. (2013) Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 62, 359–366 [DOI] [PubMed] [Google Scholar]
- 11. Kim S. Y., Dietz P. M., England L., Morrow B., Callaghan W. M. (2007) Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 15, 986–993 [DOI] [PubMed] [Google Scholar]
- 12. Armitage J. A., Taylor P. D., Poston L. (2005) Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J. Physiol. 565, 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogden C. L., Carroll M. D., Curtin L. R., Lamb M. M., Flegal K. M. (2010) Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 303, 242–249 [DOI] [PubMed] [Google Scholar]
- 14. Catalano P. M., Ehrenberg H. M. (2006) The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 113, 1126–1133 [DOI] [PubMed] [Google Scholar]
- 15. Frias A. E., Morgan T. K., Evans A. E., Rasanen J., Oh K. Y., Thornburg K. L., Grove K. L. (2011) Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152, 2456–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCurdy C. E., Bishop J. M., Williams S. M., Grayson B. E., Smith M. S., Friedman J. E., Grove K. L. (2009) Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 119, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grant W. F., Gillingham M. B., Batra A. K., Fewkes N. M., Comstock S. M., Takahashi D., Braun T. P., Grove K. L., Friedman J. E., Marks D. L. (2011) Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One 6, e17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Comstock S. M., Pound L. D., Bishop J. M., Takahashi D., Kostrba A. M., Smith M. S., Grove K. L. (2013) High-fat diet consumption during pregnancy and the early post-natal period leads to decreased α cell plasticity in the nonhuman primate. Mol. Metab. 2, 10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams L. D., Burdock G. A., Edwards J. A., Beck M., Bausch J. (2009) Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 47, 2170–2182 [DOI] [PubMed] [Google Scholar]
- 20. Poudel R., Stanley J. L., Rueda-Clausen C. F., Andersson I. J., Sibley C. P., Davidge S. T., Baker P. N. (2013) Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS One 8, e64401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh C. K., Kumar A., Lavoie H. A., Dipette D. J., Singh U. S. (2013) Diabetic complications in pregnancy: is resveratrol a solution? Exp. Biol. Med. (Maywood) 238, 482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konje J. C., Kaufmann P., Bell S. C., Taylor D. J. (2001) A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am. J. Obstet. Gynecol. 185, 608–613 [DOI] [PubMed] [Google Scholar]
- 23. Acharya G., Sitras V., Erkinaro T., Makikallio K., Kavasmaa T., Pakkila M., Huhta J. C., Rasanen J. (2007) Experimental validation of uterine artery volume blood flow measurement by Doppler ultrasonography in pregnant sheep. Ultrasound Obstet. Gynecol. 29, 401–406 [DOI] [PubMed] [Google Scholar]
- 24. Varlamov O., Somwar R., Cornea A., Kievit P., Grove K. L., Roberts C. T., Jr. (2010) Single-cell analysis of insulin-regulated fatty acid uptake in adipocytes. Am. J. Physiol. Endocrinol. Metab. 299, E486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lagerstedt S. A., Hinrichs D. R., Batt S. M., Magera M. J., Rinaldo P., McConnell J. P. (2001) Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol. Genet. Metab. 73, 38–45 [DOI] [PubMed] [Google Scholar]
- 26. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henson M. C., Castracane V. D. (2000) Leptin in pregnancy. Biol. Reprod. 63, 1219–1228 [DOI] [PubMed] [Google Scholar]
- 28. Timmers S., Konings E., Bilet L., Houtkooper R. H., van de Weijer T., Goossens G. H., Hoeks J., van der Krieken S., Ryu D., Kersten S., Moonen-Kornips E., Hesselink M. K., Kunz I., Schrauwen-Hinderling V. B., Blaak E. E., Auwerx J., Schrauwen P. (2011) Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crandall J. P., Oram V., Trandafirescu G., Reid M., Kishore P., Hawkins M., Cohen H. W., Barzilai N. (2012) Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 67, 1307–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 32. Roberts V. H., Rasanen J. P., Novy M. J., Frias A., Louey S., Morgan T. K., Thornburg K. L., Spindel E. R., Grigsby P. L. (2012) Restriction of placental vasculature in a non-human primate: a unique model to study placental plasticity. Placenta 33, 73–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wallace J. M., Bourke D. A., Aitken R. P., Leitch N., Hay W. W., Jr. (2002) Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am. J. Physiol. 282, R1027–1036 [DOI] [PubMed] [Google Scholar]
- 34. Bourque S. L., Dolinsky V. W., Dyck J. R., Davidge S. T. (2012) Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 33, 449–452 [DOI] [PubMed] [Google Scholar]
- 35. Arrick D. M., Sun H., Patel K. P., Mayhan W. G. (2011) Chronic resveratrol treatment restores vascular responsiveness of cerebral arterioles in type 1 diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 301, H696–H703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naderali E. K., Doyle P. J., Williams G. (2000) Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guinea-pigs. Clin. Sci. (Lond.) 98, 537–543 [PubMed] [Google Scholar]
- 37. Shiozaki M., Hayakawa N., Shibata M., Koike M., Uchiyama Y., Gotow T. (2011) Closer association of mitochondria with lipid droplets in hepatocytes and activation of Kupffer cells in resveratrol-treated senescence-accelerated mice. Histochem. Cell Biol. 136, 475–489 [DOI] [PubMed] [Google Scholar]
- 38. Um J. H., Park S. J., Kang H., Yang S., Foretz M., McBurney M. W., Kim M. K., Viollet B., Chung J. H. (2010) AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59, 554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halban P. A., Wollheim C. B., Blondel B., Meda P., Niesor E. N., Mintz D. H. (1982) The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology 111, 86–94 [DOI] [PubMed] [Google Scholar]
- 40. Hauge-Evans A. C., Squires P. E., Persaud S. J., Jones P. M. (1999) Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 48, 1402–1408 [DOI] [PubMed] [Google Scholar]
- 41. Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Jr., Walle U.K. (2004) High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 32, 1377–1382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.