Abstract
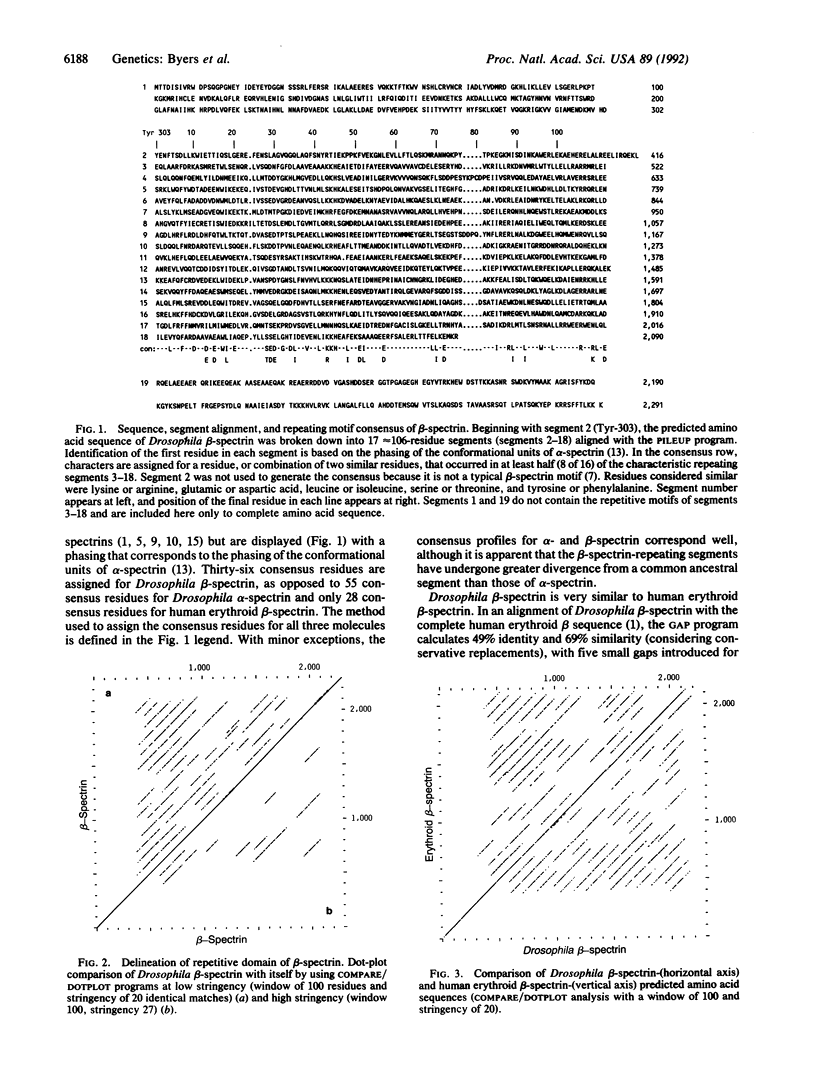
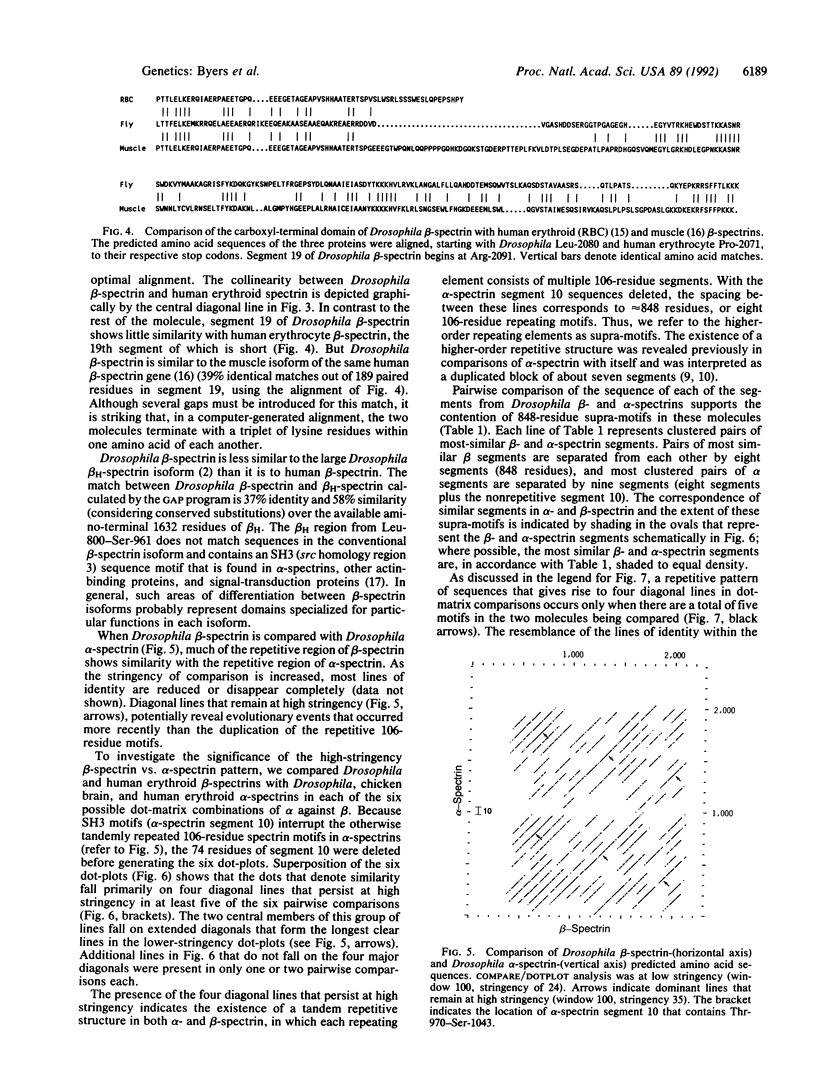
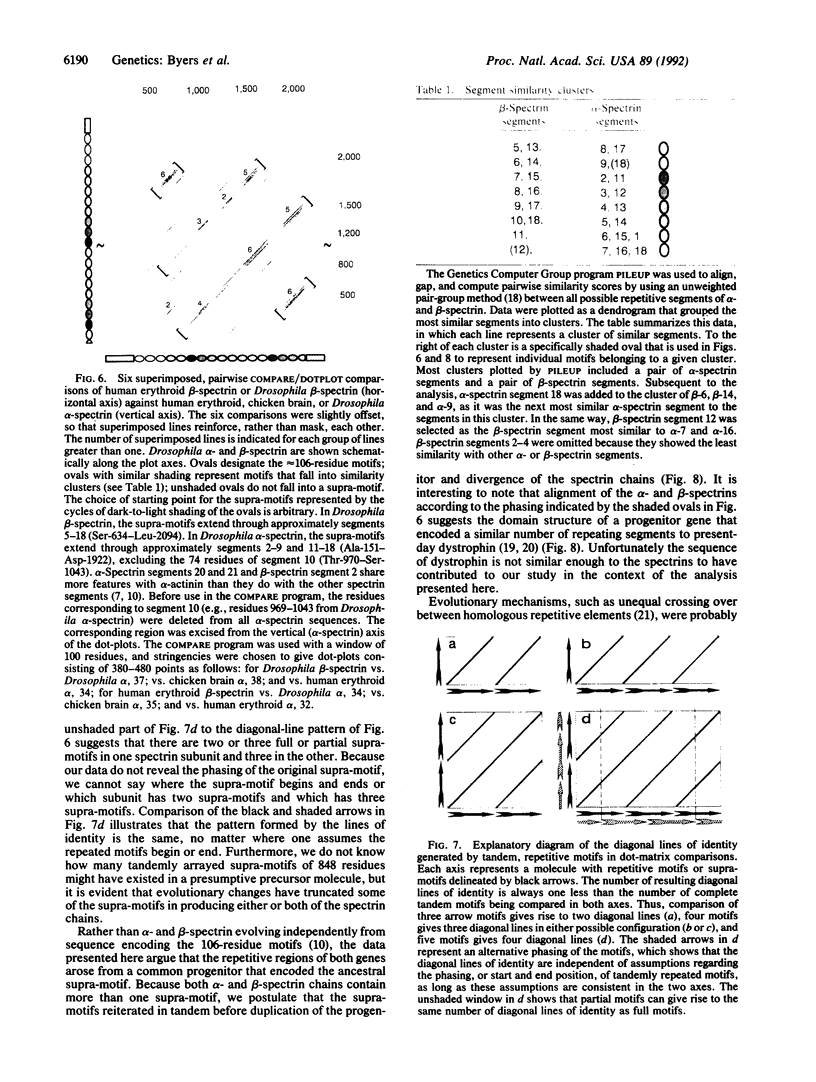
The alpha and beta chains of spectrin are homologous, yet they have acquired different structural features that work in synergy to give the multimer its overall properties. The primary amino acid sequence of each spectrin subunit is dominated by tandemly repeated 106-residue motifs. By comparing the complete Drosophila beta-spectrin sequence with other spectrins we have discovered evidence that a higher-order, 848-amino acid supra-motif is tandemly repeated in both alpha- and beta-spectrin. These data argue that alpha- and beta-spectrin, rather than evolving independently from sequences encoding the ancestral 106-residue motifs, must have arisen after the establishment of a large supra-motif composed of eight of the 106-residue motifs. Our data suggest the segment structure of a progenitor gene that gave rise to both alpha- and beta-spectrin as well as dystrophin. The structural differences that evolved after the split between the alpha- and beta-spectrin genes confer the independent functions that exist in their products today.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. Sequence similarity of the amino-terminal domain of Drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol. 1989 Oct;109(4 Pt 1):1633–1641. doi: 10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman T. R., Fishkind D. J., Mooseker M. S., Morrow J. S. Contributions of the beta-subunit to spectrin structure and function. Cell Motil Cytoskeleton. 1989;12(4):248–263. doi: 10.1002/cm.970120406. [DOI] [PubMed] [Google Scholar]
- Coleman T. R., Fishkind D. J., Mooseker M. S., Morrow J. S. Functional diversity among spectrin isoforms. Cell Motil Cytoskeleton. 1989;12(4):225–247. doi: 10.1002/cm.970120405. [DOI] [PubMed] [Google Scholar]
- Davison M. D., Baron M. D., Critchley D. R., Wootton J. C. Structural analysis of homologous repeated domains in alpha-actinin and spectrin. Int J Biol Macromol. 1989 Apr;11(2):81–90. doi: 10.1016/0141-8130(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Mulholland J., Zhu Z. M., Botstein D. Homology of a yeast actin-binding protein to signal transduction proteins and myosin-I. Nature. 1990 Jan 18;343(6255):288–290. doi: 10.1038/343288a0. [DOI] [PubMed] [Google Scholar]
- Dubreuil R. R., Byers T. J., Sillman A. L., Bar-Zvi D., Goldstein L. S., Branton D. The complete sequence of Drosophila alpha-spectrin: conservation of structural domains between alpha-spectrins and alpha-actinin. J Cell Biol. 1989 Nov;109(5):2197–2205. doi: 10.1083/jcb.109.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R., Byers T. J., Stewart C. T., Kiehart D. P. A beta-spectrin isoform from Drosophila (beta H) is similar in size to vertebrate dystrophin. J Cell Biol. 1990 Nov;111(5 Pt 1):1849–1858. doi: 10.1083/jcb.111.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R. Structure and evolution of the actin crosslinking proteins. Bioessays. 1991 May;13(5):219–226. doi: 10.1002/bies.950130504. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Koenig M., Kunkel L. M. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem. 1990 Mar 15;265(8):4560–4566. [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Kotula L., Laury-Kleintop L. D., Showe L., Sahr K., Linnenbach A. J., Forget B., Curtis P. J. The exon-intron organization of the human erythrocyte alpha-spectrin gene. Genomics. 1991 Jan;9(1):131–140. doi: 10.1016/0888-7543(91)90230-c. [DOI] [PubMed] [Google Scholar]
- Maeda N., Smithies O. The evolution of multigene families: human haptoglobin genes. Annu Rev Genet. 1986;20:81–108. doi: 10.1146/annurev.ge.20.120186.000501. [DOI] [PubMed] [Google Scholar]
- Ohta T. Multigene families and the evolution of complexity. J Mol Evol. 1991 Jul;33(1):34–41. doi: 10.1007/BF02100193. [DOI] [PubMed] [Google Scholar]
- Sahr K. E., Laurila P., Kotula L., Scarpa A. L., Coupal E., Leto T. L., Linnenbach A. J., Winkelmann J. C., Speicher D. W., Marchesi V. T. The complete cDNA and polypeptide sequences of human erythroid alpha-spectrin. J Biol Chem. 1990 Mar 15;265(8):4434–4443. [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W. The present status of erythrocyte spectrin structure: the 106-residue repetitive structure is a basic feature of an entire class of proteins. J Cell Biochem. 1986;30(3):245–258. doi: 10.1002/jcb.240300306. [DOI] [PubMed] [Google Scholar]
- Wasenius V. M., Saraste M., Salvén P., Erämaa M., Holm L., Lehto V. P. Primary structure of the brain alpha-spectrin. J Cell Biol. 1989 Jan;108(1):79–93. doi: 10.1083/jcb.108.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11827–11832. [PubMed] [Google Scholar]
- Winkelmann J. C., Costa F. F., Linzie B. L., Forget B. G. Beta spectrin in human skeletal muscle. Tissue-specific differential processing of 3' beta spectrin pre-mRNA generates a beta spectrin isoform with a unique carboxyl terminus. J Biol Chem. 1990 Nov 25;265(33):20449–20454. [PubMed] [Google Scholar]
- Winograd E., Hume D., Branton D. Phasing the conformational unit of spectrin. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10788–10791. doi: 10.1073/pnas.88.23.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]







