Abstract
OBJECTIVE
Transverse relaxation time (T2) imaging provides the opportunity to examine membrane fluidity, which can affect a number of cellular functions. The objective of the present work was to examine T2 abnormalities of children with unmodified DSM-IV-TR Bipolar disorder (BD) in bilateral cingulate-paracingulate (CPC) white matter.
METHOD
Twenty-one children and adolescents with BD and sixteen controls underwent magnetic resonance imaging at 1.5 Tesla and compared using a region-of-interest analysis. A post-hoc diffusion tensor imaging (DTI) analysis was also performed on selected subjects.
RESULTS
The T2 values were significantly decreased on the right hand side of the subjects with BD compared with controls. Hemispheric difference was also observed in BD group, with decreased T2 on the right hand side compared with the left. No significant difference was observed between left and right CPC T2 in controls. For those participants who had both T2 and DTI measurements, significant DTI differences were observed: On the left, fractional anisotropy was reduced and trace and radial diffusivity were increased, whereas on the right trace was increased and T2 was decreased in BD subjects compared with the controls.
CONCLUSIONS
Our findings suggest that the observed T2 difference is a reflection of cerebral blood flow rather than the alteration of the fluidity of cell membranes. It is possible that myelin damage happens on the left hand side in early onset BD in addition to changes in the blood flow. Prospective studies with larger numbers of subjects are warranted to further explore the relevance of the presented results.
Keywords: t2 relaxometry, dti, early onset bipolar disorder
INTRODUCTION
Bipolar disorder (BD) is a common medical illness that causes extreme shifts in mood, energy, and functioning, and is estimated to affect 3% of adults in the United States alone1, but also can affect children and adolescents.2,3 Untreated, this disorder is associated with greater risk for the development of alcohol and substance abuse addictions, interpersonal and academic problems as well as suicide.2–4 It was reported that delayed treatment of childhood-onset BD results in negative outcome in adults.5 Despite the great technical advancements in neuroimaging and increasing numbers of studies, the underlying precise neuro-circuitry of early onset BD remains unknown.
Several studies have used magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) to investigate anatomical and neurochemical abnormalities potentially involved in early onset BD (below the age of 18 years). Although differences in a number of brain structures and metabolites have been reported using MRI and MRS, the frontal lobes (including the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex) and the basal ganglia have been the most extensively studied regions. In these regions, abnormalities have been reported suggesting that neuronal integrity, neurotransmission, bioenergetic markers, and cell membrane metabolism are impaired in youths with BD.6,7
Image-based measurement of transverse relaxation time (T2) is another MR based methodology for the in vivo investigation of tissue abnormalities. This relaxation time refers to the decay of signal detected by the scanner due to energy loss to the environment and is commonly measured by the acquisition of series of images at different echo times. The time constant of the exponential decay represents the rate of T2 relaxation. This T2 relaxation time is influenced by cerebral blood flow, the presence of tissue iron deposition and/or the lipid structure of brain cell membranes.
Membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane and myelin sheath. When the lipid concentrations vary, changes in the fluidity occurs.
Lipids alter the geometric properties of membranes and affect a number of cellular functions, such as controlling the protein traffic and providing messenger molecules that mediate communication between cells. The role of lipids in tissue physiology and cell signaling is demonstrated in many neurological disorders, including schizophrenia, neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases8 and central nervous system injuries such as stroke.9 BD may also be characterized by changes in the lipid structure of brain cell membranes, resulting in decreased membrane fluidity causing the membrane to become more rigid and eventually to malfunction.9,10 Quantitative image-based measurements of T2 relaxation provide the opportunity to examine membrane fluidity in vivo, with decreased T2 reflecting increased membrane fluidity.11,12
Diffusion tensor imaging (DTI) can also be used to examine changes in the cell membranes in vivo: In DTI, the magnetic resonance signal is generated in proportion to the diffusion of water molecules in tissue and this diffusion is restricted by macromolecules and cell membranes.13 Movement of water becomes less restricted when a change occurs in the structure of brain cell membranes, resulting in decreased membrane fluidity with changes in the diffusion coefficients. Indeed, reduced Fractional Anisotropy (FA) in the bilateral cingulate-paracingulate (CPC) has been previously shown in a study involving a cohort of children subjects with BD compared with healthy control subjects.14
In this study, we compared bilateral CPC white matter T2 values in children and adolescents with BD with healthy comparison subjects (HCS) using region-of-interest (ROI) analyses. The region of interest was chosen because of the extensive research implicating anterior cingulate cortical and frontal white matter in the pathophysiology of childhood and adolescent BD.14–17 Since decreased T2 is thought to reflect increased membrane fluidity, we hypothesized that children and adolescents with BD would have increased bilateral CPC white matter T2 reflecting decreased membrane fluidity. In order to better understand our T2 data, a post-hoc DTI analysis was also performed on selected subjects who had both DTI and T2 scans.
METHOD
Subjects
A total of 21 children with unmodified DSM-IV-TR BD were included in this study (10 male (mean age 11.5 ± 3.3) years, and 11 female (mean age: 11.6 ± 4.2 years). All of the subjects were receiving medication treatment for their BD and were in the mixed or manic state: none were depressed. Sixteen healthy comparison subjects (HCS) were also examined [9 male (mean age: 10.9 ± 4.3) and 7 female (mean age: 13.3 ± 2.1)]. There was no significant difference in age between these two groups. For the DTI analysis, 10 subjects with BD [3 male (mean age: 16.6 ± 4.8 years) and 7 female (mean age: 11.8 ± 4.5 years)] and 10 HCS [6 male (mean age: 10.6 ± 4.0 years) and 4 female (mean age: 14.3 ± 1.9 years)] were selected who were also part of the T2 study. There was no significant difference in age between these two groups.
Diagnosis was determined using the KSADS-E (parent and child) for Bipolar I Disorder, administered by a board certified child and adolescent psychiatrist. Measures of current mood symptoms and impairment ratings on all patients were obtained using the Young Mania Rating Scale18 (YMRS) and the Children’s Depression Rating Scale-Revised19, 20 (CDRS). Prior to participating subjects received a physical examination to ensure that they fulfilled all of the physical inclusion and exclusion criteria. All of the subjects were recruited through McLean Hospital and the Cambridge Health Alliance (outpatient, partial, and inpatient programs).
Inclusion criteria for BD subjects were age 6 to 18 years, diagnosis of unmodified DSM-IV-TR Bipolar I Disorder. Exclusion criteria were presence of chronic medical illness, history of neurological illness (including seizure disorder, multiple sclerosis, cerebral ischemia or infarction, neoplasia), a history of uncontrolled general medical disorder and a history of head trauma with loss of consciousness, contraindication to MR scan (including claustrophobia), presence of metal pins or braces, and evidence of white matter hyperintensities or structural brain abnormalities on brain MRI. Additional exclusion criteria were co-morbid diagnoses of substance dependence, autism, tic disorders and pervasive developmental disorder which was ruled out with a Pervasive Developmental Disorders checklist based on the DSM criteria to complement the KSADS. None of the participants had eating disorders. Co-morbid diagnosis of anxiety disorder, attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD) and conduct disorder were not exclusion criteria. The comorbid diagnosis rates are shown in Table 1. Exclusion criteria for HCS included any Axis 1 diagnosis and a family history of major depressive disorder or bipolar disorder in first-degree relatives.
Table 1.
Subject demographic information (Mean±SD where appropriate)
| Normal Control (N=14) | Bipolar (N=19) | Statistical Evaluation | |
|---|---|---|---|
| Age | 11.5 ± 3.3 | 9.7 ± 3.4 | F(32,1)=0.50; p=0.483 |
| Gender | 7M, 7F | 10M, 9F | χ2: 0.054; p=0.816 |
| CDRS | 40.0 ± 16.3 | ||
| YMRS | 23.6 ± 6.5 | ||
| Comorbid diagnosis, % | |||
| Panic disorder | 11.8 | ||
| Agoraphobia | 17.6 | ||
| Social phobia | 17.6 | ||
| Specific phobia | 41.2 | ||
| OCD | 17.6 | ||
| GAD | 41.2 | ||
| ADHD | 35.3 | ||
| Separation anxiety | 58.8 | ||
| Conduct disorder | 35.3 | ||
| ODD | 76.5 | ||
Note: ADHD = Attention De cit Hyperactivity Disorder; CDRS = Children’s Depression Rating Scale-Revised; GAD = Generalized Anxiety Disorder; OCD = Obsessive compulsive disorder; ODD = Oppositional Defiant Disorder; YMRS = Young Mania Rating Scale.
The study was approved for human subjects research by the Institutional Review Board at McLean Hospital and the Cambridge Health Alliance. After a complete description of the study, written informed assent were obtained from all subjects and written informed consent from parent/ legal guardians.
MRI acquisition
T2 relaxometry data were acquired on a 1.5-Tesla magnetic resonance scanner (Signa, General Electric Medical Systems, Milwaukee, WI) equipped with a proton birdcage headcoil. Localizer images, high-resolution T1-matched axial images through 27 planes for which T2 maps were generated, and T2 echoplanar image sets were acquired. T1 scans were acquired with a field of view of 200 mm and an acquisition matrix of 256×256. Parameters used for T2 images were as follow: 32 spin-echo steps per axial plane, 4 ms TE increments, TR = 6 s, field of view (FOV) = 200 mm, slice thickness = 5 mm with no skip, and matrix size =128 × 128.
Diffusion weighted images were acquired using the single-shot echo planar imaging technique with gradients applied in 6 non-collinear directions. Axial images were acquired using the following parameters: TE = 59 ms, TR = 10000 ms, matrix = 128×128, FOV = 200× 200 mm2, 25 continuous slices of 5 mm thickness, b value = 1125 s/mm2 and one T2 weighted ‘b0’ image with b value = 0 s/mm2. To enhance the signal to noise ratio, imaging was repeated 8 times. The total acquisition time for the entire study (anatomical images, T2 and DTI) was 1 hour and 15 minutes. All of the data presented in this study represent T2 and DTI data acquired during the same scanning session. None of the subjects were sedated.
Image Processing and Analysis
Data were processed and analyzed with a multi step procedure: The 32 TE-stepped images were first transferred to an offline workstation and corrected for in-plane motion using the DART image registration algorithm.21 A single T2 value for each pixel was then calculated using linear least squares regression and a mono-exponential decay function. Diffusion-weighted images were corrected for eddy current distortions and head motion using linear image registration (automated image registration [AIR] algorithm).22 Thereafter, DtiStudio23 (Radiology Department, John Hopkins University, Baltimore, Maryland, https://www.mristudio.org/) was used to generate diffusivity images following methods described by Pierpaoli and Basser.24 Using the computed eigenvalues (L1, L2, and L3), the magnitude images for radial diffusivity [(L2 + L3)/2], axial diffusivity (L1), trace of the diffusion tensor (apparent diffusion coefficient or mean diffusivity) (L1 + L2 + L3), and FA were calculated. For those subjects with both T2 and DTI data, spatial transformation of each participant’s b0 image to T2 maps and resampling of diffusivity images were performed. After transforming all the images into the common space, calculations of regional T2, Trace, axial diffusivity, radial diffusivity and FA values were made for the ROI drawn manually on the cingulum under the guidance of color FA images for each subject.
Representative right and left cingulum voxels were chosen as voxels having blue color (i.e. superior–inferior diffusion) located behind the corpus callosum (identified as a strip of red colour, i.e. right–left diffusion). The slice has been selected such that the branches of the cingulum turn from green to blue as they arch around the genu anteriorly. Nine voxel ROI (Fig 1) was conservatively limited to square regions (3 × 3 voxels) to provide consistency between slices and subjects. Pixels having extreme values (e.g., cerebral spinal fluid containing substantial T2 values > 120 ms) were excluded in order to minimize partial volume artifacts. Measurements were performed blinded to diagnosis and clinical findings of the participants.
Figure 1.
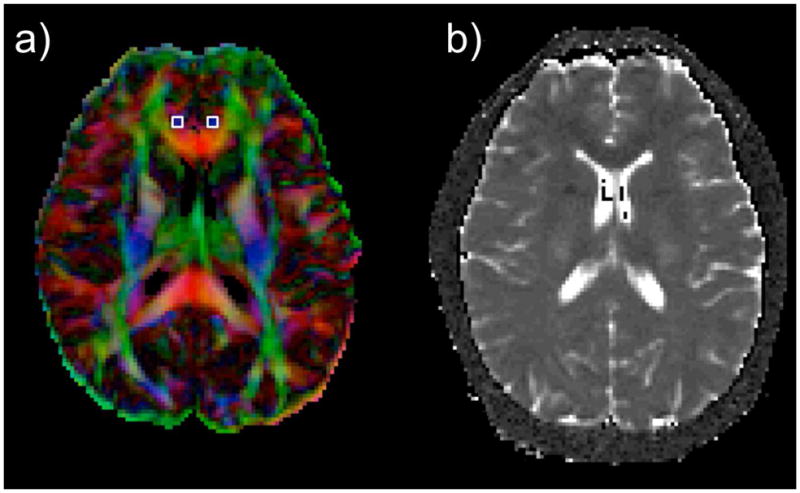
Representative slices from a subject showing images used to extract the values in cingulate-paracingulate white matter. Note: (a) Color Fractional Anisotropy images with left and right regions of interest on the cingulum (b) Transverse relaxation time (T2) map obtained by fitting the multi spin-echo steps.
Reliability of ROI measurement
Intra-rater reliability was assessed using the intraclass correlation coefficient (ICC). Since only one rater made the measurements, inter-rater reliability was not calculated. Intra-rater reliability was determined with repeated measurements on only the T2 images. The ICC was first calculated separately for measurements on normal subjects and bipolar subjects, and subsequently for all subjects (normal and BD) combined. The ICCs of ROI measurements in normal and BD subjects were 0.92 and 0.87, respectively. The overall ICC was 0.89. As these ICC values were each greater than 0.80, they constitute excellent agreement for intra-rater reliability.
Statistical Analysis
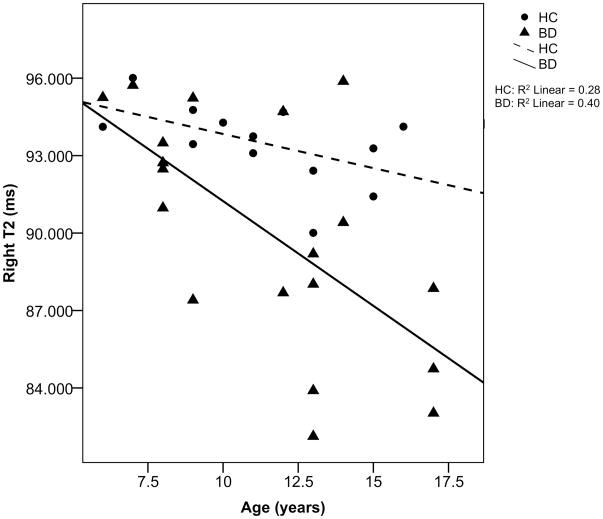
Statistical analysis was performed using the PASW software (PASW 18.0 for Macintosh). Univariate analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were used to test possible FA, trace, radial diffusivity, axial diffusivity, and T2 differences, adjusted for age and sex as covariates. There was no effect of sex on any of the measures. Age showed a negative correlation with radial diffusivity (R = 0.57, p = 0.014) and right T2 values (R=0.537, p = 0.001) for subjects with BD and HCS combined. When examined separately the negative correlation between right T2 and age reached significance in subject with BD (R = 0.634, p = 0.004) but not in HCS (R = 0.529, p = 0.063) (Fig 2). Therefore, the nal analysis of group differences was conducted with an ANCOVA with age as a covariate. Each measurement was also compare statistically between the 2 hemispheres using repeated-measures ANOVA with age as covariate. A p-value of < 0.05 was considered statistically signi cant.
Figure 2.
Correlation between transverse relaxation time (T2) values on the right with age in patients with bipolar disorder (BD) and healthy controls (HC). Note: Age negatively correlates with T2 values in patients with bipolar (R2=0.402).
RESULTS
We found four subjects whom we classi ed as “outliers” and excluded from the analysis because their T2 values did not fall within 2 standard deviations of the mean value of their corresponding groups. Adjusted subject demographics are presented in Table 1.
T2 data
The T2 values for right and left CPC are shown in Table 2. For the BD subjects there was significant difference between left and right CPC T2. This was not the case for the HCS. T2 was significantly decreased on the right of the subjects with BD compared with the left (F = 4.566, df = (1,17), p < 0.047 for the interaction between the factor (T2) and covariate (age), main effect was not significant (p > 0.1)). Comparing the 19 subjects with BD and 14 HCS showed significant between group differences only in the right CPC T2. T2 was significantly decreased on the right of the subjects with BD compared with the HCS (F = 9.347, df = (1,32), p < 0.005, covariate: age). Correlation coefficient showed no relationship between T2 values and CDRS, and YMRS scores in the BD group (uncorrected for multiple comparisons in an exploratory analysis). There were no statistically significant T2 differences between patients with comorbid ADHD and without (p = 0.195). Similarly, there were no statistically significant differences between patients with comorbid ODD and without (p = 0.203) or patients with comorbid anxiety and without (p = 0.779).
Table 2.
Transverse relaxation time (T2) values for right and left cingulate-paracingulate.
| T2 (ms) | ||
|---|---|---|
| Left | Right | |
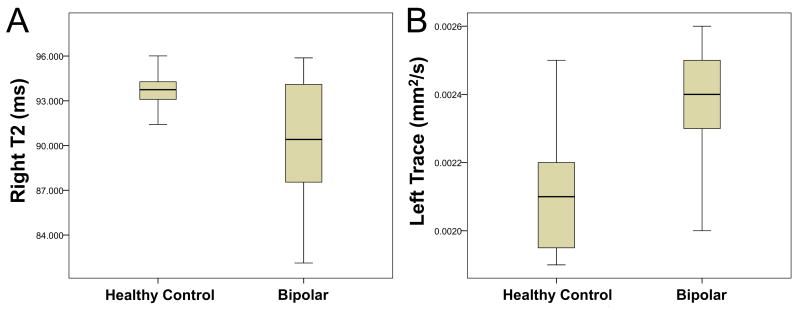
| Bipolar (N=19) | 93.58 ± 2.48 | 90.04 ± 4.52 |
| Normal Control (N=14) | 92.23 ± 2.66 | 93.18 ± 1.89 |
Note: Right sided T2 is significantly lower in the subjects with Bipolar Disorder compared with left (F(1,17) = 4.566, p = 0.047, covariates: age) and with healthy comparison subjects (F(1,32) = 9.347, p < 0.005, covariates: age).
DTI data
For those participants who had both T2 and DTI measurements, the values for right and left CPC are shown in Table 3. Examined measures were FA, axial and radial diffusivities, trace and T2. For the BD subjects, there was significant difference only in the T2 measures between the left and right CPC white matter (F = 12.453, df = (1,6), p < 0.012 for the interaction between the factor (T2) and covariate (age), main effect was not significant (p > 0.05)). When BD subjects were compared with the HCS, left FA was reduced (F = 7.503, df = (1,15), p < 0.017, covariate: age), and both left Trace and radial diffusivity were increased (Trace: F = 6.884, df = (1,15), p = 0.021 and Radial: F = 7.513, df = (1,15), p = 0.017, covariate: age). On the right, Trace was increased (F = 6.396, df = (1,15), p = 0.025, covariate: age), and T2 was decreased (F = 13.340, df = (1,15), p = 0.003, covariate: age), when BD subjects were compared with the HCS (Fig 3). Significant differences were observed only in FA between the left and right in HCS [F = 12.342, df = (1,6), p < 0.013 for the main effect, interaction with age was also significant (p < 0.012)]. On the right hand-side axial diffusivity correlated inversely with CDRS, controlling for age and sex. A series of correlation coefficients showed no relationship between DTI measures and CDRS and YMRS scores in the BD group (uncorrected for multiple comparisons in an exploratory analysis).
Table 3.
Transverse relaxation time (T2), Fractional Anisotropy (FA) and Trace comparison.
| T2 (ms) | FA | Trace Diffusivity | ||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| Bipolar (N=8) | 94.15 ± 2.65 | 90.39 ± 5.13 | 0.39 ± 0.12 | 0.45 ± 0.12 | 2.40E-03 ± 2.00E-04 | 2.27E-03 ± 1.03E-04 |
| Normal Control (N=8) | 91.58 ± 3.23 | 93.29 ± 1.93 | 0.50 ± 0.12 | 0.49 ± 0.10 | 2.12E-03 ± 1.98E-04 | 2.12E-03 ± 1.31E-04 |
Note: The trace diffusivity is higher in the left and right cingulate-paracingulate compared with the healthy control subjects (Left: F(1,15) = 6.884, p = 0.021; Right: F(1,15) = 6.396, p = 0.025, covariates : age).
Figure 3.
Box plots of transverse relaxation time (T2) and Trace showing a) decreased T2 on the right and b) increased Trace on the left cingulum in subjects with bipolar compared to healthy controls.
Medication Effects
At the time of the scan, all participants with BD were being treated with one or more type of medications: 14 were being treated with antipsychotics, 11 with mood stabilizers, 6 with antidepressant, 3 with stimulants, 3 with selective-serotonin re-uptake inhibitors, and 4 with lithium. The association between the T2 values and classes of medication was examined using linear regression model controlling for age. Results showed no association between T2 values and classes of medication (Antidepressants: β = 0.024, t = 0.063, p = 0.951; Antipsychotics: β = −0.132, t = −0.400, p = 0.696; Mood stabilizers: β = 0.108, t = 0.335, p = 0.743 ; Lithium: β = 0.043, t = 0.144, p = 0.888).
DISCUSSION
To the best of our knowledge, this is the first study to use T2 imaging to investigate membrane fluidity abnormalities associated with early onset BD. Our hypothesis of increased T2 in children and adolescents with BD was not confirmed. However a significant T2 reduction on the right was observed.
T2 is influenced by many factors, in particular iron content, cerebral blood flow or volume, water content and/or membrane fluidity.12, 25, 26 While T2 may be affected by iron content, it is unclear if it would be sufficient enough to account for the T2 differences observed in this study. The major effect of iron in the brain is T2 reduction, i.e., higher brain iron concentrations result in shorter T2.26 Based on the only study we could find that includes a convenient equation allowing approximate regional iron determination based on the T2, for the 2.86% T2 decrease observed in this study there would have been 74% increase in iron content. However, keeping in mind that the equation is derived for much older adults (mean age 62.3 ± 7.1) for subcortical deep gray matter regions, we expect lesser effects for children and adolescents since the amount of iron deposition in the brain increases with age. It is known from the work of Hallgren and Sourander27 that the iron distribution inside the brain is heterogeneous with highest content found in globus pallidus, 5 times more than in frontal WM. Thus, we believe it is more likely that the T2 difference observed in the WM region studied in this analysis is a reflection of other factors than the iron deposition which would amount to only 15% in children according to our predictions.
Regarding the water content, we are not able to specify the source of the decreased T2 in subjects with BD as the current T2 mapping methodology does not distinguish between intracellular (axonal), extracellular or myelin water (between the layers of myelin surrounding axons). T2 decay is composed of roughly three units - myelin water which has a T2 of approximately 20 ms, extracellular water has a decay of about 80 ms, and axonal water a T2 of 250 ms.28 However, since the sequence used in this study acquired echoes at relatively long echo times (58 ms to 186 ms, which were chosen to effectively allow sufficient magnetization decay and thus more accurately capture T2 relaxation times), the short T2 of the myelin water could not be quantified, and thus, the results here likely do not reflect abnormalities of the T2 of myelin water (based on the simulation of Whittall,29 the contribution of the short T2 component to the long T2 component would be very small, less than approximately 2%).
In our analysis, T2 values were calculated using linear least squares regression and a mono-exponential decay function. Double-exponential decay function was not explored since we incorporated the intermediate component of T2, which can generally be characterized by a single-exponential decay in the absence of signi cant partial volume averaging. Since we conservatively limited our ROIs to one tissue type and excluded voxels with extreme T2 values, partial volumes artifacts were minimized and hence making the mono-exponential decay function a reasonable choice.
Decreases in intracellular or extracellular water could lead to shorter T2 times. During typical development, water T2 shortens perhaps due to decreasing axonal water secondary to increased microfilament and microtubule production or as a result of decreased extracellular fluid secondary to myelin production (as myelinated axons occupy more space than unmyelinated axons). In the subset of subjects that had both T2 and DTI data, no significant changes in DTI measures were observed. DTI derived radial diffusivity was previously shown to reflect myelin integrity.30 Assuming myelination has a direct effect on the membrane fluidity (myelin is made up of a lipid bilayer and can cause the membrane to become more rigid and eventually to malfunction), we could not prove our original hypothesis of impaired membrane fluidity in patients with BD due to no changes in DTI measures. Thus, it is more likely that the T2 difference is a reflection of cerebral blood flow in patients with BD.
A literature search was conducted to investigate how cerebral blood flow in typically development children varies in the right and left hemispheres, but we were unable to find any relevant studies. One report has indicated reduced white matter density on the right subgenual portion of the anterior cingulate and in the subgyral WM surrounding this area in adult patients with bipolar disorder relative to controls using magnetization transfer imaging.31 There are also reports that relate magnetization transfer ratio reduction to neuronal integrity32 and white matter neuronal integrity to cerebral flood flow. 33 Based on these reports, we can hypothesize that subjects with BD have abnormalities of neural integrity in the right cingulate-paracingulate reflected by T2 changes. However, this hypothesis would be highly speculative and will require further testing and studies. In our study we did not see any FA changes that represent neuronal integrity probably due to small sample size. Larger studies are therefore needed. The T2 decrease with age may be a consequence of increased white matter organization in bipolar disorder, however, we feel further interpretation would be highly speculative.
Our DTI results may be suggesting that myelin damage also occurs on the left in early onset BD in addition to changes in the blood flow. Decreased blood flow could damage myelination, and progressive myelin damage could result in decreased lipid and increased water content. In terms of DTI measures, deficit in myelin could result in increased radial diffusivity, trace and T2, in addition to decreased FA. It could be that there is a relationship between the cerebral blood flow and myelination: Blood flow in the white matter has been reported to be much higher in developing brains, possibly because of the demand related to myelination.34 Taking into account the small number of subjects, this pathologic explanation and interpretation should be considered speculative.
Regarding the DTI results, we included a broader range of diffusion measures (i.e., axial diffusivity, radial diffusivity and trace) and not just FA to characterize the WM abnormalities. Furthermore we performed the ROI analysis on a major fiber bundle where there would be fewer numbers of fiber crossings, and therefore eliminate the potential problems related to FA reduction in crossing fiber areas. Even though we realize that using 6 gradient directions is not considered state-of-the-art by today’s standards, no significant differences were found for the mean FA among different DTI protocols that used 6, 21, and 31 gradient directions and same MRI machine as ours.35
This study has several limitations, including the small sample size. For analyzing regional differences, we have performed only a ROI analysis, which does not provide information on more global changes in the brain. A voxel-based analysis, rather than the ROI approach, would have been more proper to study the global changes. However, the modest number of subjects included in this study did not permit this approach. Another important limitation of this study is that the patients were medicated. It is conceivable that medications may have considerable effects on the results. However, although an MRS study, other investigators have observed no metabolic brain differences in medicated compared with unmedicated early onset BD patients (medicated with mood stabilizers and/or antipsychotics).17 Nevertheless, we examined whether there was any association between T2 values and classes of medication using linear regression model controlling for age and found no association probably due to small sample sizes. In addition, no detailed handedness, neuropsychological testing or socioeconomic status information were available for our analyses, therefore they have not been further investigated.
In conclusion, the T2 and DTI results of this study taken together appear to suggest that increased CPC blood flow is associated with BD, which results in a developmental difference in blood flow on the right in subjects with BD. Although our preliminary data on a small group require replication in a larger sample of subjects, these data may serve to stimulate further research into the biological basis of early onset BD.
Acknowledgments
This study was funded by MH001978 (CMM), MH073998 (CMM), MH01573 (JAF) and “Private Individual Anonymous Donor” (JAF).
Footnotes
Disclosure: Dr. Frazier has received research support from Bristol-Myers Squibb, Glaxo Smith Kline, Johnson and Johnson, Neuropharm, Otsuka America Pharmaceutical and Pfizer, Inc. Drs. Gönenç and Moore, and Mr. Crowley report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geller B, Fox LW, Clark KA. Rate and predictors of prepubertal bipolarity during follow-up of 6- to 12-year-old depressed children. J Am Acad Child Adolesc Psychiatry. 1994;33:461–468. doi: 10.1097/00004583-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- 4.Findling RL. Dosing of Atypical Antipsychotics in Children and Adolescents. Primary Care Companion. J Clin Psychiatry. 2003;5(suppl 6):10–13. [PubMed] [Google Scholar]
- 5.Leverich GS, Post RM, Keck PE, Jr, et al. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 6.Terry J, Lopez-Larson M, Frazier JA. Magnetic resonance imaging studies in early onset bipolar disorder: an updated review. Child Adolesc Psychiatr Clin N Am. 2009;18:421–39. doi: 10.1016/j.chc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Frazier JA, Ahn MS, DeJong S, Bent EK, Breeze JL, Giuliano AJ. Magnetic resonance imaging studies in early-onset bipolar disorder: a critical review. Harv Rev Psychiatry. 2005;13(3):125–140. doi: 10.1080/10673220591003597. Review. [DOI] [PubMed] [Google Scholar]
- 8.Adibhatla RM, Hatcher JF. Role of Lipids in Brain Injury and Diseases. Future Lipidol. 2007;2:403–422. doi: 10.2217/17460875.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adibhatla RM, Dempsy R, Hatcher JF. Integration of cytokine biology and lipid metabolism in stroke. Front Biosci. 2008;13:1250–1270. doi: 10.2741/2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Molecular Psychiatry. 2004;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 12.Hirashima F, Parow AM, Stoll AL, et al. Omega-3 fatty acid treatment and T(2) whole brain relaxation times in bipolar disorder. Am J Psychiatry. 2004;161:1922–1924. doi: 10.1176/ajp.161.10.1922. [DOI] [PubMed] [Google Scholar]
- 13.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Frazier JA, Breeze JL, Papadimitriou G, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9:799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 15.DelBello MP, Cecil KM, Adler CM, Daniels JP, Strakowski SM. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31:1264–1273. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- 16.Blumberg HP, Krystal JH, Bansal R, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Moore CM, Frazier JA, Glod CA, et al. Glutamine and glutamate levels in children and adolescents with bipolar disorder: a 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adolesc Psychiatry. 2007;46:524–534. doi: 10.1097/chi.0b013e31802f5f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 19.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Poznanski EO, Mokros HB. Children’s depression rating scale, revised (CDRS-R) Los Angeles: Western Psychological Services; 1984. [Google Scholar]
- 21.Maas LC, Frederick BD, Renshaw PF. Decoupled automated rotational and translational registration for functional MRI time series data: the DART registration algorithm. Magn Reson Med. 1997;37:131–139. doi: 10.1002/mrm.1910370119. [DOI] [PubMed] [Google Scholar]
- 22.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 25.Anderson CM, Kaufman MJ, Lowen SB, Rohan M, Renshaw PF, Teicher MH. Brain T2 relaxation times correlate with regional cerebral blood volume. MAGMA. 2005;18:3–6. doi: 10.1007/s10334-004-0076-2. [DOI] [PubMed] [Google Scholar]
- 26.Vymazal J, Righini A, Brooks RA, et al. T1 and T2 in the brain of healthy subjects, patients with Parkinson disease, and patients with multiple system atrophy: relation to iron content. Radiology. 1999;211:489–495. doi: 10.1148/radiology.211.2.r99ma53489. [DOI] [PubMed] [Google Scholar]
- 27.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- 28.MacKay A, Laule C, Vavasour I, Bjarnason T, Kolind S, Madler B. Insights into brain microstructure from the T2 distribution. Magn Reson Imaging. 2006;24:515–525. doi: 10.1016/j.mri.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Whittall KP, MacKay AL, Li DK. Are mono-exponential fits to a few echoes sufficient to determine T2 relaxation for in vivo human brain? Magn Reson Med. 1999;41:1255–1257. doi: 10.1002/(sici)1522-2594(199906)41:6<1255::aid-mrm23>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 30.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 31.Bruno SD, Barker GJ, Cercignani M, Symms M, Ron MA. A study of bipolar disorder using magnetization transfer imaging and voxel-based morphometry. Brain. 2004;127(11):2433–2440. doi: 10.1093/brain/awh274. [DOI] [PubMed] [Google Scholar]
- 32.Pendlebury ST, Lee MA, Blamire AM, Styles P, Matthews PM. Correlating magnetic resonance imaging markers of axonal injury and demyelination in motor impairment secondary to stroke and multiple sclerosis. Magn Res Imag. 2000;18:369–378. doi: 10.1016/s0730-725x(00)00115-6. [DOI] [PubMed] [Google Scholar]
- 33.Kimelberg HK. Functions of Mature Mammalian Astrocytes: A Current View. Neuroscientist. 2010;16(1):79–106. doi: 10.1177/1073858409342593. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy C, Grave GD, Jehle JW, Sokoloff L. Blood flow to white matter during maturation of the brain. Neurology. 1970;20:613–618. doi: 10.1212/wnl.20.6.613. [DOI] [PubMed] [Google Scholar]
- 35.Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol. 2006;27:1776–1781. [PMC free article] [PubMed] [Google Scholar]