Abstract
Background:
T lymphocyte infiltration has been detected in glioma, although its significance remains unclear. The purpose of the present study was to explore the prognostic value of CD4+ and CD8+ tumour-infiltrating lymphocytes (TILs) in glioma, and the prognostic value of infiltrating Forkhead box protein 3 (FoxP3+) regulatory T cells were also investigated.
Methods:
CD4+, FoxP3+ and CD8+ TILs were assessed by immunohistochemical staining of tissue microarray cores from 284 gliomas. Kaplan–Meier analysis and Cox proportional hazards models were used to examine the survival function of these TILs in 90 glioblastoma patients.
Results:
The number of CD8+ TILs was inversely correlated with tumour grade (P=0.025), whereas the number of CD4+ TILs was positively correlated with tumour grade (P=0.002). FoxP3+ TILs were only observed in glioblastomas, but not in low-grade astrocytomas or oligodendroglial tumours. Among patients with glioblastoma, none of CD4+ TILs, FoxP3+ TILs and CD8+ TILs alone was significantly associated with patient prognosis. However, the presence of high CD4+ and low CD8+ TIL levels was an independent predictor of poor progress-free survival (multivariate hazard ratio (HR) 1.618, 95% confidence interval (CI) 1.245–2.101, P<0.001) and poor overall survival (multivariate HR 1.508, 95% CI 1.162–1.956, P=0.002). Moreover, pseudoprogression was more often found in patients with high CD4+ TILs and high CD8+ TILs.
Conclusions:
The combination of CD4+ and CD8+ TILs is a predictor of clinical outcome in glioblastoma patients, and a high level of CD4+ TILs combined with low CD8+ TILs was associated with unfavourable prognosis.
Keywords: glioma, tumour-infiltrating lymphocytes, prognosis
Previous studies have shown that lymphocyte infiltration occurs in glioma and that the presence of tumour-infiltrating lymphocytes (TILs) is predictive of clinical outcome (Brooks et al, 1978; von et al, 1984). However, the findings of these studies were not consistent (El Andaloussi and Lesniak, 2006; Heimberger et al, 2008; Lohr et al, 2011; Kim et al, 2012; Yue et al, 2013). Because of the wide application of standard radiotherapy and/or chemotherapy for the treatment of glioma patients, the effect of immune factors on clinical outcome and the potential prognostic value of TILs are important clinical issues that need to be elucidated.
Functionally distinct lymphocytes are distinguished by their mutually exclusive expression of CD4 or CD8 co-receptors (Yu et al, 2003). CD8+ cytotoxic T lymphocytes are crucial components of the tumour-specific adaptive immunity that attacks tumour cells (Smyth et al, 2006; Mahmoud et al, 2011). The role of CD4+ T cells is complicated (Gu-Trantien et al, 2013). CD4+ helper T cells have a central role in initiating and maintaining anti-cancer immune responses, which significantly affects the function of CD8+ T cells (Ho et al, 2002). However, CD4+ regulatory T cells (Tregs) suppress anti-tumour immunity and promote tumour progression (Gobert et al, 2009; Zamarron and Chen, 2011). Forkhead box protein 3 (FoxP3+) is a specific molecular marker for Tregs (El Andaloussi and Lesniak, 2006; Yue et al, 2013).
In the present study, we investigated the infiltration of CD4+, FoxP3+ and CD8+ lymphocytes in a large series of glioma samples, and examined the effects of these TILs, alone and in combination, on the prognosis of glioblastoma patients who received standard therapy.
Materials and methods
Study population
We examined clinical samples and patient records corresponding to 284 patients who had been diagnosed with glioma (153 with grade II, 41 with grade III and 90 with grade IV) from the Chinese Glioma Cooperative Group sample database. Grade II gliomas included 92 astrocytomas, 27 oligodendrogliomas and 34 oligoastrocytomas. Grade III gliomas included 10 anaplastic astrocytomas, 13 anaplastic oligodendrogliomas and 18 anaplastic oligoastrocytomas. Grade IV gliomas included 90 primary glioblastoma multiformes (GBMs). Molecular classification information was available for 49 GBM samples from Chinese Glioma Genome Atlas (CGGA, http://www.cgcg.org.cn; proneural 4, neural 7, classical 15 and mesenchymal 23). The patients had no other cancers or diseases, such as acute infection or diabetes, and they had not received previous radiotherapy or chemotherapy at the time of the study. Patients underwent surgical resection by neurosurgeons who used similar operational techniques and principles from January 2007 to December 2009. According to the 2007 World Health Organization classification guidelines, the histological diagnosis was established and verified by two neuropathologists. Samples were immediately snap-frozen in liquid nitrogen after resection. One part of each sample was fixed with formalin, embedded with paraffin wax and kept at room temperature. All patients with GBM received radiotherapy and chemotherapy postoperatively according to the Stupp protocol (Stupp et al, 2005).
The 90 patients with GBM were included in the survival analysis. Patients underwent routine contrast-enhanced magnetic resonance imaging (MRI) examination before and after surgery. Tumour size was calculated based on preoperative MRI scans as follows: longest diameter × widest diameter × thickness (section thickness × the number of layers) × 1/2. Clinicopathologic data were retrospectively collected from medical records and are summarised in Table 1. Progress-free survival (PFS) was defined from the date of surgery to the first MRI-confirmed recurrence. Tumour progression and pseudoprogression were defined according to RANO criteria (Brandsma and van den Bent (2009)). In the case that the MRI changes were equivocal, the therapy was continued observing the patient closely, and MRI was repeated every 4 week. Confirming the progress in a 4-week control MRI, the initial MRI was considered as a progression time point at which the initial suspicion of progression was expressed. Overall survival (OS) was defined as the interval between surgery and death from GBM. The median follow-up period was 15.7 months (range, 2–53.7 months), during which 79 patients were dead from GBM. No patient with GBM was lost to follow-up. Data were censored at the last follow-up for patients without death at the time of the analysis. This study was approved by the institutional review board of our hospital, and written informed consent was obtained from each glioma tissue donor, who consented to the use of the tumour tissue and clinical data for future research.
Table 1. Correlation between combined CD4+ and CD8+ lymphocytic infiltration status and clinicopathologic features in 90 glioblastomas.
|
Combined CD4+
and CD8+
Lymphocytic Infiltration Status |
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
All cases |
Low CD4+/low CD8+ |
Low CD4+/high CD8+ |
High CD4+/high CD8+ |
High CD4+/ low CD8+ |
|
||||||||||
| Clinical feature | No. | % | No. | % | No. | % | No. | % | No. | % | P | |||||
| Total patients |
90 |
|
100 |
24 |
|
26.7 |
21 |
|
23.3 |
23 |
|
25.6 |
22 |
|
24.4 |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.975 |
| Male | 46 | 51.1 | 13 | 54.2 | 10 | 47.6 | 12 | 52.2 | 11 | 50.0 | ||||||
| Female |
44 |
|
48.9 |
11 |
|
45.8 |
11 |
|
52.4 |
11 |
|
47.8 |
11 |
|
50.0 |
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.268 |
| Mean | 45.7 | 45.1 | 45.1 | 42.7 | 50.2 | |||||||||||
| s.d. |
|
13.0 |
|
|
13.6 |
|
|
11.1 |
|
|
11.7 |
|
|
14.9 |
|
|
| Pre-op KPS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.181 |
| Mean | 77.8 | 77.7 | 77.4 | 81.3 | 74.8 | |||||||||||
| s.d. |
|
10.0 |
|
|
11.2 |
|
|
6.2 |
|
|
8.0 |
|
|
12.6 |
|
|
| Resection |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.538 |
| GTR | 45 | 50 | 12 | 50.0 | 8 | 38.1 | 14 | 65.2 | 11 | 50.0 | ||||||
| Residue <30% | 22 | 24.4 | 7 | 29.2 | 5 | 23.8 | 3 | 13.0 | 7 | 31.8 | ||||||
| Residue >30% |
23 |
|
25.6 |
5 |
|
20.8 |
8 |
|
38.1 |
6 |
|
26.1 |
4 |
|
18.2 |
|
| Tumour size, cm3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.382 |
| Mean | 62.3 | 69.6 | 60.2 | 62.8 | 55.7 | |||||||||||
| s.d. |
|
27.4 |
|
|
25.0 |
|
|
25.4 |
|
|
30.6 |
|
|
28.3 |
|
|
| MGMT promoter |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.481 |
| Methylated | 30 | 33.3 | 11 | 45.8 | 6 | 28.6 | 6 | 26.1 | 7 | 31.8 | ||||||
| Unmethylated |
60 |
|
66.7 |
13 |
|
54.2 |
15 |
|
71.4 |
17 |
|
73.9 |
15 |
|
68.2 |
|
| psPD |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0.007 |
| psPD | 15 | 16.7 | 1 | 4.2 | 3 | 14.3 | 9 | 39.1 | 2 | 9.1 | ||||||
| non-psPD | 75 | 83.3 | 23 | 95.8 | 18 | 85.7 | 14 | 60.9 | 20 | 90.9 | ||||||
Abbreviations: GTR=gross total resection; KPS=Karnofsky performance status; MGMT=O(6)-methylguanine-DNA-methyltransferase; pre-op=preoperative; psPD=pseudoprogression.
Tissue microarrays and immunohistochemistry
Tissue microarrays were constructed using 284 glioma clinical samples and analysed by immunohistochemical staining as we previously described (Han et al, 2013). Briefly, tissue microarrays were constructed with tissue microarrayer (Beecher Instruments, Silver Springs, MD, USA). Each tumour was sampled in duplicate from representative areas using a 0.6-mm punch, yielding composite array blocks comprising a total of 568 tissue cores.
Paraffin-embedded specimens were cut into 4-μm sections. After deparaffinization with xylene and rehydration, antigen retrieval was performed by microwave treatment in 10 mmol l−1 sodium citrate buffer (pH 6.0) for 20 min. The endogenous peroxidase was blocked with 3% H2O2 in methanol. Non-specific binding was blocked for 10 min using protein-blocking buffer. The sections were washed in phosphate-buffered saline (PBS). Diluted primary antibodies against CD4 (clone 4B12, 1 : 40; Novocastra Laboratories Ltd., Newcastle, UK), CD8 (clone 144B, 1 : 50; Abcam, Cambridge, UK) or FoxP3 (clone mAbcam 450, 1 : 50; Abcam) were added to the tissue and incubated overnight at 4 °C. For negative controls, the primary antibody was replaced by normal mouse serum. Human normal tonsil was used as positive control. Samples were then incubated with the horseradish peroxidase labelled secondary antibody in the immunohistochemical kit (KIT-5930, MaxVision, Fu Zhou, China) for 30 min at room temperature. Diaminobenzidine was used for colour development and hematoxylin as counterstain. Results were visualised and photographed under a light microscope (Olympus BX-51; Olympus Optical Co., Ltd., Tokyo, Japan).
Quantitative evaluation was performed by examining each section using at least five different high-power fields ( × 40 objective and × 10 eyepiece) with the most abundant TILs. Because other populations occasionally stain with CD4 antibody, CD4+cells with apparent morphological appearance different from T cells were excluded from the count. The number of CD4+, FoxP3+ and CD8+ TILs was manually counted three times for each photograph independently by two investigators (JD and YL) and an experienced neuropathologist (QL) blinded to the clinical background of the patients. Scores were re-examined after a period of time to ensure reproducibility. An excellent intra-observer and inter-observer agreement was reached. When strong differences in scoring between observers occurred, the core was re-evaluated to reach a concordant scoring. The average of CD4+, FoxP3+ and CD8+ TIL counts per field for each patient was used for statistical analysis (Sato et al, 2005).
MGMT promoter methylation analysis by methylation-specific PCR
Methylation-specific PCR was performed as described previously (Esteller et al, 1999) to detect O(6)-methylguanine-DNA-methyltransferase (MGMT) promoter methylation in the 90 GBMs. Briefly, tissue samples were lysed with 490 μl lysis buffer containing 20 mM Tris-Cl (pH 8.0), 5 mM EDTA (pH 8.0), 400 mM NaCl and 1% (w/v) sodium dodecyl suphate, and digested with 10 μl proteinase K at 10 mg ml−1 at 37 °C for 12 h. Genomic DNA was purified from the lysate by phenol/chloroform extraction. One microgram of DNA was denatured by NaOH and modified by sodium bisulfite. Methylation-specific PCR was performed using primer sequences for MGMT as follows: 5′-TTT GTG TTT TGA TGT TTG TAG GTT TTT GT-3′ (forward) and 5′-AAC TCC ACA CTC TTC CAA AAA CAA AAC A-3′ (reverse) for the unmethylated reaction; and 5′-TTT CGA CGT TCG TAG GTT TTC GC-3′ (forward) and 5′-GCA CTC TTC CGA AAA CGA AAC G-3′ (reverse) for the methylated reaction. Each PCR reaction (10 μl) was loaded onto non-denaturing 6% polyacrylamide gels, stained with ethidium bromide and visualised under ultraviolet illumination. The PCR reaction was repeated at least three times.
Statistical analysis
Cox proportional hazards models were used to calculate hazard ratios (HRs) of recurrence or death according to the number of CD4+, FoxP3+ and CD8+ TILs in GBMs, unadjusted and adjusted for age, sex, preoperative Karnofsky performance status (KPS), tumour size, degree of resection and MGMT promoter methylation. To adjust for potential confounders, age, preoperative KPS and tumour size were used as continuous variables and all of the other covariates were used as categorical variables. We dichotomised MGMT promoter methylation status (methylation vs unmethylation) and TIL levels (high vs low, the cutoff values are defined in Results). Tumour resection was defined as follows: (0) gross total resection, (1) partial removal with residual tumour <30% and (2) residual tumour >30% or biopsy. The combination of CD4+ TILs and CD8+ TILs was defined as: (1) low CD4+ TILs with low CD8+ TILs; (2) low CD4+ TILs with high CD8+ TILs; (3) high CD4+ TILs with high CD8+ TILs; (4) high CD4+ TILs with low CD8+ TILs. The combination of FoxP3+ TILs and CD8+ TILs was defined in the same pattern. Kaplan–Meier survival analysis was used to determine the distribution of OS and PFS time, and the results were analysed with the log-rank test.
The χ2 test and analysis of variance were used to determine statistical significance. Statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA). A 2-tailed P-value of <0.05 was regarded as significant.
Results
Glioma infiltration by CD4+, FoxP3+ and CD8+ lymphocytes
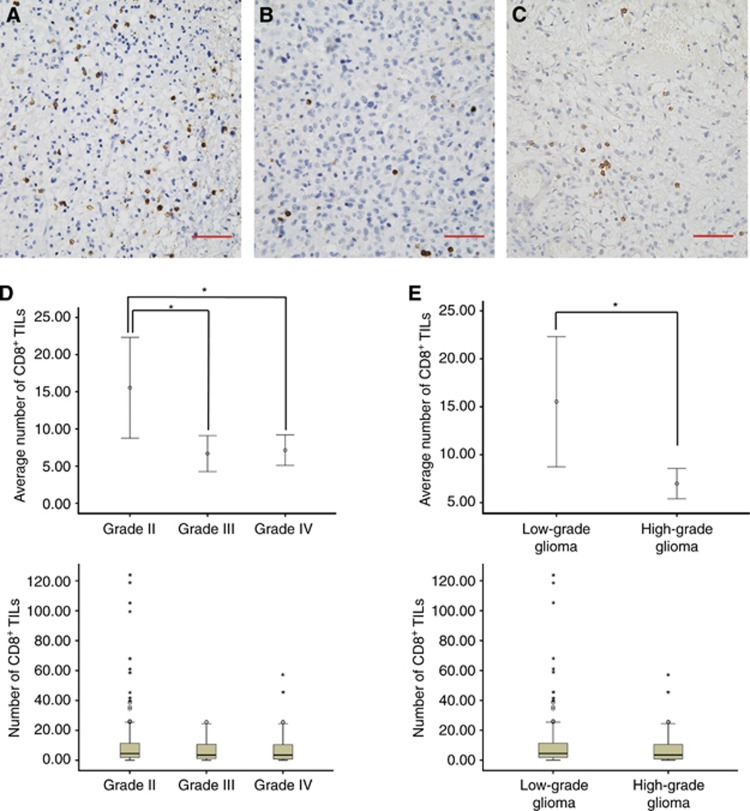
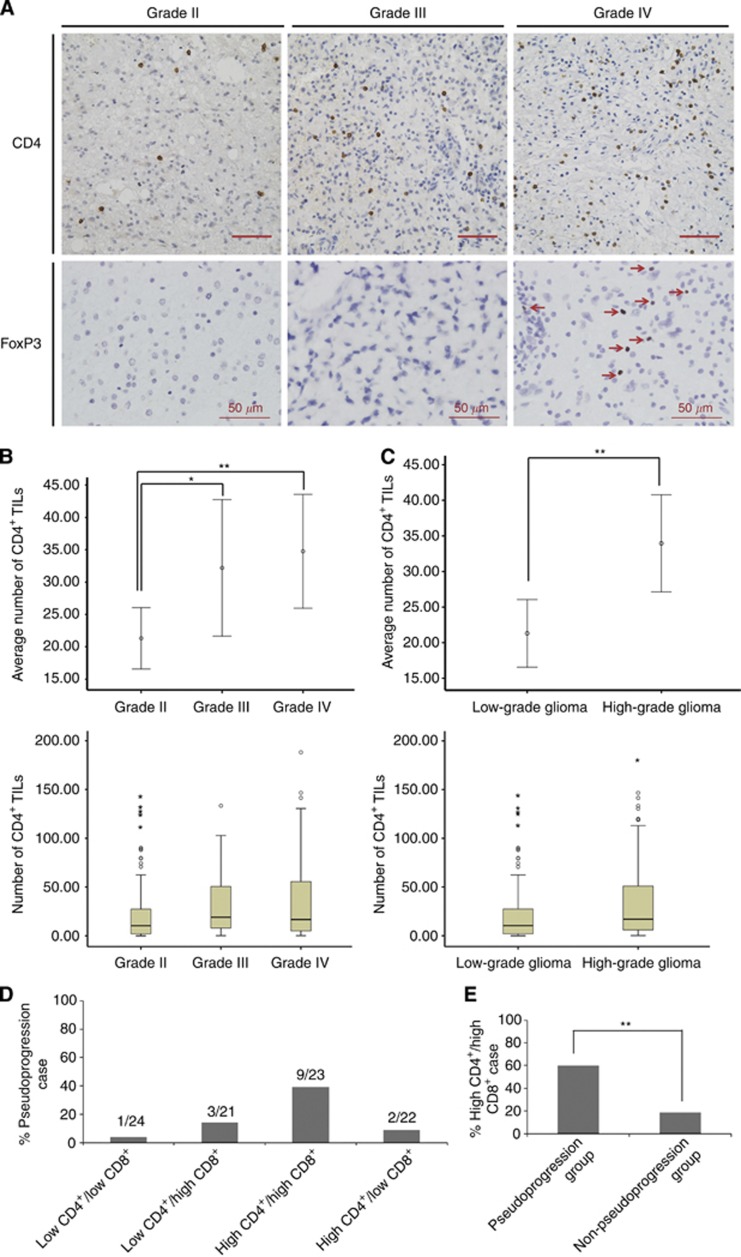
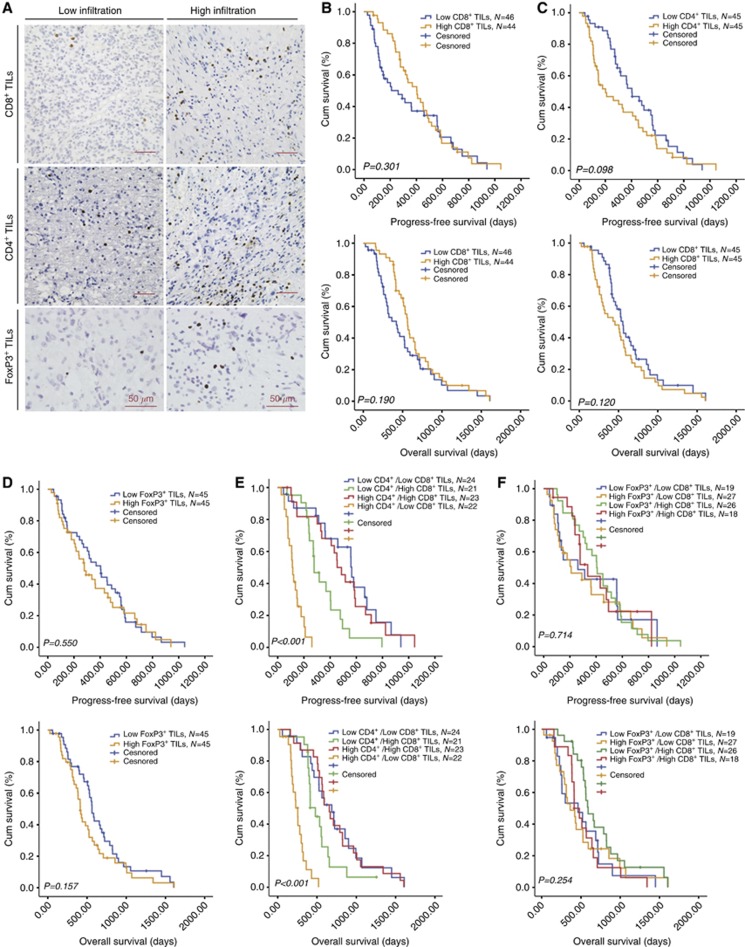
As shown in Figure 1, the number of CD8+ TILs was significantly lower in high-grade gliomas (grade III and IV, 6.9±9.1) than in low-grade gliomas (grade II, 15.5±42.5, P=0.023). In contrast, the number of CD4+ TILs was higher in high-grade gliomas (34.0±39.4) than in low-grade gliomas (21.3±29.7, P=0.002, Figures 2A–C). No significant differences in the number of CD4+ and CD8+ TILs were observed between patients with grade III and grade IV gliomas (Figures 1D and 2B). FoxP3+ TILs were not observed in astrocytomas and anaplastic astrocytomas. Moreover, we did not found FoxP3+ TILs in oligodendroglial tumours. FoxP3+ TILs were only present in glioblastomas and the number was small (3.8±8.7, Figure 2A). Statistics of averaged TILs counts are shown in Table 2. The median number of CD4+, FoxP3+ and CD8+ TILs was 16.7, 2.1 and 3.4 per high-power field, respectively in the 90 grade IV GBM tissues, which was used as the cutoff point to define a high infiltration group and a low infiltration group for survival analysis (Figure 3A).
Figure 1.
CD8+ tumour-infiltrating lymphocytes (TILs) in gliomas. Representative immunohistochemical images of CD8+ TILs in glioma ( × 400): (A) Grade II (B) Grade III (C) Grade IV. (D and E): The number of CD8+ TILs in different grades of glioma. Scale bars, 50 μm. *P<0.05.
Figure 2.
CD4+ and FoxP3+ tumour-infiltrating lymphocytes (TILs) in gliomas. (A) Representative immunohistochemical images of CD4+ and FoxP3+ TILs in glioma. Arrows, FoxP3-positive cells. (B and C): The number of CD4+ TILs in different grades of glioma. (D) The distribution of pseudoprogression cases among different groups. (E) The percentage of high CD4+/high CD8+ cases in pseudoprogression and non-pseudoprogression groups. Scale bars, 50 μm. *P<0.05, **P<0.01.
Table 2. The number of CD4+ and CD8+ tumour-infiltrating lymphocytes in glioma (n=284).
| Cell type | Mean | Median | Interquartile range | s.d. | Skewness |
|---|---|---|---|---|---|
| CD4+ T cell |
27.1 |
13.0 |
3.4–35.4 |
35.1 |
1.83 |
| CD8+ T cell | 11.6 | 4.0 | 1.4–10.9 | 32.0 | 8.13 |
Figure 3.
Prognostic significance of tumour-infiltrating lymphocytes (TILs) in glioblastoma. (A) Immunohistochemical pictures demonstrating the subgroups of low infiltration and high infiltration. (B–F) Kaplan–Meier plots of overall survival and progress-free survival, stratified by the predefined cutoff points for CD8+, CD4+ and FoxP3+ TILs.
The number of CD4+, FoxP3+ and CD8+ TILs did not vary significantly according to age, sex, preoperative KPS, degree of resection, tumour size and MGMT promoter methylation. In GBMs, the number of CD4+, FoxP3+ and CD8+ TILs did not vary greatly according to molecular classification (Supplementary Table 1 and 2).
Prognostic significance of TILs in GBM
In GBM, unexpectedly, none of CD4+, FoxP3+ and CD8+ TILs alone were significantly associated with patient outcome in the Kaplan–Meier analysis or Cox regression analysis (Figures 3B–D). Similar results were obtained when the number of CD4+, FoxP3+ or CD8+ TILs was analysed as a continuous variable.
However, as shown in Figure 3E, compared with all the other groups high CD4+ TILs in combination with low CD8+ TILs were significantly associated with shorter PFS (median PFS 112 days; 95% CI 94.8–129.2 days vs median PFS 452 days; 95% CI 373.7–530.3 days, P<0.001) and OS (median OS 255 days; 95% CI 192.7–317.4 days vs median OS 568 days; 95% CI 525.1–610.9 days, P<0.001) in GBM. Cox regression analyses demonstrated that high CD4+ TILs in combination with low CD8+ TILs was an independent predictor of poor PFS (HR 1.618, 95% CI 1.245–2.101, P<0.001) and OS (HR 1.508, 95% CI 1.162–1.956, P=0.002, Table 3) in GBM. The correlation between combined CD4+ and CD8+ TILs and clinicopathologic features in the 90 GBMs was shown in Table 1. In contrast, the combination of FoxP3+ and CD8+ TILs was not significantly associated with PFS or OS (Figure 3F).
Table 3. Multivariate analyses of different prognostic parameters for overall survival and progress-free survival of 90 glioblastoma patients.
| |
Overall survival |
Progress-free survival |
||||
|---|---|---|---|---|---|---|
| Variable | P | HR | 95% CI | P | HR | 95% CI |
| Sex |
0.253 |
1.311 |
0.824–2.088 |
0.472 |
1.191 |
0.740–1.915 |
| Size |
0.610 |
0.998 |
0.989–1.007 |
0.727 |
1.002 |
0.993–1.011 |
| Resection |
0.600 |
1.072 |
0.827–1.388 |
0.193 |
1.194 |
0.915–1.557 |
| Preoperative KPS |
0.011 |
0.969 |
0.946–0.993 |
0.105 |
0.980 |
0.957–1.004 |
| Age |
0.033 |
1.020 |
1.002–1.038 |
0.002 |
1.030 |
1.010–1.049 |
| MGMT methylation |
<0.001 |
0.346 |
0.197–0.609 |
0.057 |
0.589 |
0.341–1.017 |
| High CD4+/low CD8+ | 0.002 | 1.508 | 1.162–1.956 | <0.001 | 1.618 | 1.245–2.101 |
Abbreviations: CI=confidence interval; HR=hazard ratio; KPS=Karnofsky performance status; MGMT=O(6)-methylguanine-DNA-methyltransferase.
Our results showed that age, preoperative KPS and MGMT promoter methylation were also independent prognostic factors for patients with GBM who received surgery and postoperative standard radiotherapy plus chemotherapy. Sex, tumour size and degree of resection were not associated with prognosis in GBM patients in this study.
Stratified analysis of TILs and prognosis
We further examined the influence of TILs on PFS and OS across strata of other potential predictors, including age, preoperative KPS and MGMT promoter methylation status. High CD4+ TILs in combination with low CD8+ TILs were associated with poor PFS and OS in all subgroups (Table 4). The effect of high CD4+ TILs in combination with low CD8+ TILs was not significantly modified by age, preoperative KPS and MGMT promoter methylation status (all P for interaction ⩾0.15).
Table 4. Stratified analysis of high CD4+ TIL Levels in combination with low CD8+ TIL levels for overall survival and progress-free survival in glioblastoma patients.
|
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|
| |
Overall survival |
Progress-free survival |
||||
| Subgroups | P | HR | 95% CI | P | HR | 95% CI |
|
MGMT promoter status | ||||||
| Methylated | 0.015 | 1.889 | 1.129–3.158 | 0.001 | 2.613 | 1.496–4.561 |
| Unmethylated |
0.008 |
1.590 |
1.127–2.243 |
0.011 |
1.591 |
1.110–2.281 |
|
Age | ||||||
| Age<47 | 0.063 | 1.389 | 0.982–1.965 | 0.027 | 1.541 | 1.049–2.262 |
| Age⩾47 |
<0.001 |
2.308 |
1.494–3.566 |
0.001 |
1.813 |
1.261–2.609 |
|
KPS | ||||||
| KPS<80 | 0.002 | 3.291 | 1.567–6.914 | 0.014 | 1.915 | 1.143–3.210 |
| KPS⩾80 | 0.043 | 1.392 | 1.011–1.918 | 0.006 | 1.599 | 1.143–2.237 |
Abbreviations: CI=confidence interval; HR=hazard ratio; KPS=Karnofsky performance status; MGMT=O(6)-methylguanine-DNA-methyltransferase.
TILs and pseudoprogression
In this series of patients, pseudoprogression was identified in 15 patients (16.7%). Among 30 patients with methylated MGMT promoter, pseudoprogression was found in 10 (33.3%). Among 60 patients with unmethylated MGMT promoter, pseudoprogression was identified in 5 (8.3% P=0.003). In 90 GBM patients (15 pseudoprogression; 75 non-pseudoprogression), the pseudoprogression patients had a significantly longer OS than the non-pseudoprogression patients (median 826 vs 439 days, P<0.001). This finding is consistent with previous studies that pseudoprogression is significantly correlated with MGMT status and effect of treatment (Brandes et al, 2008).
Moreover, among the 15 pseudoprogression patients, 1 was in low CD4+/low CD8+ group (6.7%), 3 were in low CD4+/ high CD8+ group (20.0%), 9 were in high CD4+/high CD8+ group (60.0%) and 2 were in high CD4+/low CD8+ group (13.3%). Higher rate of pseudoprogression was found in patients with high CD4+ TILs and high CD8+ TILs (P=0.007, Table 1 and Figure 2D). In addition, the percentage of high CD4+/high CD8+ cases in pseudoprogression group was significantly higher than that in non-pseudoprogression group (9 out of 15 vs 14 out of 75, P=0.002, Figure 2E). This finding indicated that immune-related factors may influence the incidence of pseudoprogression.
Discussion
Surgery and postoperative radiotherapy plus chemotherapy according to the Stupp protocol have improved the survival of patients with GBM; however, the outcome of most glioma patients remains poor (Stupp et al, 2005). Therefore, additional therapies, including immunotherapy, are currently under intensive investigation. The analysis of immunological parameters at the tumour site, especially TILs, is important for the development of successful immunotherapy (Sato et al, 2005). Although the brain is an immunologically privileged organ, lymphocytic infiltration into gliomas has been documented in large patient series (Brooks et al, 1978; Palma et al, 1978). However, in glioma, whether these TILs contribute to host immunosurveillance (Smyth et al, 2006) and patients' reaction to combined therapy, or whether they participate in tumour-specific immuno-suppression and cancer immunoediting (Dunn et al, 2002, 2004) is generally unclear. The infiltration of lymphocytes and the prognostic value of TILs may partly indicate their role in glioma tissues.
In the present study, we showed that high-grade gliomas have higher levels of CD4+ TILs than low-grade gliomas, which in combination with decreased levels of CD8+ TILs indicate a decreased CD8+/CD4+ ratio in high-grade glioma patients, consistent with the results reported by Yu et al (2003). Waziri et al (2008) reported that most of the CD4+ TILs present in glioblastoma suppress the cellular immune response. Therefore, despite an increase in the total CD4+ TIL population, the immune function of high-grade glioma patients may be compromised because of the relative low level of CD8+ CTL in the same glioma tissues.
The increase of CD4+ TILs and decrease of CD8+ TILs in high-grade glioma (Figures 1 and 2) may reflect the participation of TILs in the glioma progression. Thus, we further investigated whether TILs affect the response of glioma to radiochemotherapy. We examined the prognostic effect of CD4+ TILs and CD8+ TILs in 90 glioblastoma patients with detailed clinical information and data, who received standard therapy. Previous studies have reported that CD8+ TILs have favourable effects on the survival of patients with breast cancer (Mahmoud et al, 2011), ovarian cancer (Sato et al, 2005) and colorectal cancer (Galon et al, 2006; Pages et al, 2009). However, in the present study, we showed that the number of CD8+ TILs alone cannot effectively predict patient outcome in GBM. This could be attributed to the low level of CD8+ TILs in high-grade glioma compared with that in low-grade glioma and other tumours. Consistent with our results, Kim et al (2012) reported that CD8+ TILs were not an independent predictor in an analysis of 61 glioblastomas . The lower total number of TILs in glioma (Table 2) than in other tumours such as breast cancer (Mahmoud et al, 2011) and ovarian cancer (Sato et al, 2005) could be related to the blood–brain barrier or the specific local microenvironment, which requires further research.
The function of CD4+ TILs in human malignancies is considered a double-edged immunological sword. On one hand, CD4+ helper T cells perform critical roles in the recruitment, activation and regulation of many facets of the adaptive immune response. Without adequate CD4+ T-cell help, CD8+ T cells frequently fail to fully function in vivo (Bos and Sherman, 2010; Bos et al, 2012). CD4+ helper T cells have been associated with better survival in breast cancer (Gu-Trantien et al, 2013) and in other malignancies. On the other hand, CD4+ Tregs can dampen anti-tumour immunity and promote tumour progression (Ruffell et al, 2010; Zamarron and Chen, 2011). Tregs are unfavourable prognostic markers in patients with breast cancer (Gobert et al, 2009), hepatocellular carcinoma (Gao et al, 2007) and pancreatic cancer (Hiraoka et al, 2006). In the present study, the overall fraction of CD4+ TILs had no significant prognostic value, which could be associated with the complexity of its components.
We further examined the FoxP3+ TILs, which represents the regulatory subpopulation of CD4+ TILs. FoxP3+ TILs were only found in glioblastomas, but not in low-grade astrocytomas and oligodendroglial tumours, which is consistent with previous studies (Heimberger et al, 2008; Lohr et al, 2011). However, we did not find that the level of FoxP3+ TILs was an independent prognostic factor for survival. Our study indicates that the level of FoxP3+ TILs is low in glioma as a whole, which may influence the prognostic value of FoxP3+ TILs. Consistently, Lohr et al (2011) reported that FoxP3+ Tregs were infrequently present and not associated with GBM patient outcome.
Although different subpopulations of CD4+ TILs have different functions, the overall effect of total CD4+ TILs could be assessed in combination with CD8+ TILs. In the present study, we showed that the combination of CD4+ TILs and CD8+ TILs can predict the survival of glioma patients after combined treatment. CD8+/CD4+ TIL ratios have been associated with the prognosis of ovarian cancer patients (Sato et al, 2005). Similarly, our results showed that high CD4+ TIL levels in combination with low CD8+ TIL levels was independently associated with shorter PFS and OS in GBM. In glioma patients, high CD4+ TIL levels with low CD8+ TIL levels may indicate suppressed cellular immune responses, which may lead to failure of the current treatment strategy. Interestingly, in GBM patients, low CD4+ and high CD8+ TIL levels were also associated with unfavourable prognosis, although their survival was better than that of patients with high CD4+ and low CD8+ TILs (Figure 3E). Inadequate CD4+ helper T cells and inappropriately activated CD8+ CTLs may be responsible for this poor prognosis. Similarly, Perrin et al (1999) reported that the ultimate failure of the immune system with adequate CD8+ TILs to control high-grade glioma growth could be the consequence of a deficient CD4+ helper T-cell component of the response. These results together with our findings suggest that effective anti-tumour immunity requires appropriate CD8+/CD4+ TIL ratios in glioma, which represents a normal anti-tumour immune reaction. On the contrary, imbalance of CD4+ and CD8+ TILs may reflect or result in malfunction of anti-tumour immunity. The modulation of the CD8+/CD4+ TIL ratio could therefore be therapeutically significant, although further studies are necessary to clarify these issues, in particular the effects of the different CD4+ TIL subpopulations in GBM.
In the present study, we showed that the combination of FoxP3+ TILs and CD8+ TILs may be invalid as a prognostic marker in gliomas. For one thing, high-grade gliomas have multiple mechanisms of mediating immunosuppression other than Tregs, such as immunosuppressive cytokines, antigen loss, T-cell apoptosis and induction of anergy by tumour antigen-presenting cells (Heimberger et al, 2008). In addition, besides FoxP3+ TILs, other subtypes of CD4+ TILs may exert a powerful regulatory or suppressive influence upon CD8+ TILs. For example, Waziri et al (2008) reported that a significant proportion of TILs within GBM were CD4+CD56+ immunosuppressive T cells, whereas Tregs demonstrated only a modest proportional increase within GBM. For another thing, the low number and uneven distribution of FoxP3+ TILs in glioma tissues also limited its role as prognostic marker. Thus, the lack of a prognostic effect of FoxP3+ Tregs or the combination of FoxP3+ TILs and CD8+ TILs in this setting is logical.
Although the underlying mechanism is unclear, pseudoprogression may have a clinical impact on radiochemotherapy-treated GBM (Brandes et al, 2008). Interestingly, pseudoprogression was frequently found in patients with high CD4+ TILs and high CD8+ TILs, which indicates that immune or inflammatory factors may be involved in the formation of pseudoprogression after combined therapy. However, the case number of pseudoprogression in this research is limited, and more cases are needed to confirm this correlation. The pseudoprogression data further support our result that CD4+ TILs combined with CD8+ TILs can affect outcome of patients after combined therapy. An association has also been well established between MGMT promoter methylation and GBM patients' response to radiochemotherapy as well as patient outcome. In this study, we did not found a correlation between MGMT promoter methylation and TILs, which suggests that these two prognostic factors may independently affect patient prognosis through different pathways. Moreover, in the present study, no correlation was observed between immune infiltration and molecular classification of GBMs. Nevertheless, the number of samples in each molecular subtype was also too limited to draw any strong conclusion and further large-sample researches are required.
Conclusions
In the present study, we provide evidence that immune factors can affect the outcome of GBM patients with current standard therapies. High CD4+ TIL levels in combination with low CD8+ TIL levels were associated with unfavourable prognosis. Further studies analysing the effects of different subpopulations of TILs and the relationship between pseudoprogression and TILs in glioma may help elucidate the exact mechanisms by which TILs affect the prognosis of glioma.
Acknowledgments
We thank Jingpu Shi at the Department of Clinical Epidemiology, The First Affiliated Hospital of China Medical University for superb technical assistance with statistical and epidemiological analyses. This work was supported by grants from the National High Technology Research and Development Program of China (863) (No. 2012AA02A508), National Natural Science Foundation of China (No. 81172409) and Science and Technology Department of Liaoning Province (No. 2011225034).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Bos R, Marquardt KL, Cheung J, Sherman LA. Functional differences between low- and high-affinity CD8(+) T cells in the tumor environment. Oncoimmunology. 2012;1:1239–1247. doi: 10.4161/onci.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633–638. doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- Brooks WH, Markesbery WR, Gupta GD, Roszman TL. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol. 1978;4:219–224. doi: 10.1002/ana.410040305. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8:234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Menetrier-Caux C. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le BH, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothe F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Xia J, Qin X, Han S, Wu A. Phosphorylated SATB1 is associated with the progression and prognosis of glioma. Cell Death Dis. 2013;4:e901. doi: 10.1038/cddis.2013.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, Hiraoka N, Fuller GN. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- Ho WY, Yee C, Greenberg PD. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J Clin Invest. 2002;110:1415–1417. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Jung TY, Jung S, Jang WY, Moon KS, Kim IY, Lee MC, Lee JJ. Tumour-infiltrating T-cell subpopulations in glioblastomas. Br J Neurosurg. 2012;26:21–27. doi: 10.3109/02688697.2011.584986. [DOI] [PubMed] [Google Scholar]
- Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, Korff T, von DA, Unterberg A, Beckhove P, Herold-Mende C. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- Palma L, Di LN, Guidetti B. Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas. Incidence, fate, and relevance to prognosis in 228 operated cases. J Neurosurg. 1978;49:854–861. doi: 10.3171/jns.1978.49.6.0854. [DOI] [PubMed] [Google Scholar]
- Perrin G, Schnuriger V, Quiquerez AL, Saas P, Pannetier C, de Tribolet N, Tiercy JM, Aubry JP, Dietrich PY, Walker PR. Astrocytoma infiltrating lymphocytes include major T cell clonal expansions confined to the CD8 subset. Int Immunol. 1999;11:1337–1350. doi: 10.1093/intimm/11.8.1337. [DOI] [PubMed] [Google Scholar]
- Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- von HRI, Hofman FM, Taylor CR, Apuzzo ML. Mononuclear lymphoid populations infiltrating the microenvironment of primary CNS tumors. Characterization of cell subsets with monoclonal antibodies. J Neurosurg. 1984;60:1138–1147. doi: 10.3171/jns.1984.60.6.1138. [DOI] [PubMed] [Google Scholar]
- Waziri A, Killory B, Ogden AT, 3rd, Canoll P, Anderson RC, Kent SC, Anderson DE, Bruce JN. Preferential in situ CD4+CD56+ T cell activation and expansion within human glioblastoma. J Immunol. 2008;180:7673–7680. doi: 10.4049/jimmunol.180.11.7673. [DOI] [PubMed] [Google Scholar]
- Yu JS, Lee PK, Ehtesham M, Samoto K, Black KL, Wheeler CJ. Intratumoral T cell subset ratios and Fas ligand expression on brain tumor endothelium. J Neurooncol. 2003;64:55–61. doi: 10.1007/BF02700020. [DOI] [PubMed] [Google Scholar]
- Yue Q, Zhang X, Ye HX, Wang Y, Du ZG, Yao Y, Mao Y. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J Neurooncol. 2013;116 (2:251–259. doi: 10.1007/s11060-013-1314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.