Abstract
Eight novel mononuclear and two dinuclear platinum(IV) complexes were synthesized and characterized by elemental analysis, one- and two-dimensional NMR spectroscopy, mass spectrometry, and reversed-phase HPLC (log kw) and in one case by X-ray diffraction. Cytotoxicity of the compounds was studied in three human cancer cell lines (CH1, SW480, and A549) by means of the MTT assay, featuring IC50 values to the low micromolar range. Furthermore a selected set of compounds was investigated in additional cancer cell lines (P31 and P31/cis, A2780 and A2780/cis, SW1573, 2R120, and 2R160) with regard to their resistance patterns, offering a distinctly different scheme compared to cisplatin. To gain further insights into the mode of action, drug uptake, DNA synthesis inhibition, cell cycle effects, and induction of apoptosis were determined for two characteristic substances.
INTRODUCTION
Platinum(II) complexes, namely, cis-, carbo-, and oxaliplatin (Figure S1, Supporting Information), are highly successful anticancer drugs in worldwide clinical application against a variety of tumor types.1–3 However, major limitations of these drugs are (i) dose-limiting severe toxicities, (ii) poor bioavailability, and (iii) intrinsic or acquired resistance.4–8 As a consequence, different approaches have emerged to improve the cytotoxic profile of anticancer platinum compounds, including sterically hindered platinum agents, trans configured platinum compounds, multinuclear platinum(II), and platinum(IV) complexes.9–13
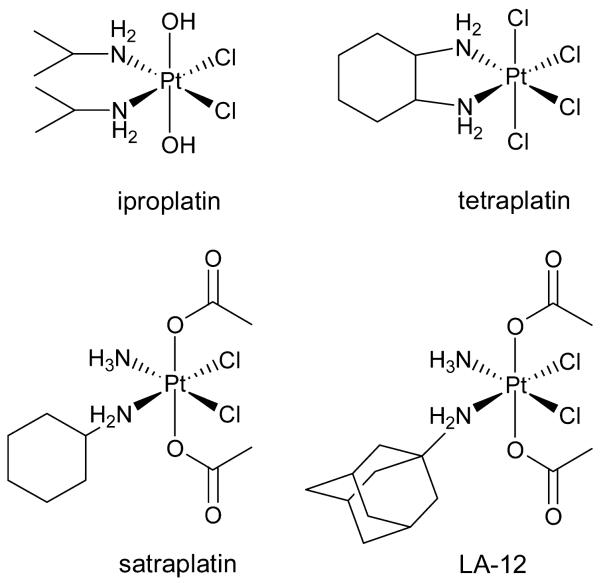
The platinum(IV) complexes seem to have the ability to overcome the drawbacks of platinum(II) drugs because of their (i) reduced systemic toxicity, as they act as prodrugs, (ii) possible oral application, and (iii) high kinetic inertness. However, only four platinum(IV) complexes (Figure 1) entered clinical trials so far and none of them have been currently approved as an anticancer drug.10,14
Figure 1.
Clinically investigated platinum(IV) drugs.
Iproplatin ((OC-6-33)-dichloridodihydroxidobis-(isopropylamine)platinum(IV), chip, JM915) was the first platinum(IV) compound that entered clinical trials, but because of insufficient activity compared to cisplatin and carboplatin, the clinical phase III studies were abandoned.16,17 High neurotoxicity led to discontinuation of the investigation of tetraplatin ((OC-6-22)-tetrachlorido(D,L-cyclohexane-1,2-diamine)platinum(IV), ormaplatin) in phase I clinical trials.18,10,17 The orally administered drug satraplatin ((OC-6-43)-diacetatoamminedichlorido(cyclohexylamine)platinum(IV), JM21619) was the most promising platinum(IV) complex in clinical trials. Nonetheless, the phase III SPARC trial (satraplatin and prednisone against refractory cancer) showed no significant increase of survival rates compared to standard treatment.20,21 The satraplatin-related complex (OC-6-43)-diacetato-(adamantylamine)amminedichloridoplatinum(IV) (LA-12, cyclohexylamine was exchanged by adamantylamine) was under clinical investigation, but results have not been published yet.22,23
It is well recognized that octahedral platinum(IV) complexes are not the active species in the human body. Therefore, these compounds are prodrugs that have to undergo extracellular or preferably intracellular reduction by agents like ascorbic acid, glutathione, or high molecular weight biomolecules, thereby resulting in the antitumor active square-planar platinum(II) metabolites.24–28
Thus, the activity of these complexes depends on a range of bioinorganic and pharmacological attributes like the reduction potential, lipophilicity and solubility, binding behavior to biomolecules, and inertness with regard to ligand exchange reactions.29–31 Several studies show that the type of axial ligands has an exceptionally high influence on the reduction potential and consequently on the cytotoxicity (chlorido ligands, high reduction potential and high cytotoxicity; hydroxido ligands, low reduction potential and low cytotoxicity).32 As platinum(IV) drugs should ideally be reduced intracellularly, drug uptake into the cancer cell must occur before the reduction process takes place. Cellular accumulation is possible via passive diffusion across the cell membrane or active transport into the cell.33 On the one hand, an increase of the lipophilic character of platinum(IV) complexes facilitates drug accumulation via the lipid bilayer of the cell membrane.34 On the other hand, a decrease of aqueous solubility impedes drug application. Oral administration can only be ensured when the platinum(IV) compound is stable enough to reach the target, especially under the broad pH range in the human gastrointestinal tract.35 The additional axial ligands in platinum(IV) complexes compared to platinum(II) enable the adjustment of all these important properties on the way to novel and more selective anticancer agents. Particularly challenging in this context is the synthesis of platinum(IV) compounds with two different axial ligands. At present, the only possibilities of receiving an unsymmetrically substituted complex is (i) oxidation in an alcohol36,37 or carboxylic acid solution38 or (ii) maintaining a free hydroxido group during the subsequent derivatization reaction.39,40
In the present work, we report on the synthesis of unsymmetrically substituted mono- and dinuclear platinum(IV) compounds including an ethylene glycol moiety leading to good water solubility that aids drug formulation. Furthermore, the complexes were investigated with regard to their cytotoxicity, drug accumulation, and lipophilic profile. Additionally, structure–activity relationships are discussed including compounds synthesized previously.40
RESULTS AND DISCUSSION
Synthesis and Characterization
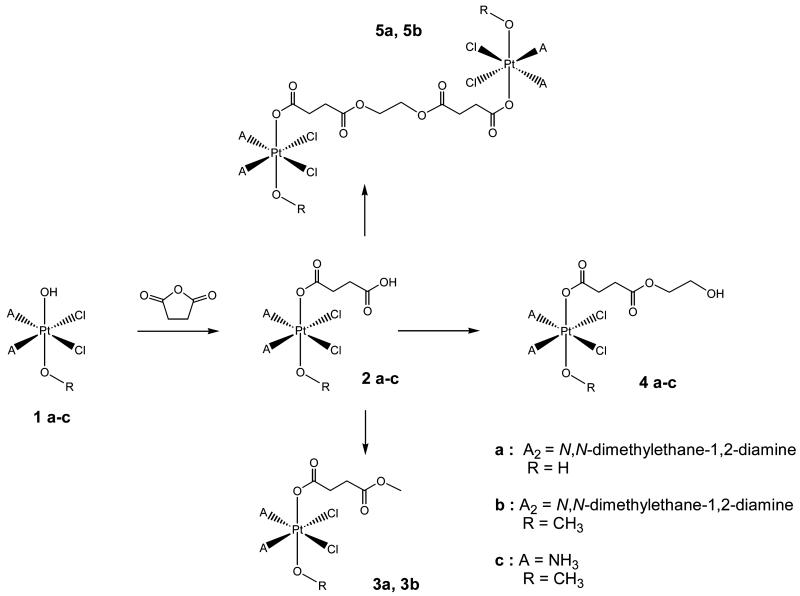
Synthesis of the starting platinum(II) complexes cisplatin and (SP-4-3)-dichlorido(N,N-dimethyl-ethane-1,2-diamine)platinum(II) was performed according to standard literature procedures starting from K2PtCl4. Subsequent oxidation with 30% H2O2 in H2O led to the symmetric complex 1a with two hydroxido groups in axial position, whereas oxidation with 50% H2O2 in absolute MeOH produced the asymmetric complex 1b or 1c possessing one hydroxido and one methoxido ligand in axial position (Scheme 1). Reaction of 1a–c with succinic anhydride (2 equiv in DMF) produced monocarboxylated products 2a–c in good yields (42–73%). Further derivatization was performed using CDI (1,1-carbonyldiimidazol) and an excess of methanol/methanolate or ethylene glycol/ethylene glycolate solution yielding the corresponding esters 3a, 3b, and 4a–c in moderate yields (28–38%) after column chromatography. Dinuclear species 5a and 5b were obtained by addition of only 0.5 equiv of the ethylene glycol/ethylene glycolate solution (yields, 26–32%). All novel platinum(IV) compounds were characterized by elemental analysis, multinuclear (1H, 13C, 15N, and 195Pt) one- and two-dimensional NMR spectroscopy, reversed-phase HPLC, and ESI mass spectrometry; furthermore, 1b was analyzed by X-ray diffraction.
Scheme 1.
Overview of the synthesis of mono- and dinuclear platinum(IV) complexes 1–5
NMR Spectroscopy
195Pt NMR chemical shifts were found at around 2500 ppm indicating a PtN2Cl2O2 coordination sphere in accordance with literature data (see Table 1).41
Table 1.
195Pt NMR Shifts and log kw Values of Synthesized Compounds
| compd | 195Pt NMR signal | log kw |
|---|---|---|
| 1a | 2412 (DMSO-d6) | |
| 2a | 2497 (DMSO-d6) | 0.46 ± 0.03 |
| 3a | 2497 (DMSO-d6) | 0.94 ± 0.01 |
| 4a | 2497 (DMSO-d6) | 0.30 ± 0.06 |
| 5a | 2496 (DMSO-d6) | 1.98 ± 0.07 |
| 1b | 2368 (DMSO-d6) | 0.09 ± 0.01 |
| 2b | 2379 (DMF-d7) | 1.00 ± 0.01 |
| 3b | 2497 (DMF-d7) | 1.58 ± 0.02 |
| 4b | 2410 (DMSO-d6) | 1.37 ± 0.06 |
| 5b | 2410 (DMSO-d6) | 2.95 ± 0.01 |
| 1c | 2559 (DMSO-d6) | |
| 2c | 2606 (DMSO-d6) | 0.39 ± 0.01 |
| 4c | 2602 (DMSO-d6) | 0.22 ± 0.01 |
| satraplatin | 2900 (DMSO-d6) | 1.79 ± 0.01 |
Expectedly, the platinum shift is only altered when deviations in the structure are close to the platinum center. The value of the platinum chemical shift increases when a methoxido group (complex 1b, 2368 ppm) is exchanged by a hydroxido group (complex 1a, 2412 ppm), and this increase can also be observed for the respective monocarboxylated complex (complex 2a, 2497 ppm). Additionally, the platinum resonance of cisplatin analogues is shifted to lower field compared to complexes with a N,N-dimethylethane-1,2-diamine chelate ligand.
In 1H NMR spectra, derivatization of the axial ligands can best be judged. All chemical shifts of the synthesized compounds were found in the expected range. NH3 signals of diammine complexes 1c, 2c, and 4c were detected at 5.5–5.9 ppm, whereas the NH2 resonances of complexes 2a–5a and 1b–5b were shifted to lower field and split into two signals which were detected between 6.7–7.3 ppm and 9.1–9.6 ppm, respectively. The appearance of these two separated NH signals indicates a monocarboxylation and as a result the formation of a chiral platinum(IV) center. The same holds true for the N(CH3)2 proton signals (complexes 2a–5a) resonating as two singulets at 2.69–2.66 and 2.66–2.59 ppm, additionally featuring 195Pt satellites with a coupling of 3J(1H,195Pt) = 24 Hz.42
The methyl ester complexes 3a and 3b as well as dimers 5a and 5b exhibit the same number of signals in proton NMR spectra because of the symmetry plane in the dinuclear species. Nevertheless, differentiation is easy, since the methyl group resonates at around 3.6 ppm, whereas the singlet of the linking ethylene group of the dimers was detected at 4.2 ppm with an intensity of four protons.
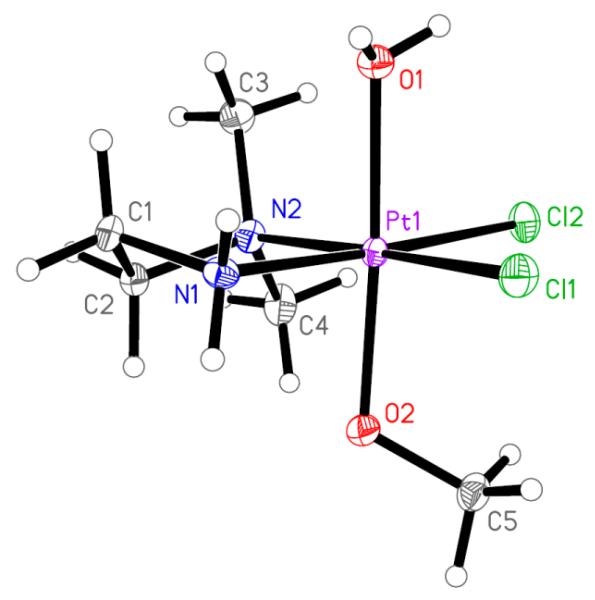
Crystal Structure
Crystals of 1b suitable for X-ray data collection were obtained by slow evaporation of an aqueous trifluoroacetic acid solution. The result of the X-ray diffraction study of 1b+·TFA− is shown in Figure 2. Crystal data, data collection parameters, and structure refinement details are given in Table S1 (Supporting Information). Complex 1b crystallized in the triclinic centrosymmetric space group . The platinum(IV) atom has an octahedral coordination geometry with a bidentate N,N-dimethylethane-1,2-diamine ligand and two chlorido ligands bound in the equatorial plane. In the axial position one methoxido and one aqua ligand are coordinated (because of the acidic conditions, the hydroxido ligand is protonated). The Pt–N, Pt–O, and Pt–Cl bond lengths are in good agreement with those of structurally similar compounds.41
Figure 2.
ORTEP view of 1b+ (TFA− counterion is omitted) with atom labeling scheme. The thermal ellipsoids have been drawn at the 50% probability level. Selected bond lengths (Å) and bond angles (deg) are as follows: Pt–N1 2.035(3), Pt–N2 2.103(3), Pt–Cl1 2.3134(9), Pt–Cl2 2.3176(8), Pt–O1 2.060(2), Pt–O2 1.983(2); O1–Pt–O2 175.81(10), N1–Pt–N2 85.31(12), Cl1–Pt–Cl2 93.34(3).
Reversed-Phase HPLC Measurements
A very important parameter in the pharmacological behavior of drugs, especially with respect to cellular accumulation, is their lipophilicity. As the commonly used shake flask method for determination of the octanol/water partition coefficient is prone to irregularities (dependency on temperature, pH, ionic strength, saturation of water with octanol and vice versa, potential hydrolysis of the compounds in the aqueous phase), we used a chromatographic method, which is known to be highly reproducible.43
The measured log kw values specify the relative lipophilicity of the synthesized compounds, but these values are altered through protonation/deprotonation steps of free polar groups. Therefore, formic acid was added to the solvents in reversed-phase HPLC to ascertain that the carboxylic acids were protonated. As expected, the order of lipophilicity (when the platinum coordination sphere PtN2Cl2(OH)(OCOR) is unchanged) is as follows: dimer > methyl ester > ethylene glycol ester > complex with free carboxylic group (Table 1, Figure S2, Supporting Information). Platinum(IV) complexes with an altered platinum core show log kw values in the following descending order: Pt(N,N-Me2en)Cl2(OCOR)(OMe) > Pt-(N,N-Me2en)Cl2(OCOR)(OH) > Pt(NH3)2Cl2(OMe)-(OCOR) > Pt(N,N-Me2en)Cl2(OH)(OMe). In comparison to satraplatin, all novel complexes exhibit lower log kw values except dinuclear complexes 5a and 5b.
Cytotoxicity in Cancer Cell Lines
The colorimetric microplate 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to study the cytotoxic potency of the novel complexes in three human tumor cell lines (CH1, A549, and SW480) in comparison to cisplatin (Table 2, Figures S3–S5, Supporting Information). The cisplatin sensitive ovarian carcinoma cell line CH1 showed the strongest response to all synthesized compounds with IC50 values in the low micromolar range. It is noticeable that in this cell line all tested compounds, independent of the ligand sphere, have IC50 values in a similar range (1a, 1b, 3a, 3b, 4a–c, 5a, 5b), with the exception of 1c and the derivatives featuring a free carboxylic group (2a, 2b). The latter have an up to 8-fold lower activity in CH1 cells. A very different picture was observed in A549 (non-small-cell lung carcinoma) and SW480 (colon adenocarcinoma) cells. In general, these two cell lines were less responsive to the tested compounds, with IC50 values mostly 1 or even 2 orders of magnitude higher than those in CH1 cells.
Table 2.
Cytotoxicity of Complexes 1–5 Compared to Cisplatin in Three Human Cancer Cell Lines
| IC50 [μM]b | |||
|---|---|---|---|
|
|
|||
| compd | CH1 | A549 | SW480 |
| 1a a | 3.3 ± 1.4 | 111 ± 15 | 115 ± 10 |
| 2a a | 8.4 ± 1.0 | 145 ± 39 | 75 ± 13 |
| 3a a | 3.1 ± 0.9 | 48 ± 12 | 8.4 ± 1.1 |
| 4a | 3.4 ± 1.2 | 319 ± 54 | 77 ± 22 |
| 5a | 6.2 ± 2.8 | 356 ± 27 | 84 ± 10 |
| 1b | 2.5 ± 0.2 | 73 ± 3 | 40 ± 14 |
| 2b | 12 ± 2 | >250 | 135 ± 28 |
| 3b | 1.5 ± 0.6 | 38 ± 4 | 4.7 ± 0.8 |
| 4b | 2.5 ± 0.6 | 120 ± 13 | 9.4 ± 2.9 |
| 5b | 2.3 ± 0.3 | 193 ± 74 | 19 ± 3 |
| 1c | 0.61 ± 0.10 | 33 ± 2 | 3.9 ± 1.0 |
| 2c | 3.8 ± 1.5 | 220 ± 27 | 129 ± 19 |
| 4c | 3.2 ± 1.0 | 132 ± 33 | 37 ± 4 |
| cisplatin | 0.16 ± 0.03 | 1.3 ± 0.4 | 3.5 ± 0.3 |
Previously published compounds.40
50% inhibitory concentrations in CH1, A549, and SW480 cells in the MTT assay (96 h exposure). Values are the mean ± standard deviations obtained from at least three independent experiments.
Moreover, the methyl ester derivatives (3a, 3b) are more potent than the analogous complexes with a terminal free polar group such as carboxyl (2a, 2b) or hydroxy (4a, 4b), as reflected by up to 7 times (A549) and 29 times (SW480) lower IC50 values. The ethylene glycol esters (especially 4b and 4c) show in turn a higher activity than their carboxylic acid counterparts (2b, 2c), especially in SW480 cells. To sum up, these findings imply that small changes in the structures of the ligands have a strong impact on the activity and presumably the mode of action. Furthermore, the novel dimeric platinum species 5a and 5b did not improve the cytotoxicity.
log kw vs IC50
In contrast to literature, where frequently a connection between cytotoxicity and lipophilicity was reported,31,44 the here presented data show no evidence for a correlation between IC50 and log kw in the cells investigated. The platinum(II) core seems to have a notable impact on the cytotoxicity, as all cisplatin derivatives show better or similar cytotoxicity than their N,N-dimethylethane-1,2-diamine counterparts featuring an increased log kw value. Also, in the case of complexes with the same platinum(II) core, the log kw values do not correlate with the cytotoxicity. For example, the dinuclear species have the highest log kw but there is no increase (in A549 and SW480 cells, a strong decrease) of cytotoxicity compared to the monomers. In contrast, the unsymmetrically oxidized cisplatin 1c is the most cytotoxic compound, although the substance is too polar for log kw measurements, because it is not retarded on the column. A plot IC50 vs log kw for cell lines CH1, SW480 and A549 is depicted in Figure S6, Supporting Information.
Impact of Drug Resistance on the Anticancer Activity of the New Complexes
Differences in the activity of the new compounds against CH1, A549, and SW480 cells indicate that there are molecular mechanisms that might render cells more sensitive or resistant to the novel platinum(IV) drugs. Notably, A549 cells are known for their high expression of ABCC1 (multidrug resistance protein 1, MRP1), ABCC2 (multidrug resistance protein 2, MRP2), and ABCG2 (breast cancer resistance protein, BCRP), while SW480 cells express low levels of ABCB1 (P-glycoprotein, P-gp).45 To evaluate whether ABC transporter expression is responsible for the above observed resistance of A549 and SW480 cells to the investigated platinum(IV) compounds, ABCC1-overexpressing 2R120 cells as well as ABCB1- and ABCC1-overexpressing 2R160 cells were investigated in comparison to their chemosensitive parental line SW1573 (Table 3). For these tests, compounds 1a–5a were chosen exemplarily. In general, the activity pattern of the platinum drugs against all three sublines was comparable to the ones in A549 and SW480 cells, with the methyl ester being the most active (factor of 4 in the cases of SW1573 and 2R160 and a factor 2 in the case of 2R120). With regard to the impact of ABC transporter expression, only a minor or no influence was observed with respect to ABCC1, and ABCB1 expression had basically no effect.
Table 3.
IC50 Values of Complexes 1a–5a Compared to Cisplatin with Regard to Their Resistance Patternsa
| IC50 [μM] | |||||
|---|---|---|---|---|---|
|
|
|||||
| compd | SW1573 | 2R120 | -fold resistance | 2R160 | -fold resistance |
| 1a | 130 ± 12 | >250.0 | >1.9 | 185 ± 6 | 1.4 |
| 2a | 151 ± 9 | 243 ± 4 | 1.6 | 196 ± 19 | 1.3 |
| 3a | 30.4 ± 0.9 | 95±5 | 3.1 | 48 ± 3 | 1.6 |
| 4a | 134 ± 5 | 217 ± 6 | 1.6 | 171 ± 5 | 1.3 |
| 5a | 144±7 | 243 ± 12 | 1.7 | 193 ± 7 | 1.3 |
| cisplatin | 6.5 ± 0.1 | >20.0 | >3.0 | 12.7 ± 0.4 | 2.0 |
| IC50 [μM] | |||
|---|---|---|---|
|
|
|||
| compd | A2780 | cis 8 7 A27 | -fold resistance |
| 1a | 50 ± 4 | 66 ± 12 | 1.3 |
| 2a | 31 ± 6 | 86 ± 7 | 2.8 |
| 3a | 19.7 ± 0.4 | 37 ± 3 | 1.9 |
| 4a | 22 ± 2 | 99 ± 30 | 4.5 |
| 5a | 10±2 | 83 ± 14 | 8.3 |
| cisplatin | 0.70 ± 0.02 | 3.7 ± 0.3 | 5.3 |
| satraplatin | 0.5 ± 0.3 | 5.7 ± 0.2 | 11.4 |
| IC50 [μM] | |||
|---|---|---|---|
|
|
|||
| compd | P31 | P31/cis | -fold resistance |
| 1a | 63 ± 2 | 106 ± 8 | 1.7 |
| 2a | 164±2 | 176 ± 4 | 1.1 |
| 3a | 34.3 ± 1.2 | 76 ± 3 | 2.2 |
| 4a | 124±4 | 167±4 | 1.3 |
| 5a | 137±6 | 154 ± 5 | 1.1 |
| cisplatin | 4.0 ± 0.3 | ≫20.0 | ≫5 |
Cell lines used: SW1573, 2R120, 2R160, A2780, A2780/cis, P31, and P31/cis.
To judge the influence of acquired cisplatin resistance, the A2780 and A2780/cis cells as well as P31 and P31/cis cells were used. Multiple mechanisms are known to underlie cisplatin resistance,8 which involve (i) reduced intracellular drug accumulation, (ii) conjugation to intracellular thiols like glutathione (GSH), (iii) enhanced repair of platinum DNA adducts or enhanced tolerance to these adducts, or (iv) resistance to cell death induction. Hence, A2780/cis cells are characterized by reduced drug uptake, enhanced GSH levels, and increased repair potential46,47 while P31/cis cells are known for their general apoptosis deficiency.48 As shown in Table 3, the apoptosis deficiency of P31 and P31/cis cells had, in contrast to cisplatin (≫5-fold), basically no impact on the platinum(IV) drugs (maximum of 2.2-fold resistance). With regard to A2780/cis cells, a more complex situation was observed. In the cases of the dinuclear complex 5a and satraplatin, a resistance even stronger than the one against cisplatin was found (8.3-fold and 11.4-fold vs 5.3-fold for cisplatin). The ethylene glycol ester 4a indicated an equal resistance compared to cisplatin (4.5-fold), whereas all other tested compounds (1–3a) were only slightly (1.3- to 2.8-fold) less active in the cisplatin-resistant subline.
Role of Apoptosis Induction and Cell Cycle Arrest in the Anticancer Activity of Compounds 3a and 4a
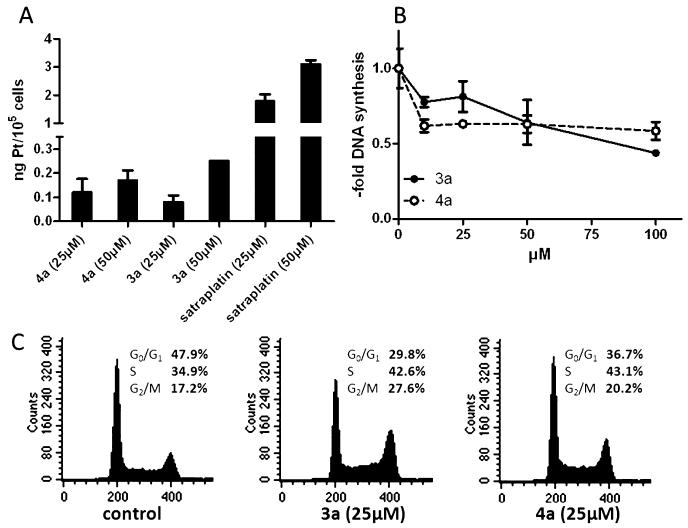
The structure–activity pattern found via MTT assays strongly indicated that there are distinct differences in the mechanisms underlying the activity of compounds with a terminal ethylene glycol ester (4a–c) in comparison to a terminal methyl ester moiety (3a,b). To gain more insights into the mode of action, compounds 3a and 4a were chosen for further experiments. As shown in Figure 3A, in good correlation to the data of the MTT assay no significant differences in the cellular drug uptake were found. In addition, these experiments revealed that the stronger activity of satraplatin can be explained at least partially by enhanced drug uptake.
Figure 3.
Role of cell cycle arrest in the anticancer activity of compound 3a and 4a. (A) Total Pt levels of treated A2780 cells were determined after 2 h of drug exposure by ICP-MS. Values given are the relative mean and SD from at least two independent experiments. (B) DNA synthesis levels were determined in A2780 cells by [3H]thymidine incorporation after 24 h of drug treatment at the indicated concentrations. (C) Cell cycle analysis was evaluated by flow cytometry determining the DNA content of PI-stained A2780 cells after 24 h of treatment with the indicated drug concentrations. Percentages of 25 000 cells in G0/G1, S, and G2/M phases of cell cycle were calculated by Cell Quest software.
Satraplatin has been recently shown to induce G2/M arrest in colorectal cancer cells via p53 and p21 induction.49 In accordance, also for the two novel platinum(IV) compounds a stop of DNA synthesis and distinct cell cycle disturbances were found (Figure 3B and Figure 3C). Hence, both compounds induced an increase of the S- and the G2/M population in A2780 cells. Interestingly, the activity of 3a in general revealed a dose-dependent pattern while 4a had stable activity over a wide concentration range (10–100 μM). Consequently, we hypothesize that treatment with compound 3a might lead to apoptosis induction in addition to the cytostatic activity.
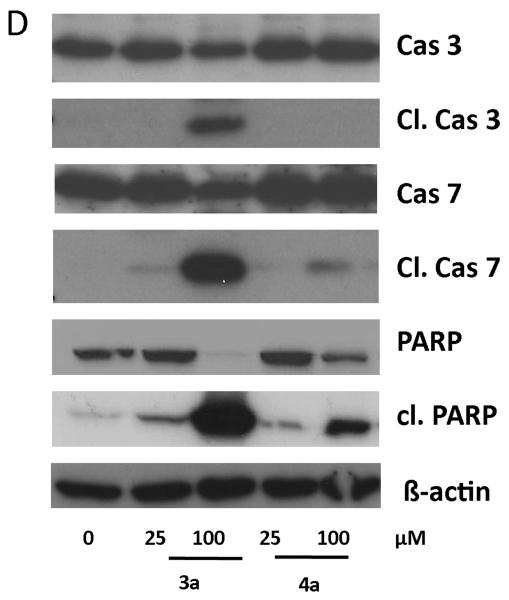
To investigate whether 3a and 4a substantially differ in their apoptosis-inducing potential, total protein extracts of treated cells were analyzed by Western blotting for activation of the apoptotic caspase cascade. Indeed, after 24 h of incubation with 100 μM 3a a distinct induction of apoptotic signaling via the mitochondrial pathway (cleavage of caspase 3 and 7 as well as the caspase substrate PARP) was observed, while for cells treated with 4a or low concentrations of 3a (25 μM) no apoptosis induction was found (Figure 4). Together with the results above, these data confirm that in the case of compound 3a in addition to the cytostatic a cytotoxic mode of action component also exists. This implies distinct differences in the mechanisms underlying the anticancer activities of compounds 3a and 4a, which might explain the differences observed in the sensitivity patterns in the MTT screening.
Figure 4.
Apoptosis induction of compounds 3a and 4a. Apoptosis-induced cleavage of PARP, caspase 7, and caspase 3 in A2780 cells after 24 h of treatment was determined via Western blot. Antibodies used are described in Experimental Section.
CONCLUSION
Ten novel platinum(IV) compounds featuring an unsymmetrical coordination sphere were synthesized and characterized in detail. Cytotoxicity in three cancer cell lines (CH1, A549, and SW480) shows strong differences in the activity pattern dependent on the terminal group of the axial ligands but not on the lipophilicity. This behavior immediately suggests that the polarity of the terminal group might profoundly influence the mode of action. Consequently, a set of compounds were investigated regarding resistance pattern, drug uptake, induction of apoptosis, and cell cycle distribution. The major difference of the monomeric compounds with a terminal methyl ester compared to that with a terminal ethylene glycol ester is the stronger apoptosis induction of the former. In contrast, drug uptake and cell cycle arrest pattern did not differ substantially. However, further investigations are needed to better define the influences of axial ligands on the mode of action of platinum(IV) prodrugs.
EXPERIMENTAL SECTION
Materials and Methods
K2PtCl4 was obtained from Johnson Matthey (Switzerland). Reverse osmosis water was distilled twice before use, and absolute methanol was dried using standard procedures. All other solvents and reagents were obtained from commercial sources and were used as received. Silica gel 60 (Fluka) was used for column chromatography. All platinum(II) starting compounds were synthesized according to standard literature procedures.
Physical Measurements
1H, 13C, 15N, and 195Pt one- and two-dimensional NMR spectra were recorded with a Bruker Avance III 500 MHz instrument at 500.32 (1H), 125.81 (13C), 50.70 (15N), and 107.55 (195Pt) MHz or 500.10 (1H), 125.75 (13C), 50.68 (15N), and 107.51 (195Pt) MHz in DMSO-d6 at 298 K. For NMR numbering scheme, see Figure S7, Supporting Information.
The solvent residual peak for 1H and 13C was used as internal reference, whereas 195Pt chemical shifts were referenced relative to external K2PtCl4 and 15N chemical shifts relative to external NH4Cl. Electrospray ionization mass spectrometry was carried out with a Bruker Esquire3000 ion trap using MeOH as solvent. Elemental analyses were performed using a Perkin-Elmer 2400 CHN elemental analyzer at the Microanalytical Laboratory of the University of Vienna, Austria, and are within ±0.4% of the calculated values, confirming their ≥95% purity (for summary of elemental analysis results, see Table S2). Preparative reversed phase HPLC was performed on a Agilent 1200 series system controlled by Chemstation software.
Synthesis
1a, 2a, and 3a were synthesized as published recently.40
General Procedure A
A mixture of succinic anhydride and 1a, 1b, or 1c in DMF was heated to 50 °C until the solid material dissolved to form a yellow solution. The solvent was removed under reduced pressure, and the yellow residue was suspended in acetone. The solution was concentrated under reduced pressure, and subsequent addition of diethyl ether led to precipitation of a yellow solid.
General Procedure B
1,1′-Carbonyldiimidazole (CDI) in DMF was added to a solution of 2a, 2b, or 2c in DMF, and the mixture was heated to 50 °C. After 15 min of stirring and simultaneous flushing with argon to remove the formed CO2, the solution was cooled to room temperature. Sodium alcoholate in absolute alcohol was added to the solution, and the mixture was stirred at room temperature. The solvents were removed under reduced pressure, and the obtained solids were purified by column chromatography.
(OC-6-54)-Dichlorido(N,N-dimethylethane-1,2-diamine)-hydroxidomethoxidoplatinum(IV) (1b)
(SP-4-3)-Dichlorido(N,N-dimethylethane-1,2-diamine)platinum(II) (3.550 g, 0.010 mol) was suspended in absolute MeOH (500 mL), and subsequently 50% H2O2 (50 mL) was added. The reaction mixture was stirred at room temperature until the solid material was dissolved. The solution was filtered and the solvent removed under reduced pressure (caution: explosive). The residue was washed with cold ethanol and ether. The crude product was purified by reversed phase HPLC. Yield: 2.74 g (68%). C5H16N2O2Cl2Pt (402.2): calcd C 14.93, H 4.01, N 6.97; found C 14.73, H 3.73, N 6.69. 1H NMR (DMSO-d6): δ = 6.96 (bs, 1H, H5a), 6.76 (bs, 1H, H5b), 3.08 (s + d, 3H, H1, 3JH,Pt = 20.5 Hz), 2.77–2.55 (bm, 4H, H3/H4), 2.69 (s + d, 3H, H2a, 3JH,Pt = 26.5 Hz), 2.60 (s + d, 3H, H2b, 3JH,Pt = 26.5 Hz) ppm. 13C NMR (DMSO-d6): δ = 66.6 (C4), 58.4 (C1), 48.4 (C2a), 47.9 (C2b), 45.2 (C3) ppm. 15N NMR: δ = 3.4 ppm. 195Pt NMR (DMSO-d6): δ = 2368 ppm. ESI-MS: m/z 375.8 [(N,N-Me2enPtCl2) + Na+]+.
(OC-6-44)-Diamminedichloridohydroxido-methoxidoplatinum(IV) (1c)
Cisplatin (0.676 g, 0.010 mol) was suspended in absolute MeOH (500 mL), and subsequently 50% H2O2 (50 mL) was added. The reaction mixture was stirred at room temperature until the solid material dissolved. The solution was filtered and the solvent removed under reduced pressure (caution: explosive). The residue was washed with cold ethanol and ether. The crude product was purified by reversed phase HPLC. Yield: 0.55 g (70%). CH10Cl2N2O2Pt·0.5MeOH (364.11): calcd C 4.95, H 3.32, N 7.69; found C 4.94, H 3.12, N 7.37. 1H NMR (DMSO-d6): δ = 5.76–5.53 (bm, 6H, H2a/H2b), 2.99 (s + d, 3H, H1, 3JH,Pt = 20.5 Hz) ppm. 13C NMR (DMSO-d6): δ = 58.7 ppm. 15N NMR (DMSO-d6): δ = −30.9 ppm. 195Pt NMR (DMSO-d6): δ = 2559 ppm.
(OC-6-54)-(3-Carboxypropanoato)dichlorido(N,N-dimethylethane-1,2-diamine)methoxidoplatinum(IV) (2b)
General procedure A was used, with 1b (0.700 g, 1.740 mmol), succinic anhydride (0.435 g, 4.341 mmol), and DMF (15 mL). Yield: 0.58 g (66%). C9H20Cl2N2O5Pt (502.2): calcd C 21.52, H 4.01, N 5.58; found C 21.95, H 3.93, N 5.36. 1H NMR (DMF-d7): δ = 9.54 (bs, 1H, H5a), 6.96 (bs, 1H, H5b), 3.10–2.80 (m, 4H, H4/H3), 2.81 (s + d, 3H, H2a, 3JH,Pt = 24.0 Hz), 2.79 (s + d, 3H, H2a, 3JH,Pt = 24.0 Hz), 2.73 ((s + d, 3H, H1, 3JH,Pt = 30.5 Hz), 2.62–2.41 (m, 4H, H7/H8) ppm. 13C NMR (DMF-d7): δ = 181.3 (C6), 174.3 (C9), 67.0 (C4 or C3), 60.3 (C1), 49.5 (C2a), 49.1 (C7), 48.1 (C2b), 45.1 (C4 or C3), 32.6 (C8) ppm. 15N NMR (DMF-d7): δ = −1.5 ppm. 195Pt-NMR (DMF-d7): δ = 2379 ppm. ESI-MS: m/z 375.8 [(N,N-Me2enPtCl2) + Na+]+; 524.7 [M + Na+]+; 540.7 [M + K+]+.
(OC-6-44)-Diammine(3-carboxypropanoato)-dichloridomethoxidoplatinum(IV) (2c)
General procedure A was used, with 1c (3.400 g, 9.773 mmol), succinic anhydride (3.960 g, 0.0396 mol), and DMF (72 mL). Yield: 1.84 g (42%). C5H14Cl2N2O5Pt (448.2): calcd C 13.40; H 3.15; N 6.25; found C 13.45, H 2.95, N 6.00. 1H NMR (DMSO-d6): δ = 11.99 (bs, 1H, H7), 5.86 (bm, 6H, H2), 2.63 (s + d, 3H, H1, 3JH,Pt = 30.5 Hz), 2.43–2.35 (bm, 4H, H4/H5) ppm. 13C NMR (DMSO-d6): δ = 180.4 (C3), 174.5 (C6), 61.0 (C1), 32.0 (C4 or C5), 30.7 (C4 or C5) ppm. 15N NMR (DMSO-d6): δ = −33.2 ppm. 195Pt NMR (DMSO-d6): δ = 2606 ppm. ESI-MS: m/z 470.9 [M + Na+]+.
(OC-6-54)-Dichlorido(N,N-dimethylethane-1,2-diamine)-methoxido(4-methoxy-4-oxobutanoato)platinum(IV) (3b)
General procedure B was used, with 2b (0.400 g, 0.796 mmol) in DMF (8 mL), CDI (0.194 g, 1.195 mmol) in DMF (16 mL), and sodium methanolate (5 mL). The crude product was purified by column chromatography (MeOH/EtOAc, 1:4) to yield yellow crystals, which were dried in vacuo. Yield: 115 mg (28%). C10H22Cl2N2O5Pt (516.3): calcd C 23.26; H 4.30; N 5.43; found C 23.34, H 4.04, N 5.37. 1H NMR (DMF-d7): δ = 9.55 (bs, 1H, H5a), 6.95 (bs, 1H, H5b), 3.64 (s, 3H, H10), 3.12–2.84 (bm, 4H, H3/H4), 2.80 (s + d, 3H, H2a, 3JH,Pt = 24.0 Hz), 2.79 (s + d, 3H, H2b, 3JH,Pt = 24.0 Hz), 2.3 (s + d, 3H, H1, 3JH,Pt = 30.5 Hz), 2.63–2.47 (bm, 4H, H7/H8) ppm. 13C NMR (DMF-d7): δ = 180.9 (C6), 173.4 (C9), 67.1 (C4 or C3), 60.3 (C1), 51.1 (C10), 49.1 (C2a), 48.1 (C2b), 45.1 (C4 or C3), 32.4 (C7), 29.9 (C8) ppm. 15N NMR (DMF-d7): δ = −1.0 ppm. 195Pt-NMR (DMSO-d6): δ = 2497 ppm. 195Pt NMR (DMF-d7): δ = 2377 ppm. ESI-MS: m/z 374.8 [(N,N-Me2enPtCl2) + Na+]+, 538.8 [M + Na+]+, 554.7 [M + K+]+.
(OC-6-54)-Dichlorido(N,N-dimethylethane-1,2-diamine)-hydroxido(4-(2-hydroxyethoxy)-4-oxobutanoato)platinum(IV) (4a)
General procedure B was used, with 2a (0.400 g, 0.819 mmol) in DMF (4 mL), CDI (0.146 g, 0.901 mmol) in DMF (4 mL), and ethylene glycolate (0.5 mL). The crude product was purified by column chromatography (MeOH/EtOAc, 1:1) to yield yellow crystals, which were dried in vacuo. Yield: 167 mg (38%). C10H22Cl2N2O6Pt (532.3): calcd C 22.56; H 4.17; N 5.26; found C 22.69, H 3.96, N 5.04. 1H NMR (DMSO-d6): δ = 9.30 (bs, 1H, H5a), 7.12 (bs, 1H, H5b), 4.78 (t, 1H, H12, J = 5.5 Hz), 4.00 (t, 2H, H10, J = 5.3 Hz), 3.55 (m, 2H, H11), 2.91–2.76 (bm, 4H, H3/H4), 2.66 (s + d, 3H, H2a, 3JH,Pt = 25.0 Hz), 2.59 (s + d, 3H, H2b, 3JH,Pt = 25.0 Hz), 2.52–2.39 (bm, 4H, H7/H8), 1.55 (s + d, 1H, H1, 3JH,Pt = 18,0 Hz) ppm. 13C NMR (DMSO-d6): δ = 180.9 (C6), 173.2 (C9), 67.8 (C3), 66.2 (C10), 59.4 (C11), 50.1 (C2a), 48.6 (C2b). 45.2 (C4), 32.4 (C7 or C8), 30.3 (C7 or C8) ppm. 15N NMR (DMSO-d6): δ = −5.2 ppm. 195Pt-NMR (DMSO-d6): δ = 2497 ppm. ESI-MS: m/z 374.8 [(N,N-Me2enPtCl2) + Na+]+; 554.7 [M + Na+]+; 570.7 [M + K+]+.
(OC-6-54)-Dichlorido(N,N-dimethylethane-1,2-diamine)(4-(2-hydroxyethoxy)-4-oxobutanoato)methoxidoplatinum(IV) (4b)
General procedure B was used, with 2b (0.200 g, 0.398 mmol) in DMF (2 mL), CDI (0.071 g, 0.438 mmol) in DMF (2 mL), and ethylene glycolate (0.2 mL). The crude product was purified by column chromatography (MeOH/EtOAc, 1:5) to yield yellow crystals, which were dried in vacuo. Yield: 60 mg (28%). C11H24Cl2N2O6Pt (546.3): calcd C11H24Cl2N2O6Pt·0.5MeOH C 24.56; H 4.66; N 4.98; found C 24.80, H 4.40, N 4.67. 1H NMR (DMSO-d6): δ = 9.16 (bs, 1H, H5a), 7.06 (bs, 1H, H5b), 4.78 (t, 1H, H12, 3JH,H = 5.6 Hz), 3.99 (t, 2H, H10, 3JH,H = 5.3 Hz), 3.55 (m, 2H, H11), 2.89–2.69 (bm, 4H, H3/H4), 2.64 (bs, 9H, H1/H2a/H2b), 2.52–2.38 (bm, 4H, H7/H8)ppm. 13C NMR (DMSO-d6): δ = 180.8 (C6), 173.2 (C9), 67,2 (C3 or C4), 66.2 (C10), 60.9 (C1), 59.4 (C11), 49.6 (C2a or C2b), 48.5 (C2a or C2b), 45.2 (C3 or C4), 32.6 (C8), 30.3 (C7) ppm. 15N NMR (DMF-d7): δ = −1.2 ppm 195Pt-NMR (DMSO-d6): δ = 2410 ppm. ESI-MS: m/z 374.7 [(N,N-Me2enPtCl2) + Na+]+; 568.7 [M + Na+]+; 584.7 [M + K+]+.
(OC-6-44)-Diamminedichlorido(4-(2-hydroxyethoxy)-4-oxobutanoato)methoxidoplatinum(IV) (4c)
General procedure B was used, with 2c (0.300 g, 0.669 mmol) in DMF (6 mL), CDI (0.119 g, 0.736 mmol) in DMF (6 mL), and ethylene glycolate (0.5 mL). The crude product was purified by column chromatography (MeOH/EtOAc, 1:8) to yield yellow crystals, which were dried in vacuo. Yield: 112 mg (34%). C7H18Cl2N2O6Pt (492.2): calcd C 17.08, H 3.69, N 5.69; found C 17.08, H 3.45, N 5.91. 1H NMR (DMSO-d6): δ = 5.85 (bm, 6H, H2), 4.78 (t, 1H, H9, 3JH,H = 5.3 Hz), 4.01 (t, 2H, H7, 3JH,H =5.2 Hz), 3.56 (m, 2H, H8), 2.63 (s + d, 3H, H1, 3JH,Pt = 30.5 Hz), 2.46 (s, 4H, H4/H5) ppm. 13C NMR (DMSO-d6): δ = 180.1 (C3), 173.1 (C6), 66.1 (C7), 61.1 (C1), 59.4 (C8), 31.9 (C4 or C5), 30.6 (C4 or C5) ppm. 15N NMR (DMSO-d6): δ = −33.2 ppm. 195Pt NMR (DMSO-d6): δ = 2602 ppm. ESI-MS: m/z 514.6 [M + Na+]+, 490.6 [M − H+]−, 526.4 [M + Cl−]−.
(OC-6-54)-μ-{4,4′-[Ethane-1,2-diylbis(oxy)]bis(4-oxobutanoato)}bis[dichlorido(N,N-dimethylethane-1,2-diamine)hydroxidoplatinum(IV)] (5a)
General procedure B was used, with 2a (0.300 g, 0.614 mmol) in DMF (6 mL), CDI (0.105 g, 0.645 mmol) in DMF (3 mL), and ethylene glycolate (16 μL). The crude product was purified by column chromatography (MeOH/CHCl3, 1:1) to yield yellow crystals, which were dried in vacuo. Yield: 81 mg (26%). C18H38Cl4N4O10Pt2 (1002. 49): calcd C18H38Cl4N4O10Pt2·MeOH C 22.06, H 4.09, N 5.42; found C 21.98, H 3.74, N 5.02. 1H NMR (DMSO-d6): δ = 9.27 (bs, 2H, H5a), 7.15 (bs, 2H, H5b), 4.18 (s, 4H, H10), 2.92–2.73 (m, 8H, H3/H4), 2.66 (bs, H6, H2a), 2.60 (bs, H6, H2b), 2.47–2.37 (m, H8, H7/H8), 1.55 (s, 2H, H1) ppm. 13C NMR (DMSO-d6): δ = 180.8 (C6), 173.1 (C9), 67.8 (C3 or C4), 62.4 (C10), 50.1 (C2a), 48.6 (C2b), 45.2 (C3 or C4), 32.3 (C7 or C8), 30.2 (C7 or C8) ppm. 15N NMR (DMSO-d6): δ = −5.4 ppm. 195Pt NMR (DMSO-d6): δ = 2496 ppm. ESI-MS: m/z (positive) 1023.9 [M + Na+]+, 654.8 [M − C4H13OCl2Pt + Na+]+, 374.9 [(N,N-Me2enPtCl2) + Na+]+ (negative) 999.0 [M − H+]−.
(OC-6-54)-μ-{4,4′-[Ethane-1,2-diylbis(oxy)]bis(4-oxobutanoato)}bis[dichlorido(N,N-dimethylethane-1,2-diamine)methoxidoplatinum(IV)] (5b)
General procedure B was used, with 2b (0.300 g, 0.597 mmol) in DMF (6 mL), CDI (0.102 g, 0.627 mmol) in DMF (4 mL), and ethylene glycolate (15 μL). The crude product was purified by column chromatography (MeOH/EtOAc, 1:1) to yield yellow crystals, which were dried in vacuo. Yield: 99 mg (32%). C20H42Cl4N4O10Pt2 (1030.54): calcd C20H42Cl4N4O10Pt2·0.6EtOAc C 24.83, H 4.35, N 5.17; found C 24.70, H 4.48, N 5.09. 1H NMR (DMSO-d6): δ = 9.13 (bs, H5a, 2H), 7.06 (bs, H5b, 2H), 4.18 (s, H10, 4H), 2.90–22.58 (bm, H4–H3, 8H), 2.64 (s, H1, 6H), 2.63 (bm, H1 H2b/H2a, 12H), 2.54–2.39 (bm, H7/H8) ppm. 13C NMR (DMSO-d6): δ = 180.7 (C6), 173.1 (C9), 67.2 (C3 or C4), 62.4 (C10), 60.9 (C1), 49.6 (C2a), 48.6 (C2a), 45.2 (C3 or C4), 32.5 (C7 or C8), 30.2 (C7 or C8) ppm. 15N NMR (DMSO-d6): δ = −1.3 ppm. 195Pt NMR (DMSO-d6): δ = 2410 ppm. ESI-MS: m/z (positive) 1052.0 [M + Na+]+, 1021.9 [M − MeOH + Na+]+, 988.0 [M − MeO−]+, 668.9 [M − C5H15Cl2OPt + Na+]+, 636.9 [M − C5H15Cl2OPt − MeOH + Na+]+; (negative) 1028.3 [M − H+]−.
Crystallographic Structure Measurements
X-ray diffraction measurements of 1b+·TFA− were performed on a Bruker X8 APEXII CCD diffractometer. Single crystals were positioned at 35 mm from the detector, and 1836 frames were measured each for 20 s, over 1° scan width. The data were processed using SAINT software.50 Crystal data, data collection parameters, and structure refinement details are given in Table S1 (Supporting Information). The structure was solved by direct methods and refined by full-matrix least-squares techniques. Non-H atoms were refined with anisotropic displacement parameters. H atoms were inserted in calculated positions and refined with a riding model. The H atoms at O1 were originally located from difference Fourier map and then calculated using DFIX restraint. Structure solution was achieved with SHELXS-97 and refinement with SHELXL-97,51 and graphics were produced with ORTEP-3.52
Cell Lines and Culture Conditions
Human CH1 (ovarian carcinoma) cells were kindly provided by Lloyd R. Kelland (CRC Centre for Cancer Therapeutics, Institute of Cancer Research, Sutton, U.K.). The mesothelioma cell model P31 and its respective cisplatin-resistant subline P31/cis have been generously donated by K. Grankvist (Umeå University, Sweden).48 The non-small-cell lung cancer cell model SW1573 with its MRP1- and LRP-overexpressing subline 2R120 and its P-gp-overexpressing subline 2R160 is from H. Broxterman (Department of Medical Oncology, Free University Hospital, Amsterdam, The Netherlands).53 The ovarian carcinoma model A2780 with its cisplatin-selected subline A2780/cis was purchased from Sigma-Aldrich. The lung cancer cell line A549 and the colon cancer cell line SW480 were purchased from the American Type Culture Collection (ATCC). P31, CH1, and SW480 cells were grown in minimal essential medium (MEM), SW1573 cell lines in Dulbecco’s minimal essential medium (DMEM), and other cell lines in RPMI 1640, all supplemented with 10% fetal bovine serum. Cultures were regularly checked for Mycoplasma contamination. Cultures were maintained at 37 °C in a humidified atmosphere containing 95% air and 5% CO2.
Cytotoxicity Tests in Cancer Cell Lines
Cytotoxicity in CH1, A549, and SW480 cells was determined by the MTT assay (MTT = 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, purchased from Fluka). Cells were seeded in 100 μL of MEM into 96-well plates (Iwaki/Asahi Technoglass) at the appropriate densities: 1.5 × 103 (CH1), 4.0 × 103 (A549), and 2.5 × 103 (SW480) viable cells per well. Cells were allowed for 24 h to resume exponential growth, followed by the addition of serial dilutions of the test compounds in 100 μL/well MEM. After exposure for 96 h, the medium was replaced with a 100 μL/well RPMI 1640 medium plus 20 μL/well phosphate-buffered saline containing MTT (5 mg/mL) (all purchased from Sigma-Aldrich). After 4 h, medium/MTT mixtures were replaced with DMSO (150 μL/well), and optical densities at a wavelength of 550 nm were measured with a microplate reader (Tecan Spectra Classic). For each setting, at least three independent experiments were performed, each comprising three replicates per concentration level.
For tests in resistance models, cells were plated (2 × 103 cells in 100 μL/well) in 96-well plates and allowed to recover for 24 h. Drugs were added in another 100 μL of growth medium and cells exposed for the indicated time periods (96 h). After drug treatment, the proportion of viable cells was determined with the EZ4U (Biomedica, Vienna, Austria) assay kit following the manufacturer’s recommendations.
Total Pt Uptake Levels
A2780 cells (4 × 105/well) were seeded into six-well plates, allowed to settle for 24 h, and drug-exposed for 2 h at 37 °C. After two washes with ice-cold PBS, cells were lysed at room temperature in 400 μL of tetramethylammonium hydroxide. Lysates were diluted in 0.6 N HNO3 and platinum concentrations determined by ICP-MS. The ICP-MS instrument (Agilent 7500ce, Waldbronn, Germany) was equipped with a CETAC ASX-520 autosampler (Neuss, Germany), a Scott double pass spray chamber, and a MicroMist nebulizer operating at a sample delivery rate of approximately 0.25 mL/min. Platinum and indium standards were obtained from CPI International (Amsterdam, The Netherlands). The instrument was tuned with a solution containing 1 ppb each of lithium, yttrium, cerium, thallium, and cobalt in 2% HNO3 (Agilent Technologies, Vienna, Austria). Standards were matrix matched with regard to nitric acid and tetramethylammonium hydroxide, and indium (1 ppb) was used as internal standard for quantification. As unspecific binding to cell culture plastic may occur especially in the case of lipophilic compounds,54 results were corrected for platinum levels of a blank well containing no cells.
Western Blot Analyses
After 24 h of drug exposure, proteins were isolated, resolved by SDS–PAGE, and transferred onto a polyvinylidene difluoride membrane for Western blotting as described.55,56 The anti poly(ADP-ribosyl)polymerase (PARP), cleaved PARP, caspase 3, caspase 7, cleaved caspase 3, and cleaved caspase 7 antibodies (all polyclonal rabbit) from the apoptosis sampler kit (Cell Signaling Technology, Beverly, MA) and the anti-β-actin monoclonal mouse AC-15 (Sigma) were used in a 1:1000 dilution. Additionally, horseradish peroxidase labeled antibodies from Santa Cruz Biotechnology were used at working dilutions of 1:10000.
Cell Cycle Analysis
A2780 cells (2 × 105 per well) were seeded into six-well plates and cultured for 24 h and treated for another 24 h with the test substances. Then cells were collected by trypsinization, washed with PBS, fixed in 70% ethanol, and stored at −20 °C. To determine the cell cycle distribution, cells were transferred into PBS, incubated with RNase (10 μg/mL) for 30 min at 37 °C, treated with 5 μg/mL propidium iodide (PI) for 30 min, and then analyzed by flow cytometry using a fluorescence-activated cell sorting (FACS) Calibur (Becton Dickinson, Palo Alto, CA). The resulting DNA histograms were quantified using the ModeFit software (BD).
[3H]Thymidine Incorporation Assay
A2780 cells (5 × 104 cells/ml) were seeded in a 96-well plate, allowed to recover for 24 h, and treated with the test compounds for another 24 h. Medium was replaced by a 2 nM [3H]thymidine solution (diluted in full culture medium; radioactivity, 25 ci/mM). After 1 h of incubation at 37 °C, cells were washed three times with PBS. Cell lysates were prepared, and the radioactivity was determined as described.57
Reversed-Phase HPLC Measurements58
Analytical reversed-phase HPLC analysis was performed on a Dionex Summit system controlled by Dionex Chromeleon 6.60 software. The experimental conditions and parameters were as follows: stationary phase, silica-based C18 gel (Zorbax SB-Aq, 4.6 mm × 250 mm); mobile phase, isocratic (methanol/15 mM aqueous formic acid ranging from 5:95 for the most hydrophilic compounds to 50:50 for the most lipophilic compounds); flow rate, 1.00 mL/min; injection volume, 25 μL; column temperature, 25 °C; UV–vis detection, 210 nm. KI (0.1 mM) was chosen as the internal reference to determine the column dead time (t0). All tested compounds were measured at least twice in at least three mobile phase conditions. The capacity factor k’ is defined as the partition of a compound between a solvent (polar) mobile phase and a solid (apolar) stationary phase (tr, retention time of the investigated compounds; t0, retention time of KI)
As the capacity factor is dependent on the solvents used in the mobile phase, extrapolating to 0% organic solvent is necessary.
Here, log k’ is the capacity factor in the measured mobile phase composition, log kw is the extrapolated capacity factor in 100% H2O, SMeOH is a constant for a given substance and the given HPLC system, and φ is the volume of the organic modifier (in our case MeOH).
Values for 1a and 1c could not be verified, as the compounds are not retarded and thus too polar for reversed phase chromatography.
Supplementary Material
Contains: Table listing crystal data, data collection parameters, and structure refinement details of 1b+·TFA−; elemental analysis results of all compounds; figure of platinum(II) drugs in clinical application; figure with RP-HPLC chromatograms of compounds 1b, 2a–c, 3a, 3b, 4a–c, and satraplatin; figures with concentration–effect curves of all complexes in CH1, SW480, and A549 cells; figure with IC50 vs log kw plots; figure with the NMR numbering scheme; X-ray crystallographic file in CIF format for 1b+·TFA−. This material is also available free of charge via the Internet at http://pubs.acs.org. Crystallographic data have been deposited with the Cambridge Crystallographic Data Center with No. CCDC 909320. Copies of data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K. (deposit@ccdc.com.ac.uk).
ACKNOWLEDGMENTS
The authors are indebted to the FFG—Austrian Research Promotion Agency (Grant 811591), the Austrian Council for Research and Technology Development (Grant IS526001), the FWF (Austrian Science Fund, Grant L568 to W.B.), COST D39, and the Fond der Stadt Wien für Innovative Interdisziplinäre Krebsforschung (to P.H.). We thank Prof. Arion for X-ray structure analysis and Mahsa Adib-Razavi for support with the MTT measurements.
Footnotes
Notes: The authors declare no competing financial interest.
REFERENCES
- (1).Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur. J. Cancer. 1998;34:1522–1534. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- (2).O’Dwyer PJ, Stevenson JP, Johnson SW. Clinical Status of Cisplatin, Carboplatin, and Other Platinum-Based Antitumor Drugs. In: Lippert B, editor. Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. Helvetica Chimica Acta; Zurich, Switzerland: 1999. pp. 31–69. [Google Scholar]
- (3).Jakupec MA, Galanski M, Keppler BK. Tumour-inhibiting platinum complexes—state of the art and future perspectives. Rev. Physiol. Biochem. Pharmacol. 2003;146:1–53. doi: 10.1007/s10254-002-0001-x. [DOI] [PubMed] [Google Scholar]
- (4).Jakupec MA, Galanski M, Arion VB, Hartinger CG, Keppler BK. Antitumour metal compounds: more than theme and variations. Dalton Trans. 2008;2:183–194. doi: 10.1039/b712656p. [DOI] [PubMed] [Google Scholar]
- (5).Fricker SP. Metal based drugs: from serendipity to design. Dalton Trans. 2007;43:4903–4917. doi: 10.1039/b705551j. [DOI] [PubMed] [Google Scholar]
- (6).Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat. Res. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- (7).Brabec V, Kasparkova J. Molecular aspects of resistance to antitumor platinum drugs. Drug Resist. Updat. 2002;5:147–161. doi: 10.1016/s1368-7646(02)00047-x. [DOI] [PubMed] [Google Scholar]
- (8).Heffeter P, Jungwirth U, Jakupec M, Hartinger C, Galanski M, Elbling L, Micksche M, Keppler BK, Berger W. Resistance against novel anticancer metal compounds: differences and similarities. Drug Resist. Updat. 2008;11:1–16. doi: 10.1016/j.drup.2008.02.002. [DOI] [PubMed] [Google Scholar]
- (9).Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem. Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- (10).Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005;12:2075–2094. doi: 10.2174/0929867054637626. [DOI] [PubMed] [Google Scholar]
- (11).Lovejoy KS, Lippard SJ. Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting. Dalton Trans. 2009;48:10651–10659. doi: 10.1039/b913896j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113–8127. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- (13).Olszewski U, Hamilton G. A better platinum-based anticancer drug yet to come. Anticancer Agents Med. Chem. 2010;10:293–301. doi: 10.2174/187152010791162306. [DOI] [PubMed] [Google Scholar]
- (14).Harper BW, Krause-Heuer AM, Grant MP, Manohar M, Garbutcheon-Singh KB, Aldrich-Wright JR. Advances in platinum chemotherapeutics. Chem.—Eur. J. 2010;16:7064–7077. doi: 10.1002/chem.201000148. [DOI] [PubMed] [Google Scholar]
- (15).Chawla SP, Yap BS, Tenney DM, Bodey GP, Benjamin RS. Phase I study of weekly-administered iproplatin [cis-dichloro-transdihydroxy-bis-isopropylamine platin (chip, JM9)] Invest. New Drugs. 1988;6:311–317. doi: 10.1007/BF00173650. [DOI] [PubMed] [Google Scholar]
- (16).Harstrick A, Casper J, Guba R, Wilke H, Poliwoda H, Schmoll HJ. Comparison of the antitumor activity of cisplatin, carboplatin, and iproplatin against established human testicular cancer cell lines in vivo and in vitro. Cancer. 1989;63:1079–1083. doi: 10.1002/1097-0142(19890315)63:6<1079::aid-cncr2820630607>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- (17).Screnci D, McKeage MJ. Platinum neurotoxicity: clinical profiles, experimental models and neuroprotective approaches. J. Inorg. Biochem. 1999;77:105–110. doi: 10.1016/s0162-0134(99)00135-x. [DOI] [PubMed] [Google Scholar]
- (18).Schilder RJ, LaCreta FP, Perez RP, Johnson SW, Brennan JM, Rogatko A, Nash S, McAleer C, Hamilton TC, Roby D. Phase I and pharmacokinetic study of ormaplatin (tetraplatin, NSC 363812) administered on a day 1 and day 8 schedule. Cancer Res. 1994;54:709–717. [PubMed] [Google Scholar]
- (19).Poon GK, Mistry P, Raynoud FI, Harrap KR, Murrer BA, Barnard CF. Determination of metabolites of a novel platinum anticancer drug JM216 in human plasma ultrafiltrates. J. Pharm. Biomed. Anal. 1995;13:1493–1498. doi: 10.1016/0731-7085(95)01571-x. [DOI] [PubMed] [Google Scholar]
- (20).Choy H, Park C, Yao M. Current status and future prospects for satraplatin, an oral platinum analogue. Clin. Cancer Res. 2008;14:1633–1638. doi: 10.1158/1078-0432.CCR-07-2176. [DOI] [PubMed] [Google Scholar]
- (21).Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero J-M, Eymard J-C, Falcon S, Calabro F, James N, Bodrogi I, Harper P, Wirth M, Berry W, Petrone ME, McKearn TJ, Noursalehi M, George M, Rozencweig M. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J. Clin. Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- (22).Prochazka L, Turanek J, Tesarik R, Knotigova P, Polaskova P, Andrysik Z, Kozubik A, Zak F, Sova P, Neuzil J, Machala M. Apoptosis and inhibition of gap-junctional intercellular communication induced by LA-12, a novel hydrophobic platinum(IV) complex. Arch. Biochem. Biophys. 2007;462:54–61. doi: 10.1016/j.abb.2007.03.021. [DOI] [PubMed] [Google Scholar]
- (23).Sova P, Mistr A, Kroutil A, Zak F, Pouckova P, Zadinova M. Comparative anti-tumor efficacy of two orally administered platinum-(IV) drugs in nude mice bearing human tumor xenografts. Anti-Cancer Drugs. 2006;17:201–206. doi: 10.1097/00001813-200602000-00012. [DOI] [PubMed] [Google Scholar]
- (24).Weaver EL, Bose RN. Platinum(II) catalysis and radical intervention in reductions of platinum(IV) antitumor drugs by ascorbic acid. J. Inorg. Biochem. 2003;95:231–239. doi: 10.1016/s0162-0134(03)00136-3. [DOI] [PubMed] [Google Scholar]
- (25).Lemma K, Berglund J, Farrell N, Elding LI. Kinetics and mechanism for reduction of anticancer-active tetrachloroam(m)ine platinum(IV) compounds by glutathione. J. Biol. Inorg. Chem. 2000;5:300–306. doi: 10.1007/pl00010658. [DOI] [PubMed] [Google Scholar]
- (26).Lemma K, Sargeson AM, Elding LI. Kinetics and mechanism for reduction of oral anticancer platinum(IV) dicarboxylate compounds by L-ascorbate ions. J. Chem. Soc., Dalton Trans. 2000:1167–1172. [Google Scholar]
- (27).Galanski M, Keppler BK. Is reduction required for antitumor activity of platinum(IV) compounds? Characterisation of a platinum-(IV)-nucleotide adduct [enPt(OCOCH3)3(5′-GMP)] by NMR spectroscopy and ESI-MS. Inorg. Chim. Acta. 2000;300–302:783–789. [Google Scholar]
- (28).Zhang JZ, Wexselblatt E, Hambley TW, Gibson D. Pt(IV) analogs of oxaliplatin that do not follow the expected correlation between electrochemical reduction potential and rate of reduction by ascorbate. Chem. Commun. 2012;48:847–849. doi: 10.1039/c1cc16647f. [DOI] [PubMed] [Google Scholar]
- (29).Hall MD, Hambley TW. Platinum(IV) antitumour compounds: their bioinorganic chemistry. Coord. Chem. Rev. 2002;232:49–67. [Google Scholar]
- (30).Hall MD, Mellor HR, Callaghan R, Hambley TW. Basis for design and development of platinum(IV) anticancer complexes. J. Med. Chem. 2007;50:3403–3411. doi: 10.1021/jm070280u. [DOI] [PubMed] [Google Scholar]
- (31).Reithofer MR, Bytzek AK, Valiahdi SM, Kowol CR, Groessl M, Hartinger CG, Jakupec MA, Galanski M, Keppler BK. Tuning of lipophilicity and cytotoxic potency by structural variation of anticancer platinum(IV) complexes. J. Inorg. Biochem. 2011;105:46–51. doi: 10.1016/j.jinorgbio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- (32).Jungwirth U, Kowol CR, Keppler BK, Hartinger CG, Berger W, Heffeter P. Anticancer activity of metal complexes: involvement of redox processes. Antioxid. Redox Signal. 2011;15:1085–1127. doi: 10.1089/ars.2010.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Matsumoto S, Tanaka T, Kurokawa H, Matsuno K, Hayashida Y, Takahashi T. Effect of copper and role of the copper transporters ATP7A and CTR1 in intracellular accumulation of cisplatin. Anticancer Res. 2007:2209–2216. [PubMed] [Google Scholar]
- (34).Oldfield SP, Hall MD, Platts JA. Calculation of lipophilicity of a large, diverse dataset of anticancer platinum complexes and the relation to cellular uptake. J. Med. Chem. 2007;50:5227–5237. doi: 10.1021/jm0708275. [DOI] [PubMed] [Google Scholar]
- (35).Olszewski U, Ach F, Ulsperger E, Baumgartner G, Zeillinger R, Bednarski P, Hamilton G. In Vitro Evaluation of Oxoplatin: An Oral Platinum(IV) Anticancer Agent. Met.-Based Drugs. 2009 doi: 10.1155/2009/348916. DOI: 10.1155/2009/348916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lee Y, Yoo KH, Jung O-S. Oxidation of Pt(II) to Pt(IV) complex with hydrogen peroxide in glycols. Inorg. Chem. Commun. 2003:249–251. [Google Scholar]
- (37).Feazell RP, Nakayama-Ratchford N, Dai H, Lippard SJ. Soluble single-walled carbon nanotubes as longboat delivery systems for platinum(IV) anticancer drug design. J. Am. Chem. Soc. 2007;129:8438–8439. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Lee Y, Jung SM, Kang SW, Jung O-S. Hydrogen peroxide oxidation of di(hydroxo)platinum(II) species in carboxylic acids. Transition Met. Chem. 2004:710–713. [Google Scholar]
- (39).Dhar S, Daniel WL, Giljohann DA, Mirkin CA, Lippard SJ. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J. Am. Chem. Soc. 2009;131:14652–14653. doi: 10.1021/ja9071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Pichler V, Valiahdi SM, Jakupec MA, Arion VB, Galanski M, Keppler BK. Mono-carboxylated diaminedichloridoplatinum(IV) complexes—selective synthesis, characterization, and cytotoxicity. Dalton Trans. 2011;40:8187–8192. doi: 10.1039/c1dt10301f. [DOI] [PubMed] [Google Scholar]
- (41).Reithofer MR, Valiahdi SM, Jakupec MA, Arion VB, Egger A, Galanski M, Keppler BK. Novel di- and tetracarboxylatoplatinum(IV) complexes. Synthesis, characterization, cytotoxic activity, and DNA platination. J. Med. Chem. 2007;50:6692–6699. doi: 10.1021/jm070897b. [DOI] [PubMed] [Google Scholar]
- (42).Still BM, Anil Kumar PG, Aldrich-Wright JR, Price WS. 195Pt NMR—theory and application. Chem. Soc. Rev. 2007;36:665–686. doi: 10.1039/b606190g. [DOI] [PubMed] [Google Scholar]
- (43).Valkó K. Application of high-performance liquid chromatography based measurements of lipophilicity to model biological distribution. J. Chromatogr., A. 2004;1037:299–310. doi: 10.1016/j.chroma.2003.10.084. [DOI] [PubMed] [Google Scholar]
- (44).Varbanov H, Valiahdi SM, Legin AA, Jakupec MA, Roller A, Galanski M, Keppler BK. Synthesis and characterization of novel bis(carboxylato)dichloridobis(ethylamine)platinum(IV) complexes with higher cytotoxicity than cisplatin. Eur. J. Med. Chem. 2011;46:5456–5464. doi: 10.1016/j.ejmech.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Heffeter P, Pirker C, Kowol CR, Herrman G, Dornetshuber R, Miklos W, Jungwirth U, Koellensperger G, Keppler BK, Berger W. Impact of terminal dimethylation on the resistance profile of alpha-N-heterocyclic thiosemicarbazones. Biochem. Pharmacol. 2012;83:1623–1633. doi: 10.1016/j.bcp.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Jansen BA, Brouwer J, Reedijk J. Glutathione induces cellular resistance against cationic dinuclear platinum anticancer drugs. J. Inorg. Biochem. 2002;89:197–202. doi: 10.1016/s0162-0134(02)00381-1. [DOI] [PubMed] [Google Scholar]
- (47).Hector S, Bolanowska-Higdon W, Zdanowicz J, Hitt S, Pendyala L. In vitro studies on the mechanisms of oxaliplatin resistance. Cancer Chemother. Pharmacol. 2001;48:398–406. doi: 10.1007/s002800100363. [DOI] [PubMed] [Google Scholar]
- (48).Janson V, Andersson B, Behnam-Motlagh P, Engstrom KG, Henriksson R, Grankvist K. Acquisition of cisplatin-resistance in malignant mesothelioma cells abrogates Na+,K+,2Cl(−)-cotransport activity and cisplatin-induced early membrane blebbing. Cell. Physiol. Biochem. 2008;22:45–56. doi: 10.1159/000149782. [DOI] [PubMed] [Google Scholar]
- (49).Kalimutho M, Minutolo A, Grelli S, Federici G, Bernardini S. Platinum(IV)-derivative satraplatin induced G2/M cell cycle perturbation via p53-p21(waf1/cip1)-independent pathway in human colorectal cancer cells. Acta Pharmacol. Sin. 2011;32:1387–1396. doi: 10.1038/aps.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).SAINT-Plus, version 7.06a, and APEX2. Bruker-Nonius AXS Inc.; Madison, WI: 2004. [Google Scholar]
- (51).Sheldrick GM. Acta Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- (52).Johnson GK. Report ORNL-5138. OAK Ridge National Laboratory; Oak Ridge, TN: 1976. [Google Scholar]
- (53).Kuiper CM, Broxterman HJ, Baas F, Schuurhuis GJ, Haisma HJ, Scheffer GL, Lankelma J, Pinedo HM. Drug transport variants without P-glycoprotein overexpression from a human squamous lung cancer cell line after selection with doxorubicin. J. Cell. Pharmacol. 1990;1:35–41. [Google Scholar]
- (54).Egger A, Rappel C, Jakupec MA, Hartinger CG, Heffeter P, Keppler BK. Development of an experimental protocol for uptake studies of metal compounds in adherent tumor cells. J. Anal. At. Spectrom. 2009;24:51–61. doi: 10.1039/B810481F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Heffeter P, Jakupec MA, Korner W, Wild S, Von Keyserlingk NG, Elbling L, Zorbas H, Korynevska A, Knasmueller S, Sutterluety H, Micksche M, Keppler BK, Berger W. Multidrug-resistant cancer cells are preferential targets of the new antineoplastic lanthanum compound KP772 (FFC24) Biochem. Pharmacol. 2007;73:1873–1886. doi: 10.1016/j.bcp.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Berger W, Elbling L, Micksche M. Expression of the major vault protein LRP in human non-small-cell lung cancer cells: activation by short-term exposure to antineoplastic drugs. Int. J. Cancer. 2000;88:293–300. [PubMed] [Google Scholar]
- (57).Heffeter P, Jakupec MA, Koerner W, Wild S, Von Keyserlingk NG, Elbling L, Zorbas H, Korynevska A, Knasmueller S, Sutterluety H, Micksche M, Keppler BK, Berger W. Anticancer activity of the lanthanum compound [tris(1,10-phenanthroline)-lanthanum(III)]trithiocyanate (KP772; FFC24) Biochem. Pharmacol. 2006;71:426–440. doi: 10.1016/j.bcp.2005.11.009. [DOI] [PubMed] [Google Scholar]
- (58).OECD Guidelines for the Testing of Chemicals: Partition Coefficient (n-Octanol/Water), High Performance Liquid Chromatography (HPLC) Method. OECD; Paris, France: Apr 13, 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains: Table listing crystal data, data collection parameters, and structure refinement details of 1b+·TFA−; elemental analysis results of all compounds; figure of platinum(II) drugs in clinical application; figure with RP-HPLC chromatograms of compounds 1b, 2a–c, 3a, 3b, 4a–c, and satraplatin; figures with concentration–effect curves of all complexes in CH1, SW480, and A549 cells; figure with IC50 vs log kw plots; figure with the NMR numbering scheme; X-ray crystallographic file in CIF format for 1b+·TFA−. This material is also available free of charge via the Internet at http://pubs.acs.org. Crystallographic data have been deposited with the Cambridge Crystallographic Data Center with No. CCDC 909320. Copies of data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K. (deposit@ccdc.com.ac.uk).