Abstract
Background:
Transforming growth factor-beta (TGF-β) induces the epithelial-to-mesenchymal transition (EMT) leading to increased cell plasticity at the onset of cancer cell invasion and metastasis. Mechanisms involved in TGF-β-mediated EMT and cell motility are unclear. Recent studies showed that p53 affects TGF-β/SMAD3-mediated signalling, cell migration, and tumorigenesis. We previously demonstrated that Nox4, a Nox family NADPH oxidase, is a TGF-β/SMAD3-inducible source of reactive oxygen species (ROS) affecting cell migration and fibronectin expression, an EMT marker, in normal and metastatic breast epithelial cells. Our present study investigates the involvement of p53 in TGF-β-regulated Nox4 expression and cell migration.
Methods:
We investigated the effect of wild-type p53 (WT-p53) and mutant p53 proteins on TGF-β-regulated Nox4 expression and cell migration. Nox4 mRNA and protein, ROS production, cell migration, and focal adhesion kinase (FAK) activation were examined in three different cell models based on their p53 mutational status. H1299, a p53-null lung epithelial cell line, was used for heterologous expression of WT-p53 or mutant p53. In contrast, functional studies using siRNA-mediated knockdown of endogenous p53 were conducted in MDA-MB-231 metastatic breast epithelial cells that express p53-R280K and MCF-10A normal breast cells that have WT-p53.
Results:
We found that WT-p53 is a potent suppressor of TGF-β-induced Nox4, ROS production, and cell migration in p53-null lung epithelial (H1299) cells. In contrast, tumour-associated mutant p53 proteins (R175H or R280K) caused enhanced Nox4 expression and cell migration in both TGF-β-dependent and TGF-β-independent pathways. Moreover, knockdown of endogenous mutant p53 (R280K) in TGF-β-treated MDA-MB-231 metastatic breast epithelial cells resulted in decreased Nox4 protein and reduced phosphorylation of FAK, a key regulator of cell motility. Expression of WT-p53 or dominant-negative Nox4 decreased TGF-β-mediated FAK phosphorylation, whereas mutant p53 (R280K) increased phospho-FAK. Furthermore, knockdown of WT-p53 in MCF-10A normal breast epithelial cells increased basal Nox4 expression, whereas p53-R280K could override endogenous WT-p53 repression of Nox4. Remarkably, immunofluorescence analysis revealed MCF-10A cells expressing p53-R280K mutant showed an upregulation of Nox4 in both confluent and migrating cells.
Conclusions:
Collectively, our findings define novel opposing functions for WT-p53 and mutant p53 proteins in regulating Nox4-dependent signalling in TGF-β-mediated cell motility.
Keywords: NADPH oxidase, reactive oxygen species (ROS), cell migration, transforming growth factor-beta (TGF-β), epithelial-to-mesenchymal transition (EMT), mutant p53
Transforming growth factor-beta (TGF-β) is a pluripotent cytokine with dual tumour-suppressive and tumour-promoting effects. During tumour progression, transformed cells become unresponsive to the tumour-suppressive effects of TGF-β and respond with tumour-promoting effects by undergoing changes in morphology leading to increasing cell mobility, invasion, and metastasis (Xu et al, 2009; De Craene and Berx, 2013). Transforming growth factor-β has been considered a master regulator of the epithelial-to-mesenchymal transition (EMT). Transforming growth factor-β-induced transcriptional changes convert adherent polarised epithelial cells to a migratory mesenchymal phenotype associated with organ development as well as tumour progression (Valcourt et al, 2005; Tian et al, 2011). Interestingly, recent reports indicate that wild-type P53 (WT-p53) and mutant p53 proteins can cooperate with TGF-β signalling to co-regulate genes involved in both TGF-β tumour-suppressive and tumour promoter pathways, respectively (Dupont et al, 2004; Adorno et al, 2009; Termen et al, 2013).
The tumour suppressor p53 acts primarily as a transcription factor responsible for inducing target genes that are responsible for apoptosis. p53 is the most commonly mutated gene in many cancers with over 50% of tumours expressing an inactive tumour suppressor protein. These mutations reside primarily within the DNA-binding domain that may produce a dominant-negative effect or an acquired tumour-promoting gain of function (Vousden and Prives, 2009). Recently, Cordenonsi et al (2003) demonstrated that WT-p53 can synergise with TGF-β-activated SMAD2/3 to promote cell cycle arrest and apoptosis. In contrast, Adorno et al (2009) demonstrated how mutated p53 affects the switch between TGF-β acting as a tumour suppressor to a tumour promoter whereby mutant p53/SMAD complexes work together to promote tumour cell migration and metastasis.
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that has an important role in linking integrin receptors to intracellular signalling pathways involved in cell adhesion, migration, and invasion (Zhao and Guan, 2009). Focal adhesion kinase is the primary link between extracellular matrix-activated integrin receptors and intracellular signalling pathways involved in transcriptional up-regulation of mesenchymal and invasive markers (Thannickal et al, 2003). Previous studies have shown that TGF-β-induced activation of FAK is essential for EMT progression and tumour metastasis (Luo and Guan, 2010). Interestingly, recent studies have demonstrated that TGF-β as well as mutant p53 expression can enhance FAK promoter activation, mRNA, and protein levels (Cicchini et al, 2008; Walsh et al, 2008). Moreover, FAK overexpression and p53 mutations are highly correlated in human breast cancer (Golubovskaya et al, 2009).
Transforming growth factor-β induces cellular reactive oxygen species (ROS) in many cell types. Increased ROS have been primarily associated with cytotoxicity and apoptosis; however, studies have revealed the importance of ROS as regulators of signalling pathways and gene transcription involved in EMT progression, cell migration, and metastasis (Cannito et al, 2010). Reactive oxygen species are signalling mediators acting through multiple mechanisms including cysteine oxidation and inhibition of protein tyrosine phosphatases, activation of redox-sensitive transcription factors (NFκ-B, p53, and AP-1), and oxidation of cytoskeletal proteins involved in cell motility (actin, cofilin, and vimentin) (Allen and Tresini, 2000; Fratelli et al, 2002; Chiarugi and Cirri, 2003; Kwon et al, 2004; Boivin et al, 2010; Frijhoff et al, 2013; Leoni et al, 2013). Previous studies have reported a correlation between increased ROS production and tumour progression (Wu, 2006). Intracellular ROS-generating sources include NADPH oxidase enzymes (Nox family), mitochondria, xanthine oxidase, and nitric oxide synthase (Thannickal and Fanburg, 2000).
The ROS-generating enzymes in the Nox family (Nox1–5 and Duox1–2) are critical mediators of redox signalling. Previous studies have indicated that NADPH-oxidase-dependent ROS production can alter cell motility or potentiate metastatic progression (Schroder et al, 2007; Sadok et al, 2008, 2009; Shinohara et al, 2010; Leoni et al, 2013). Of the seven Nox enzymes, Nox4 is unique in being induced by TGF-β. Transforming growth factor–β-induced Nox4 has been implicated in osteoblast differentiation, fibroblast proliferation, endothelial cell cytoskeletal rearrangement, cell motility, EMT, pulmonary fibrosis, and hepatitis C virus-induced hepatocyte oxidative stress (Hu et al, 2005; Li et al, 2008; Boudreau et al, 2009; Amara et al, 2010; Carnesecchi et al, 2011; Mandal et al, 2011; Boudreau et al, 2012). Nox4 is a 578 amino acid, six transmembrane domain flavocytochrome that functions by transporting electrons from cytosolic NADPH across biological membranes to oxygen producing ROS. Geiszt et al (2000) first described Nox4 in the kidney, but Nox4 mRNA and protein expression have been detected in other human and murine tissues including bone, vascular tissue, heart, liver, and lung (Cheng et al, 2001; Hilenski et al, 2004; Yang et al, 2004; Sturrock et al, 2006, 2007; Kuroda et al, 2010). Although TGF-β is a regulator of Nox4 in many tissues susceptible to fibrosis and tumorigenesis, little is known about the mechanisms involved.
Previously, we reported Nox4 as the primary source of TGF-β-SMAD3-induced ROS in normal and metastatic breast epithelial cells (Boudreau et al, 2012). We demonstrated how Nox4 functions as a critical mediator of EMT-related events including cell mobility and fibronectin gene regulation. Here we demonstrate that WT-p53 and mutant p53 can differentially regulate Nox4 expression and activity. We found that WT-p53 suppresses while cancer-associated mutant p53 (R175H and R280K) proteins enhance Nox4 expression in both TGF-β-dependent and independent processes. Moreover, we show that mutant p53 and Nox4 are necessary for TGF-β-mediated FAK activation and migration of human breast and lung epithelial cells.
Materials and methods
Cell culture
Human lung epithelial H1299 (p53-null), immortalised human breast epithelial MCF-10A (p53-WT), and human metastatic breast epithelial MDA-MB-231 (p53-R280K) cell lines were obtained from the ATCC (Rockville, MD, USA). H1299 cells were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (HyClone/Thermo Scientific, Logan, UT, USA) and 100 mg ml−1 of penicillin–streptomycin. MCF-10A cells were maintained in Dulbecco's minimal essential medium/F12 (DMEM/F12; Invitrogen) supplemented with 5% horse serum (Sigma, St Louis, MO, USA), 500 ng ml−1 hydrocortisone (Sigma), 20 ng ml−1 EGF (R&D Systems, Minneapolis, MN, USA), 100 ng ml−1 cholera toxin (Sigma), 10 μg ml−1 insulin (Sigma), and 100 mg ml−1 of penicillin–streptomycin (Invitrogen). MDA-MB-231 cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum and 100 mg ml−1 of penicillin–streptomycin. All cells were grown in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. In some experiments, cell cultures were treated with 10 μM 616451 (EMD Millipore, Billerica, MA, USA), a TGF-β receptor I-specific inhibitor, or 10 μM SIS3 (Sigma), a SMAD3-specific inhibitor.
Plasmids
Vectors encoding full-length human Nox4 (GenBank accession number NM_016931) and truncated, dominant-negative Nox4 (Nox4-DN; residues 1–305) were generated using cDNA subcloned into pcDNA3.1. pCMV-p53-R175H and p53-R280K were generated from pCMV-p53-WT expression vector (Clontech, Mountain View, CA, USA) by site-directed mutagenesis. All constructs were verified by automated DNA sequencing (Macrogen, Rockville, MD, USA).
Transient transfections
H1299 and MCF-10A cells were transfected with Fugene 6 (Roche, Indianapolis, IN, USA) using manufacturer's protocols. Briefly, 2.5 × 105 cells were seeded in six-well tissue culture dishes (BD Biosciences, San Jose, CA, USA) 24 h before transfection. Transfection mixtures were incubated at RT for 20 min in serum-free RPMI-1640 (H1299) or DMEM/F12 (MCF-10A) and then added dropwise to cells. MDA-MB-231 cells were transfected with Lipofectamine 2000 (Invitrogen) according to manufacturer's protocols. Briefly, 2 × 105 cells were seeded on six-well plates 24 h before transfection. The transfection mixtures were incubated at RT for 20 min in serum-free DMEM and added dropwise to cells containing serum-free medium. After 4 h, the medium was changed to DMEM containing serum.
siRNA-mediated gene knockdown
To knockdown endogenous p53 in MDA-MB-231 and MCF-10A cells, we used Dharmacon ON-TARGETplus SMARTpool siRNA TP53 (Dharmacon, Lafayette, CO, USA; L-003329-00-0005) or non-targeting control siRNA (D-001810-01-05; Dharmacon). DharmaFECT4 transfection reagent was used to transfect siRNAs according to manufacturer's protocols. The cells were transfected at a density of 1 × 104 cells per millilitre with a siRNA concentration of 50 nM and incubated in antibiotic-free complete medium for 72 h before harvesting. Nox4 siRNA was also purchased from Dharmacon (ON-TARGETplus SMARTpool siRNA Nox4 (L-010194-00-0010)) and transfected as described above.
ROS detection
Kinetic ROS detection measurements were performed by chemiluminescence in 96-well plates at 37 °C over a 45-min time course using a Luminoskan luminometer (Dharmacon) as previously described (Boudreau et al, 2009). Briefly, ∼2.5 × 104 cells were collected by trypsinisation and washed twice with Hank's balanced salt solution buffer (Invitrogen) by centrifugation. SOD3-sensitive superoxide production was measured using Diogenes reagent (National Diagnostics, Atlanta, GA, USA). Extracellular H2O2 was measured by a luminol/HRP-based chemiluminescence assay.
RNA isolation and cDNA synthesis
Total RNA from H1299, MDA-MB-231, or MCF-10A or cells was extracted from cells with Trizol (Invitrogen). One microgram of total RNA was used for Thermoscript RT-PCR. Both were conducted according to manufacturer's protocols (Invitrogen).
Quantitative real-time PCR
Gene expression was quantified by real-time PCR using an ABI Prism 7500 RT-PCR System (Applied Biosystems, Foster City, CA, USA). One microgram of total cellular RNA was reverse transcribed with ThermoScript RT-PCR kit (Invitrogen). SYBR Green PCR mix (Invitrogen) was used to detect mRNA expression. Primers designed to detect human Nox4, Fibronectin, and GAPDH were used as previously described (Boudreau et al, 2012). Results are described as relative quantification 2−(−ΔΔCt). GAPDH was used as an internal control for normalisation.
Antibodies and immunoblotting analysis
Total cell lysates were processed for western blotting as previously described and probed with the following antibodies: rabbit monoclonal anti-Nox4 (UOTR1B493) (Abcam, Cambridge, MA, USA); mouse monoclonal anti-p53 (clone DO-1; Santa Cruz Biotechnology, Dallas, TX, USA); rabbit monoclonal anti-phospho-SMAD3 (clone EP823Y; Abcam); rabbit polyclonal anti-phospho-SMAD2 (no. 3101; Cell Signaling, Beverly, MA, USA); rabbit monoclonal anti-SMAD3 (clone EP568Y; Abcam); mouse monoclonal anti-SMAD2 (no. L16D3; Cell Signaling); rabbit monoclonal anti-phospho-FAK (Y576; Invitrogen); rabbit polyclonal anti-FAK (no. 3285; Cell Signaling); (rabbit polyclonal anti-GAPDH (Trevigen); and mouse monoclonal anti-V5 (Invitrogen) antibodies.
Matrigel assay
Briefly, cells were seeded and transfected with plasmid DNA or siRNA as described above, then trypsinised and washed 24 h (plasmid DNA) or 48 h (siRNA) later. Approximately, 1.5 × 105 transfected cells were seeded in upper chambers of six-well BD Biocoat Matrigel transwell culture plates (BD Biosciences) and incubated with lower chambers containing complete medium with TGF-β (5 or 10 ng ml−1). After 24 h, non-migrating cells were scraped away and migrating cells were stained with Diff Stain (IMEB, San Marcos, CA, USA). Invading cells were counted from 10 random fields. Matrigel experiments were repeated three times.
Immunostaining
MDA-MB-231 or MCF-10A cells were seeded 3.0 × 104 per chamber of a Lab-Tek no. 1.5 borosilicate eight-chamber coverglass (Thermo Fisher Scientific, Rockville, MD, USA) 24 h before transfection. Cells were transfected with GFP to mark transfected cells in addition to Nox4-DN totalling 0.5 μg DNA per chamber. Chambers were treated with 10 ng ml−1 TGF-β 24 h post transfection for an additional 24 h. Cells were then fixed in 4% paraformaldehyde, permeabilised with 0.2% Triton X-100 in TBST, and blocked overnight at 4 °C in TBST supplemented with 5% BSA and 5% normal goat serum. After blocking, cells were incubated either with rabbit anti-pY576 FAK antibody (1 : 2000), rabbit monoclonal anti-Nox4 (1 : 1000), or mouse monoclonal anti-p53 (1 : 5000) for 1 h, washed and subsequently incubated with goat anti-rabbit Alexa Fluor conjugates (1 : 200). Nuclei were counterstained with DAPI (Life Technologies – Molecular Probes, Grand Island, NY, USA) for 5 min. Images were collected on a Zeiss LSM 780 confocal laser scanning fluorescence microscope using Zen 2010 software (Carl Zeiss Microscopy, Thornwood, NY, USA).
Statistical analysis
Data are represented as the means±s.d. of the results of at least three independent experiments. Student's t-test was used to calculate significant values. Significant values are indicated as *P-value<0.05, **P-value<0.01, or ***P-value<0.001.
Results
Wild-type p53 expression suppresses TGF-β-induced Nox4 in H1299 human lung epithelial cells
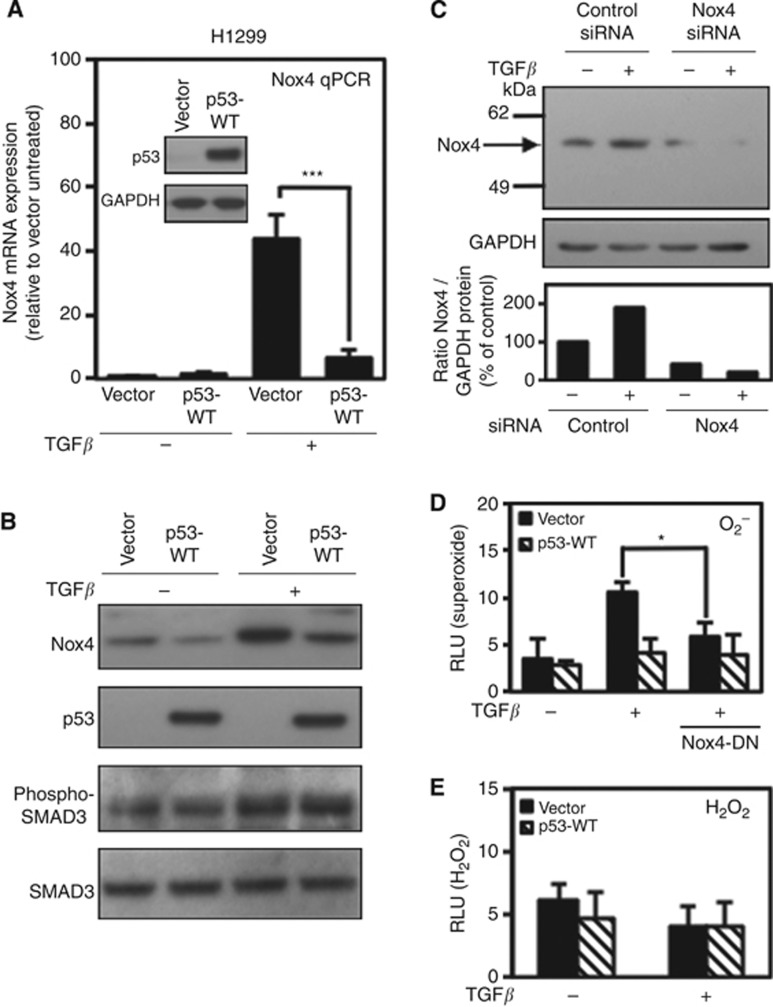
To investigate whether TGF-β-mediated Nox4 expression was subject to transcriptional regulation by p53, we transfected p53-null H1299 lung epithelial cells with WT-p53 followed by TGF-β treatment for 24 h. We found that WT-p53 expression inhibited the induction of Nox4 mRNA by TGF-β (Figure 1A). Similarly, Nox4 protein levels were suppressed in cells transfected with WT-p53 either in the absence or in the presence of TGF-β (Figure 1B). The overexpression of WT-p53 did not induce cell death or have an affect on the activation of the TGF-β/SMAD3 pathway as measured by SMAD3 phosphorylation. We used Nox4-specific siRNAs to knockdown endogenous Nox4 to evaluate the specificity of the Nox4 antibody. Using this approach, we detected a single protein band ∼59 kDa that was specifically reduced following Nox4 siRNA treatment in either the presence or the absence of TGF-β (Figure 1C).
Figure 1.
Wild-type p53 (WT-p53) suppresses TGF-β-induced Nox4 in p53-null H1299 lung epithelial cells. (A) H1299 cells were transfected with vector alone or WT-p53 cDNA. Twenty-four hours after transfection, cells were treated with TGF-β (5 ng ml−1) for 24 h. Human Nox4- and GAPDH-specific primers were used for PCR amplification of total cDNA reverse transcribed from cells (n=3). Results are described as relative quantification of Nox4 mRNA relative to vector untreated expression using GAPDH as an internal control for normalisation. Inset shows immunoblot analysis of p53 protein expression in H1299 cells transfected with vector alone or p53-WT plasmids. (B) Nox4 protein is downregulated by p53-WT expression. H1299 cells were transfected and treated as described in A. Forty micrograms of total cell lysate were analysed by western blotting. The immunoblot was probed sequentially with antibodies against Nox4, p53, phospho-SMAD3, and total SMAD3. (C) H1299 cells were transfected with non-targeting control or SMARTpool Nox4-specific siRNAs (50 nM) for 72 h, and either left untreated or treated with TGF-β (5 ng ml−1) for an additional 24 h. Nox4 protein expression was analysed by western blotting. Immunoblots were probed with anti-Nox4 followed by anti-GAPDH antibodies. (D) H1299 cells were transfected with vector alone or p53-WT or co-transfected with dominant-negative Nox4 (Nox4-DN) cDNA. Twenty-four hours after transfection, cells were treated with TGF-β (5 ng ml−1) for 24 h. Cells were collected and assayed for superoxide production with superoxide-specific Diogenes reagent for 1 h (n=3, in triplicate). (E) H1299 cells were transfected with either vector control or p53-WT plasmids and treated with TGF-β as in D and collected and assayed for H2O2 production with luminol/HRP (n=3, in triplicate). Significance values are indicated as *P-value<0.05, or ***P-value<0.001.
Next, we found that transfection of WT-p53 also suppressed TGF-β-induced oxidase activity. To qualify the source of ROS induced by TGF-β, H1299 cells were transfected with a dominant-negative form of Nox4 (Nox4-DN). The Nox4-DN lacks the C-terminal FAD and NADPH-binding domains required for enzymatic activity. We and others have shown that overexpressing Nox4-DN in different cell types significantly inhibits endogenous Nox4 oxidase activity (Mahadev et al, 2004; Boudreau et al, 2009; Mandal et al, 2011; Boudreau et al, 2012; New et al, 2012). We found that the Nox4-DN significantly reduced TGF-β-stimulated extracellular superoxide production (∼two-fold less vs vector treated) observed in the absence of WT-p53. Overexpression of WT-p53 also inhibited TGF-β-mediated superoxide production (Figure 1D). Interestingly, H2O2 was unaffected by TGF-β treatment or WT-p53 expression, indicating that the Nox4-mediated extracellular superoxide detected by this assay occurs at the plasma membrane and is a relatively small component of total cellular ROS (Figure 1E). We also found that increasing amounts of transfected WT-p53 expression alone had a dose-dependent suppressive effect on Nox4 protein expression (data not shown). These results indicate that expression of WT-p53 has a repressive effect on TGF-β-dependent and TGF-β-independent Nox4 protein levels.
Overexpression of mutant p53 supports TGF-β induction of Nox4 in human lung epithelial cells
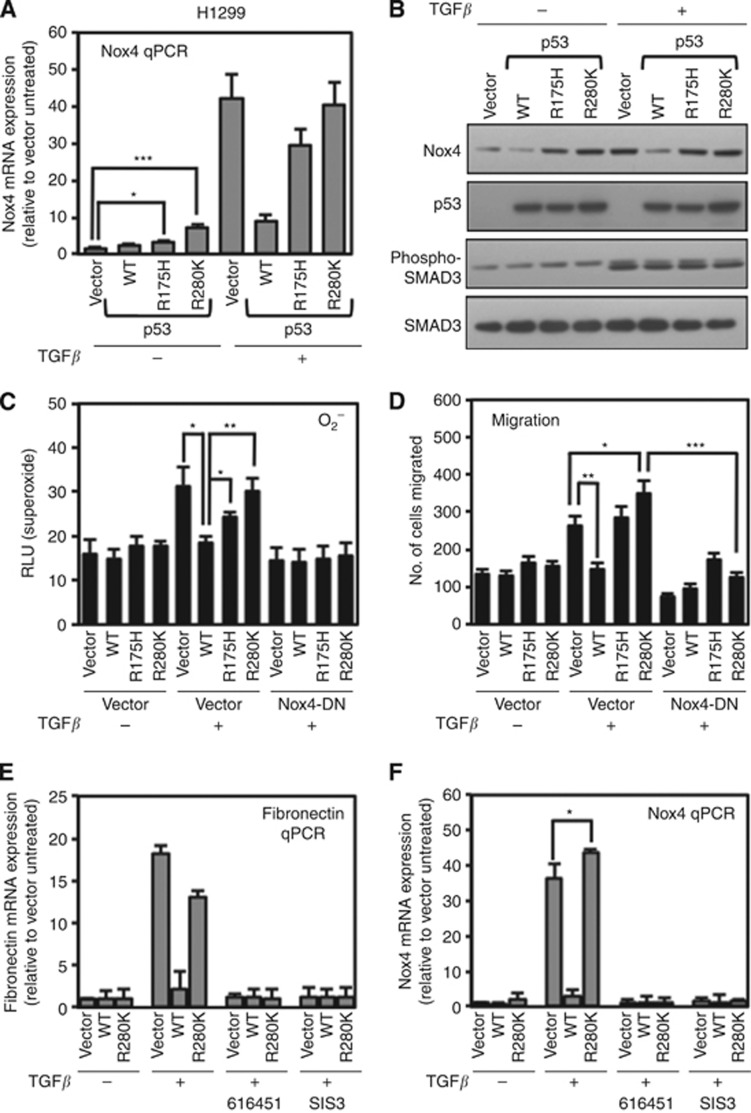
The correlation between aberrant p53 and TGF-β signalling associated with increased migration and metastasis in many cancers prompted us to evaluate the effects of mutant p53 on TGF-β induction of Nox4. To do this, we generated two different mutant p53 proteins p53-R175H and p53-R280K. p53-R175H is commonly found in p53-associated tumours, whereas R280K is endogenous to the human breast epithelial cell line MDA-MB-231, a widely-used breast cancer cell model. These missense mutations are within the p53 DNA-binding domain, considered as a ‘hot spot' for cancer-associated TP53 mutations (Strano et al, 2007). First, we investigated Nox4 mRNA expression in H1299 p53-null human lung epithelial cells transfected with p53-WT, p53-R175H, p53-R280K, or control vectors with or without TGF-β treatment for 24 h. Consistently, control cell treatment with TGF-β resulted in a robust increase in Nox4 mRNA. Nox4 mRNA was also upregulated in cells expressing mutant p53, either in the absence (p53-R175H, ∼2-fold; p53-R280K, ∼10-fold) or in the presence of TGF-β stimulation (p53-R175H, ∼30-fold; p53-R280K, ∼40-fold) (Figure 2A). In contrast, TGF-β induction of Nox4 was significantly reduced in the WT-p53-transfected cells compared with the control and p53-R175H- or p53-R280K-transfected cells. Interestingly, the p53 mutant proteins caused enhanced Nox4 protein levels either in the absence or in the presence of TGF-β, indicating a TGF-β-independent positive regulation of Nox4, whereas WT-p53 retained its repressive effect on TGF-β-induced Nox4 protein (Figure 2B). The magnitude of Nox4 protein induction observed after 24 h of TGF-β treatment was limited in comparison with Nox4 mRNA changes, suggesting significant differences in Nox4 mRNA and protein synthesis and turnover rates, consistent with reported Nox4 responses to other inducers that differentially affect Nox4 mRNA and protein levels (Peshavariya et al, 2009b). We found that TGF-β-induced SMAD3 phosphorylation was not affected by the expression of either WT-p53 or mutant p53. To determine the effect of p53 on TGF-β and Nox4-dependent superoxide generation, we co-transfected H1299 cells with Nox4-DN or control vector along with WT-p53 or mutant p53. Both p53-R175H and p53-R280K caused increased Nox4-dependent oxidase activity (i.e., sensitive to Nox4-DN) in cells treated with TGF-β (Figure 2C). In contrast, WT-p53 expression diminished TGF-β-mediated Nox4 oxidase activity comparable to cells that were untreated or cells that expressed inactive Nox4-DN.
Figure 2.
Mutant p53 proteins support TGF-β-induced Nox4 and cell migration. (A) H1299 cells were transfected with vector control plasmid, wild-type p53 (WT-p53), p53-R175H mutant, or p53-R280K mutant plasmids. Twenty-four hours post transfection, the cells were treated with TGF-β (5 ng ml−1) or left untreated for an additional 24 h. Nox4-specific primers were used for quantitative real-time PCR. Nox4- and GAPDH-specific primers were used for quantitative PCR (n=3). Results are described as relative quantification of Nox4 mRNA relative to vector untreated control. (B) Nox4 protein is differentially regulated by WT and mutant p53 expression. H1299 cells were transfected and treated as described in A. Forty micrograms of total cell lysate were analysed by western blotting. The blot was probed sequentially with antibodies against Nox4, p53, phospho-SMAD3, and total SMAD3. (C) H1299 cells were transfected with vector alone, p53-WT, p53-R175H, or p53-R280K, and co-transfected with either vector control or dominant-negative Nox4 (Nox4-DN) plasmids. Twenty-four hours after transfection, cells were treated with TGF-β (5 ng ml−1) for 24 h. Cells were then collected and assayed for superoxide generation (n=3, in triplicate). (D) H1299 cells were transfected as in C for 24 h. The cells were then re-seeded in the upper chamber of a Matrigel transwell and incubated in the lower chamber containing RPMI-1640 medium containing TGF-β 5 ng ml−1. After 24 h, the migrating cells were fixed, stained, and counted from 10 random fields (n=3). (E, F) H1299 cells were transfected with vector control, p53-WT, or p53-R280K. After 24 h, the cells were treated with 616451 (10 μM), a TGF-β receptor I-specific inhibitor or SIS3 (10 μM), a SMAD3-specific inhibitor for 4 h before treating with TGF-β for 20 h. Fibronectin-(E) or Nox4 (F)-specific primers were used for real-time quantitative PCR amplification of total cDNA reverse transcribed from cells. Results are described as relative quantification relative to vector untreated control. GAPDH-specific primers were used as an internal control (n=3, in triplicate). Significant values are indicated as *P-value<0.05, **P-value<0.01, or ***P-value<0.001.
Several reports suggest that some aberrant p53 proteins drive cell migration and tumour metastasis (Adorno et al, 2009; Muller et al, 2011). Therefore, we used the Matrigel migration assay to measure the influence of p53 on TGF-β-stimulated, Nox4-dependent cell migration. H1299 cells transfected with WT-p53 inhibited TGF-β-induced cell migration (Figure 2D). Although the p53-R175H mutant overexpressing cells responded to TGF-β simulation similar to control, cells expressing p53-R280K responded with a significant increase over vector treated cells (vector/TGF-β vs p53-R280K/TGF-β, P<0.05). When co-transfected with Nox4-DN, cells failed to migrate in response to TGF-β stimulation, further supporting a critical role for Nox4 in human epithelial cell migration.
Previously, we demonstrated Nox4-mediated regulation of fibronectin mRNA in normal and metastatic breast epithelial cells undergoing TGF-β-stimulated EMT (Boudreau et al, 2012). Nox4 has also been implicated in fibronectin expression in the diabetic kidney and lung epithelial cells (Gorin et al, 2005; Hecker et al, 2009). Fibronectin is a well-known TGF-β/SMAD3 target gene upregulated in cells during EMT (Maschler et al, 2005; Chen et al, 2013). In a previous report, WT-p53 was shown to transcriptionally repress fibronectin expression in Hela cells (Iotsova and Stehelin, 1996). To further substantiate p53 in TGF-β signalling and EMT gene expression, we examined the effect of WT and mutant p53 on TGF-β-induced fibronectin expression in H1299 cells. We observed that WT-p53 repressed TGF-β induction of fibronectin mRNA compared with empty vector or p53-R280K-transfected cells (Figure 2E). Treatment with the TGF-β receptor-1-specific pharmacological inhibitor (616451) or SMAD3-specific inhibitor (SIS3) abolished TGF-β-driven fibronectin and Nox4 mRNAs in vector and p53-R280K-treated cells, indicating a TGF-β/SMAD3-dependent mechanism (Figure 2F). Together, these data imply that mutant forms of p53 (R175H and R280K) support higher TGF-β-stimulated Nox4 mRNA and protein expression, subsequent oxidase activity as well as cell mobility, whereas WT-p53 suppresses all of these effects. Moreover, TGF-β and mutant p53-mediated Nox4 and fibronectin mRNAs are upregulated in a SMAD3-dependent manner.
Endogenous mutant p53 (R280K) is a regulator of TGF-β-induced Nox4 in MDA-MB-231 metastatic breast epithelial cells
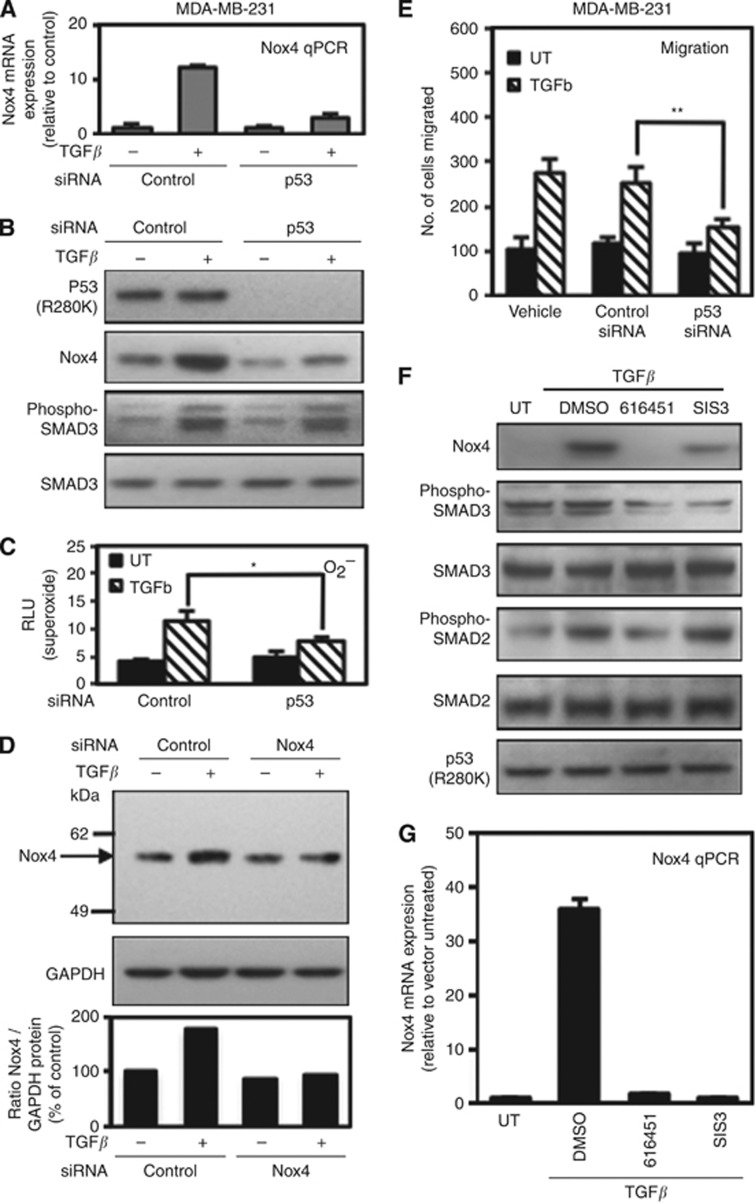
We reported that TGF-β-dependent migration of MDA-MB-231 breast epithelial cells involves enhanced Nox4 expression and oxidase activity (Boudreau et al, 2012). MDA-MB-231 is an aggressive metastatic cell line in which p53-R280K is endogenously expressed. Therefore, we wanted to confirm our findings of Nox4 induction by exogenous p53-R280K in cells expressing endogenous p53-R280K. Depletion of p53-R280K by p53-specific siRNAs substantially reduced both TGF-β-induced Nox4 mRNA and Nox4 protein expression (Figure 3A and B) and impaired Nox4-dependent superoxide production (Figure 3C). In agreement with endogenous Nox4 protein in H1299 lung epithelial cells, MDA-MB-231 breast epithelial cells also express an ∼59-kDa Nox4 protein that is induced by TGF-β and suppressed by Nox4-targeted siRNA (Figure 3D).
Figure 3.
Endogenous mutant p53 (R280K) supports TGF-β/SMAD-dependent Nox4 induction in MDA-MB-231 breast epithelial cells. (A) MDA-MB-231 cells were transfected with control siRNAs (50 nM) or p53-specific siRNAs (50 nM) for 72 h then simulated with TGF-β (5 ng ml−1) for 24 h. Real-time quantitative PCR analysis of Nox4 mRNA expression was determined from MDA-MB-231 cells treated as in A (n=3, in triplicate). (B) MDA-MB-231 cells were treated as in A followed by protein expression analysis by immunoblotting 40 μg of total cell lysate. The blot was sequentially probed with antibodies against Nox4, p53, phospho-SMAD3, and total SMAD3. (C) MDA-MB-231 cells were transfected with non-targeting control (50 nM) or SMARTpool Nox4-specific siRNAs (50 nM) for 72 h and either left untreated or treated with TGF-β (5 ng ml−1) for an additional 24 h. Nox4 protein expression was analysed by western blotting. Immunoblots were probed with anti-Nox4 followed by anti-GAPDH. (D) MDA-MB-231 cells were treated as described and assayed for superoxide production with superoxide-specific Diogenes reagent for 1 h (n=3, in triplicate). (E) MDA-MB-231 cells were treated with transfection reagent alone or transfected with control or p53-specific siRNAs. After 72 h, the cells were re-seeded in the upper chamber of a Matrigel transwell and incubated in the lower chamber containing RPMI-1640 medium containing TGF-β 5 ng ml−1 for 24 h. The migrating cells were counted from 10 random fields (n=3). (F) MDA-MB-231 cells were left untreated or treated with DMSO (vehicle), 616451 (10 μM), or SIS3 (10 μM) for 4 h before the addition of TGF-β (5 ng ml−1) for 24 h. Protein expression was analysed by immunoblotting 40 μg of total cell lysate and sequentially probed with the indicated antibodies. (G) Total RNA extracted from cells treated as in F was reverse transcribed for real-time quantitative PCR analysis of Nox4 mRNA expression. Results are described as relative quantification of Nox4 mRNA relative to untreated control (n=3, in triplicate). Significant values are indicated as *P-value<0.05, or **P-value<0.01.
Previously, we demonstrated that MDA-MB-231 cells expressing a Nox4-DN show reduced TGF-β-induced cell migration. In conjunction with our previous findings, substantially fewer MDA-MB-231 cells depleted of p53-R280K were migrated when stimulated with TGF-β (Figure 3E). To further confirm that Nox4 is modulated in a TGF-β/SMAD3-specific manner, we treated MDA-MB-231 with either TGFBR1- (616451) or SMAD3 (SIS3)-specific inhibitors, or vehicle (DMSO) followed by TGF-β stimulation. Inhibition of TGFBR1 or SMAD3 significantly reduced Nox4 protein and mRNA expression (Figure 3F and G). Collectively, these data indicate that endogenous mutant p53 (R280K) contributes to TGF-β-mediated Nox4 expression, oxidase activity, and cell motility in human breast cancer cells.
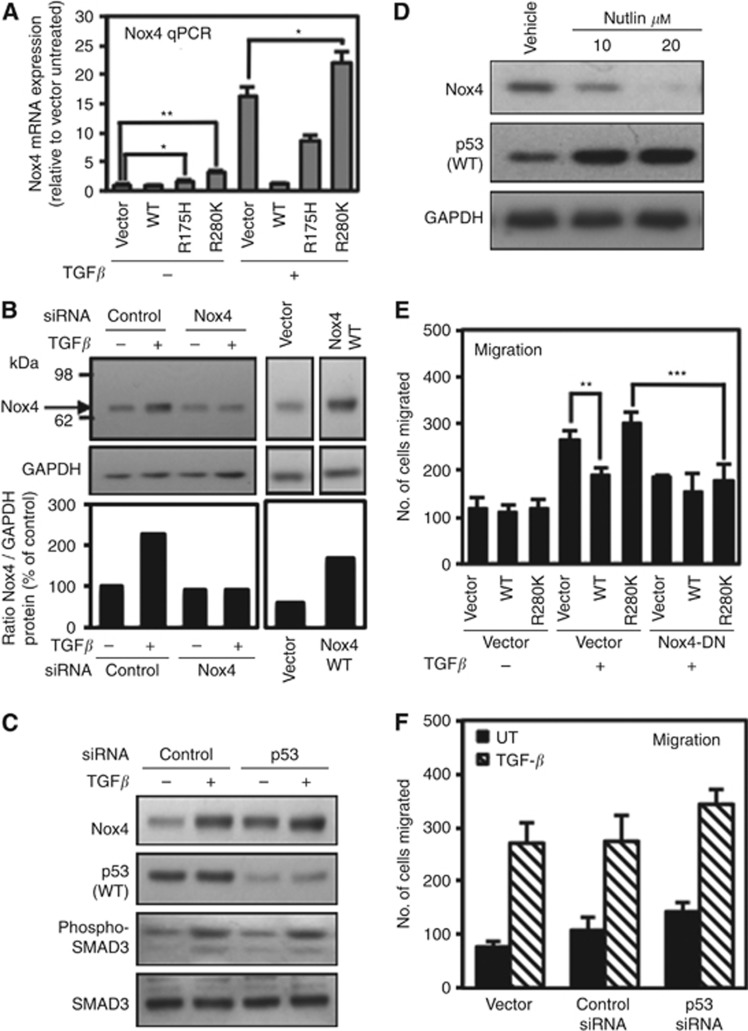
Mutant p53-R280K can overcome WT-p53 repression of Nox4 expression and cell migration in normal human mammary epithelial cells
To better understand the basis of WT-p53 repression of Nox4, we used MCF-10A cells, an immortalised human breast epithelial line that expresses endogenous WT-p53. Previously, we showed that TGF-β induces Nox4 in this cell model (Boudreau et al, 2012). First, we overexpressed WT-p53, p53-R175H, or p53-R280K to determine whether mutant p53 could overcome the repressive effect of endogenous WT-p53. We included cells overexpressing WT-p53 in these experiments, as MCF-10A cells exhibit relatively low expression of WT-p53. Remarkably, transfection of p53-R280K potentiated TGF-β transcriptional induction of Nox4 (Figure 4A). The endogenous Nox4 protein detected in the two previous cancer cell models (p53-null H1299 and the p53-R280K-expressing MDA-MB-231) was an ∼59-kDa species that was TGF-β-inducible and sensitive to Nox4 siRNA. Interestingly, we found that the normal MCF-10A breast epithelial cell line produces a Nox4 protein with a slightly higher apparent molecular mass of ∼65 kDa (Figure 4B), which is close to theoretical predictions for the full-length Nox4 sequence. The Nox4 cDNA transfected into MCF-10A also exhibited an apparent molecular mass of ∼65 kDa (Figure 4B), whereas heterologously expressed Nox4 produced the smaller species in H1299 and MDA-MB-231 (not shown). Previous studies have reported molecular masses of Nox4 protein by western blot analysis ranging from 55 to 80 kDa (Shiose et al, 2001; Hilenski et al, 2004; Hwang et al, 2005; Martyn et al, 2006; Ago et al, 2010). The Nox4 amino-acid sequence contains a putative N-terminal signal peptide cleavage site as well as predicted glycosylation sites that could account for the smallest and largest molecular weight species, respectively (Cheng et al, 2001; Martyn et al, 2006; Bedard and Krause, 2007). Thus, the variable molecular masses may be because of post-translational modification of Nox4 depending on cell types, or normal vs transformed cell phenotypes.
Figure 4.
Mutant p53 (R280K) expression correlates with Nox4 expression and overrides wild-type p53 (WT-p53) repression of Nox4 in MCF-10A breast epithelial cells. (A) MCF-10A cells were transfected with empty vector control, p53-WT, p53-R175H, or p53-R280K plasmids for 24 h followed by treatment with or without TGF-β (10 ng ml−1) for an additional 24 h. Nox4- and GAPDH-specific primers were used for real-time quantitative PCR amplification of total cDNA reverse transcribed from cells. Results are described as relative quantification of Nox4 mRNA relative to vector untreated control (n=3). (B) Detection of Nox4 protein by western blotting. MCF-10A cells were transfected with non-targeting control (50 nM) or SMARTpool Nox4-specific siRNAs (50 nM) for 72 h, and either left untreated or treated with TGF-β (5 ng ml−1) for an additional 24 h (left panel). Control blots (right panels) detect a transfected Nox4 cDNA product of the same size. Immunoblots were probed with anti-Nox4 followed by anti-GAPDH antibodies. (C) MCF-10A cells were transfected with control siRNAs (50 nM) or p53-specific siRNAs (50 nM) for 72 h followed by treatment with or without TGF-β (10 ng ml−1) for 24 h. Protein expression was analysed by immunoblotting 40 μg of total cell lysate and sequentially probed with antibodies against Nox4, p53, phospho-SMAD3, and total SMAD3. (D) MCF-10A cells were treated with 10 or 20 μM Nutlin-3 for 24 h. Protein expression was analysed by immunoblotting 40 μg of total cell lysate and sequentially probed with antibodies against Nox4, p53, and GAPDH sequentially. (E) MCF-10A cells were co-transfected with vector alone or Nox4-DN and p53-WT, or p53-R280K plasmids. Twenty-four hours post transfection, cells were re-seeded in the upper chamber of a Matrigel transwell and incubated with the lower chamber containing DMEM/F12 medium with TGF-β 10 ng ml−1. After 24 h, the migrating cells were fixed, stained, and counted from 10 random fields (n=3). (F) MCF-10A cells were treated with transfection reagent alone or transfected with control or p53-specific siRNAs. After 72 h, the cells were re-seeded in the upper chamber of a Matrigel transwell and incubated in the lower chamber containing DMEM/F12 medium with TGF-β 5 ng ml−1 for 24 h. The migrating cells were counted from 10 random fields (n=2 in triplicate).
As shown in Figure 4C, siRNA-mediated depletion of WT-p53 leads to enhanced Nox4 protein levels in both the absence and in the presence TGF-β, thus further supporting the effects of WT-p53 as a negative regulator of Nox4. These data indicate that both mutant p53 proteins can override the effects of endogenous WT and potentiate TGF-β induction of Nox4.
In an alternative approach, we treated MCF-10A cells for 24 h with increasing concentrations of Nutlin-3, an antagonist that stabilises p53 by preventing its interaction with and degradation by HDM2 E3-ligase (Vassilev et al, 2004). As demonstrated in Figure 4D, 10 μM Nutlin-3 was sufficient to protect endogenous WT-p53 from proteasomal degradation. In agreement with the effects of exogenous expression of WT-p53-reducing Nox4 protein, the enhanced stabilisation of WT-p53 correlated with reduced Nox4 protein levels. Furthermore, we observed an increase in TGF-β-mediated migration of p53-R280K-expressing MCF-10A cells, whereas overexpression of p53-WT reduced cell migration. Moreover, co-transfection with Nox4-DN reduced the number of migrating cells, further confirming the role of Nox4 in TGF-β-dependent cell migration (Figure 4E). Interestingly, siRNA-mediated depletion of WT-p53 only minimally affected cell migration with or without TGF-β treatment, indicating that additional factors are involved in MCF-10A cell motility (Figure 4F).
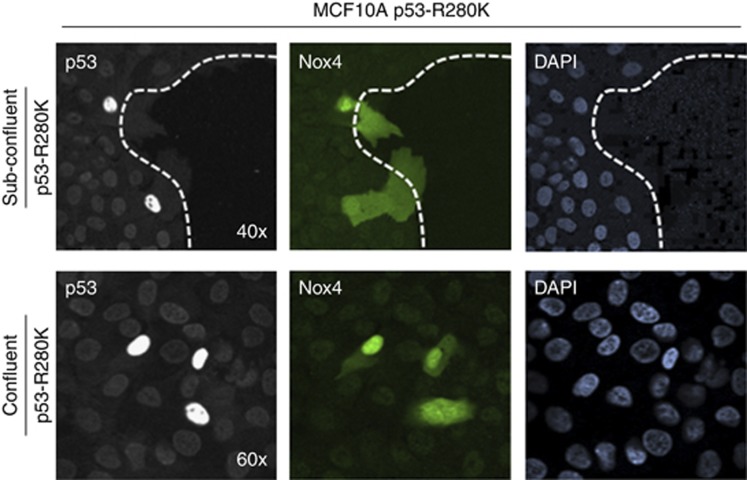
Immunofluorescence staining of MCF-10A cells exogenously overexpressing p53-R280K revealed a striking induction of endogenous Nox4 protein (Figure 5). We observed that Nox4 in the cells overexpressing p53-R280K localised to plasma membrane, perinuclear, and nuclear areas. Under sub-confluent conditions, the mutant p53-transfected cells co-stained with high Nox4 expression detected throughout spreading cells on migrating borders (upper panel). MCF-10A cells expressing p53-R280K within a confluent monolayer also co-stained with increased Nox4 in comparison with the surrounding un-transfected cells (lower panel). Taken together, these data indicate that repression of basal Nox4 by endogenous WT-p53 can be overcome by mutant p53 expression, and that p53-R280K overexpression causes increased Nox4 protein in migrating and non-migrating cells.
Figure 5.
Exogenous expression of mutant p53 (R280K) induces Nox4 in confluent and motile MCF-10A cells. MCF-10A cells were transfected with p53-R280K for 48 h. Fluorescence microscopy images were taken from the edge of a sub-confluent layer of cells (upper row) and from a confluent monolayer (lower row). From left to right, cells were stained with anti-p53 antibodies detecting high expression of transfected p53-R280K (left panel), endogenous Nox4 protein expression was detected with anti-Nox4 antibodies (middle panel), and DAPI staining of nuclei, (right panel). Dotted white line in the upper panels indicates the edge of a monolayer of cells in a sub-confluent well of a chambered coverglass.
Mutant p53 (R280K) and Nox4 modulate of TGF-β-mediated activation of FAK
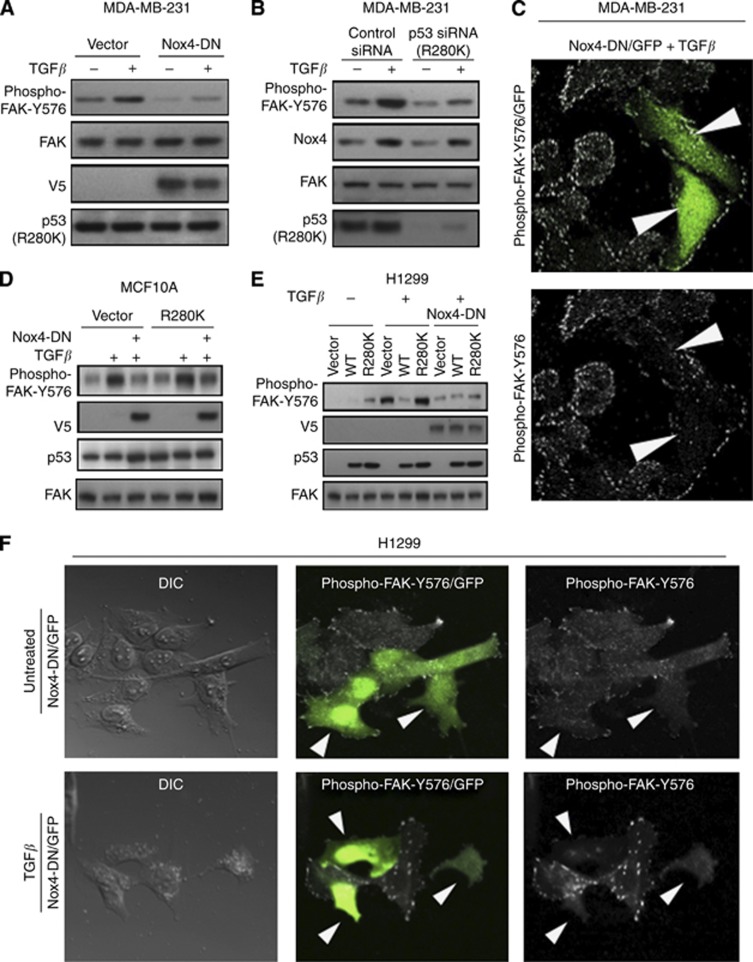
We, and others, provided evidence that Nox4 is involved in migratory processes of various cell types (Haurani et al, 2008; Meng et al, 2008; Pendyala et al, 2009; Nam et al, 2010; Boudreau et al, 2012). Previous studies indicate that Nox4 is associated with cytoskeletal alterations and focal adhesion turnover in vascular smooth muscle cells (Hilenski et al, 2004; Lyle et al, 2009). Focal adhesion kinase, a critical coordinator of signals involved in cell migration, invasion, and metastasis, is differentially regulated by p53 (Schlaepfer and Mitra, 2004; Golubovskaya et al, 2008, 2009). Based on these findings, we examined whether Nox4 had a role in TGF-β and p53 regulation of FAK-Y576 phosphorylation, which is indicative of its activation (Zhao and Guan, 2009). We addressed this by overexpressing Nox4-DN or control vector in MDA-MB-231 cells. Twenty-four hours after TGF-β treatment, expression of Nox4-DN significantly reduced the amount of FAK-Y576 phosphorylation, suggesting that Nox4 oxidase activity is involved in FAK activation (Figure 6A). Next, we used p53-targeted siRNAs to diminish the expression of endogenous p53-R280K to evaluate its effects on FAK-Y576. We found that p53-R280K depletion decreased TGF-β-induced Nox4 protein and phospho-FAK levels (Figure 6B). Furthermore, immunofluorescent imaging of phospho-FAK in MDA-MB-231 cells co-transfected with Nox4-DN, and a GFP marker displayed similar results. Expressing Nox4-DN, indicated by GFP-marked cells, reduced the amount of TGF-β-induced phospho-FAK in comparison with neighbouring GFP-negative cells (Figure 6C). We observed similar effects on FAK phosphorylation in MCF-10A cells. Mutant p53-R280K increased FAK activity in the presence or in the absence of TGF-β, which was blunted by Nox4-DN (Figure 6D). Similarly, TGF-β-mediated Nox4-dependent phospho-FAK was increased in H1299 cells expressing p53-R280K and reduced in the presence of Nox4-DN (Figure 6E). Furthermore, Nox4-DN reduced phospho-FAK immunofluoresence in TGF-β-treated H1299 cells (Figure 6F).
Figure 6.
Nox4 and mutant p53 (R280K) modulate TGF-β-induced activation of focal adhesion kinase (pFAK). (A) Overexpression of Nox4-DN reduces FAK phosphorylation. MDA-MB-231 cells were transfected with either vector control or V5-tagged Nox4-DN for 24 h followed by TGF-β (5 ng ml−1) stimulation for an additional 24 h. Total cell lysates collected for immunoblot analysis using antibodies against Nox4, p53, phospho-FAK (pFAK) and total FAK, and V5. (B) MDA-MB-231 cells were transfected with control siRNAs (25 nM) or p53-specific siRNAs (25 nM) for 72 h then simulated with TGF-β (5 ng ml−1) for 24 h. Protein expression was analysed by immunoblotting total cell lysates with antibodies against Nox4, p53, phospho-FAK (pFAK), and total FAK. (C) MDA-MB-231 cells were transfected with Nox4-DN and GFP for 24 h followed by TGF-β treatment for an additional 24 h. Upper panel: GFP (Nox4-DN) cells vs phospho-FAK staining. Lower panel: cells expressing Nox4-DN (arrows) have reduced phospho-FAK staining compared with surrounding non-transfected cells. (D) MCF-10A cells were co-transfected with vector alone or Nox4-DN, and p53-WT or p53-R280K plasmids then treated with TGF-β for 24 h. Immunoblotting analysis indicates phospho-FAK, FAK, V5-tagged Nox4-DN, and p53 protein expression. (E) p53-null H1299 cells were co-transfected transfected, treated, and analysed for protein expression and FAK phosphorylation as in D. (F) H1299 cells were transfected for 24 h as in C followed by TGF-β treatment or left untreated for an additional 24 h. Microscopy images show GFP /Nox4-DN cells along with phospho-FAK staining.
Discussion
Our work presented here reveals that TGF-β-induced Nox4 is negatively regulated by WT-p53 in human lung tumour cells and in normal and tumour breast epithelial cells. The loss of this repressive function by mutant forms of p53 (R175H, R280K) is accompanied by enhanced Nox4-derived ROS, cell migration, and FAK activation, which further elucidate the invasive behaviour commonly associated with p53 mutations. First, we observed that overexpression of WT-p53 in p53-null cells (H1299) blunts TGF-β-mediated Nox4 oxidase activity, Nox4-dependent cell migration, and FAK phosphorylation (Figures 1, 2 and 6). Conversely, normal epithelial MCF-10A cells depleted of endogenous WT-p53 by siRNA gene silencing showed an increase in basal Nox4 mRNA and protein expression (Figure 4). Furthermore, MCF-10A cells treated with Nutlin-3, a MDM2 E3-ubiquitin ligase-specific inhibitor that arrests WT-p53 degradation, showed increased p53 stability, resulting in decreased endogenous Nox4 protein (Figure 4).
Notably, common cancer-associated mutant p53 proteins, p53-R175H and p53-R280K, upregulated Nox4 in the presence or in the absence of TGF-β (Figure 2). We observed that mutant p53-R280K potentiated TGF-β-induced Nox4 expression (mRNA and protein), Nox4-depdendent cell migration, and FAK phosphorylation. These observations were further validated in MDA-MB-231 cells depleted of endogenous p53-R280K, where TGF-β-induced Nox4 expression, superoxide production, and cell migration were significantly blunted (Figure 3). Moreover, ectopic expression of p53-R280K in MCF-10A cells was sufficient to override the repressive effect of endogenous WT-p53 on Nox4 mRNA expression and Nox4-dependent cell migration (Figure 4). Immunofluorescence analysis revealed that p53-R280K expression correlated with increased Nox4 protein levels and cell migration (Figure 5). Pharmacological inhibition of TGFBR1 or SMAD3 consistently inhibited Nox4 mRNA and protein induction, affirming the importance of the TGF-β/SMAD3 axis in the transcriptional regulation of Nox4 (Figures 2 and 3).
Nox4-derived ROS have been implicated in TGF-β-mediated cell migration, proliferation, hypertrophy, wound healing, and modulation of EMT markers in a variety of cell types (Sturrock et al, 2006, 2007; Ismail et al, 2009; Bondi et al, 2010; Boudreau et al, 2012; Sancho et al, 2012; Chan et al, 2013; Hagler et al, 2013). We recently identified Nox4 as a major source of TGF-β/SMAD3-induced superoxide production that functions as an important factor in cell migration and fibronectin gene regulation in normal and metastatic breast epithelial cells (Boudreau et al, 2012). Our present study demonstrates how p53 status differentially impacts Nox4 activity in both normal WT-p53-expressing and metastatic mutant p53 (R280K)-expressing breast epithelial cell models. We also showed that expression of WT-p53 or mutant p53 differentially modulates Nox4 in H1299 p53-null lung epithelial cells. We observed that p53 can induce changes in Nox4 expression and downstream oxidase activities in both a TGF-β-dependent and TGF-β-independent manner. Furthermore, we found that mutant p53 and Nox4 are critical for TGF-β-mediated FAK phosphorylation and cell migration.
Transforming growth factor-β is a multifunctional regulatory cytokine that controls many aspects of cellular function, including cell proliferation, migration, apoptosis, and EMT progression. Epithelial-to-mesenchymal transition is a process where epithelial cells undergo transcriptional and morphological changes into mesenchymal-like cells with increased cell plasticity and mobility (Thiery et al, 2009). Several groups have shown that TGF-β can induce EMT, but the mechanisms involved are not completely understood.
Several mechanisms in the cross-talk between TGF-β and p53 have been implicated in regulating EMT and metastatic progression. Adorno et al (2009) previously reported that TGF-β along with oncogenic Ras and mutant p53 work cooperatively in a SMAD2/3-dependent manner to promote cell migration, invasion, and tumour metastasis. They further demonstrated that p63, a member of the p53 family of transcription factors, antagonises TGF-β-mediated pro-metastatic effect.
The role of p53 as a master regulator of tumour suppression has been well established. In contrast to WT-p53, aberrant p53 proteins can lose their tumour suppressor functions and give rise to highly stabilised mutant proteins with an acquired gain of function participating in EMT and tumour progression (Oren and Rotter, 2010; Kogan-Sakin et al, 2011; Termen et al, 2013). Most cancer-associated p53 mutations occur within the DNA-binding domain, rendering the proteins as defective tumour suppressors. Various p53 mutations have been linked to upregulation of genes involved in cell migration and proliferation, and downregulation of genes associated with growth inhibition and apoptosis (Kong et al, 2001; Coradini et al, 2012). The precise mechanisms involved in TGF-β and p53 regulation of Nox4 warrants further investigation, as Nox4 has been associated with cell proliferation, as well as growth inhibition and apoptosis in a variety of contexts (Geiszt et al, 2000; Peshavariya et al, 2009a; Eid et al, 2010; Weyemi et al, 2011; Sancho et al, 2012; Koziel et al, 2013).
Despite the efforts made to identify mutant p53-specific DNA-binding sites, the issues surrounding mutant forms of p53 as bona fide transcription factors remain controversial. Analyses of the promoter regions of some TGF-β/p53-responsive genes suggested the presence of overlapping p53 and SMAD regulatory elements were important for co-regulation (Sun et al, 1999; Wilkinson et al, 2008). Others indicated that WT-p53 may work by an indirect mechanism such as binding to a co-repressor affecting enhancer-mediated gene repression (Ho et al, 2005). Both SMAD2 and SMAD3 were shown to interact directly with p53 in vivo, and it was hypothesised that this interaction forms a bridge between an enhancer p53-binding site and a SMAD-binding element to regulate TGF-β-induced transcriptional repression (Cordenonsi et al, 2003; Adorno et al, 2009). Other reports suggest that p53-targeted genes may be downregulated by histone and promoter methylating enzyme recruitment in a p53-dependent manner, or that p53 may impede trans-activators from binding response elements within the promoters through protein–protein interactions or by competing for DNA binding (Murphy et al, 1999).
Previously, we demonstrated that SMAD3 is a potent regulator of the human Nox4 promoter. We showed that TGF-β-induced Nox4 promoter activity was significantly reduced by SIS3, a SMAD3-specfic inhibitor, and that expression of constitutively active or dominant-negative forms of SMAD3 substantially induced or inhibited Nox4 promoter activity, respectively (Boudreau et al, 2012). It is possible that either WT or mutant p53 serves as a transcriptional co-regulator of Nox4 mRNA, where it functions in forming a complex with other regulators of transcription such as SMAD3. Alternatively, mutant p53 could function as a transcription factor itself by binding to non-canonical p53-response elements within the Nox4 promoter. This report provides evidence that Nox4-derived ROS is required in cancer cell mobility particularly when functional WT-p53 has been disrupted or suppressed. Considering that gene transcription is a process that requires the coordination between multiple factors and co-factors, it is likely that other transcription factors, co-activators, and co-repressors are involved in p53 regulation of Nox4. Whether the DNA-binding domain of p53 is needed for direct DNA binding to the Nox4 promoter, or if the interaction of p53 with other proteins such as SMAD3 is required to repress Nox4, is currently under investigation.
Our present study provides additional insight into Nox4- and redox-dependent mechanisms involved in FAK activation facilitated by TGF-β and mutant p53. Previous reports implicated Nox4 in cytoskeletal remodelling and focal adhesion turnover (Lyle et al, 2009). Focal adhesion kinase is a non-receptor cytosolic protein tyrosine kinase that integrates growth factor and integrin signals to promote cell migration and metastasis (Zhao and Guan, 2009). Transforming growth factor–β has been linked to FAK activation and subsequent upregulation of mesenchymal and invasiveness markers (Schlaepfer et al, 2004; Cicchini et al, 2008). Golubovskaya et al (2008, 2009) recently reported that WT-p53 binds to the FAK promoter and inhibits its transcriptional activity, whereas mutant p53 promotes FAK expression. Furthermore, they reported a correlation between increased FAK expression in breast cancer tumours with p53 mutations. Recent studies have described Nox and redox-dependent regulation of cell migration involving FAK in other contexts and by other agonist (Schroder et al, 2007; Sadok et al, 2008; Shinohara et al, 2010; Leoni et al, 2013).
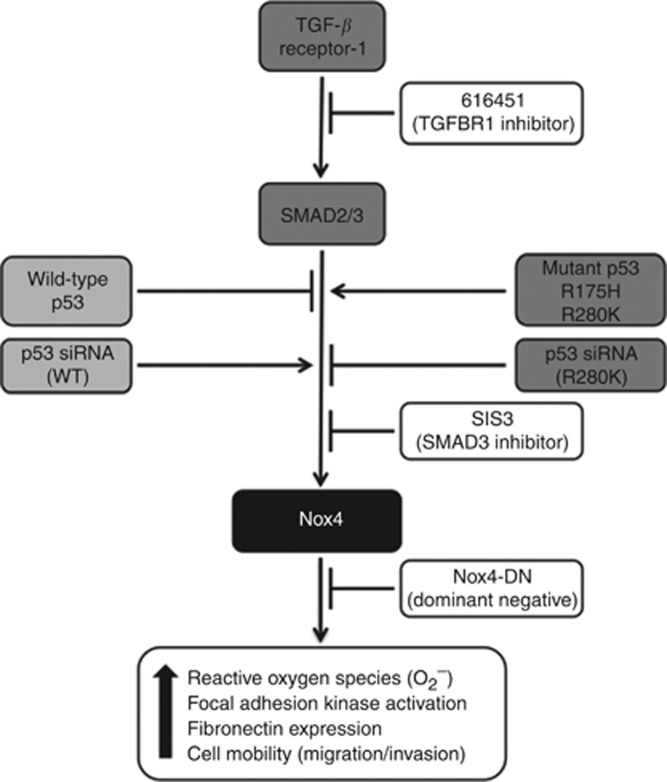
In conclusion, we emphasise that TGF-β and p53 have a critical role in regulating Nox4-dependent cell signalling and motility. As p53 has been established as a SMAD2/3 transcriptional co-factor downstream of TGF-β, we suggest a model in which mutant p53 cooperates with SMAD3 to enhance Nox4 transcription to promote subsequent Nox4 oxidase activity that participates in redox regulation of FAK activation and cell motility (Figure 7). In contrast, but possibly in a mechanistically related process, WT-p53 protects epithelial cells from TGF-β-induced EMT and metastasis. A better understanding of the Nox4-activated signalling pathways involved in EMT and tumorigenesis may uncover new molecular targets for prevention or treatment of tumour progression and metastasis.
Figure 7.
Wild-type p53 (WT-p53) and mutant p53 differentially regulate Nox4 expression in both TGF-β-dependent and independent mechanisms. Wild-type p53 suppresses basal Nox4 and TGF-β-induced Nox4 expression, whereas mutant p53 (R175H and R280K) positively regulates or enhances the TGF-β/SMAD3 effect on Nox4. Expression of mutant p53 alone can upregulate Nox4 mRNA and protein expression. The downstream effects of Nox4-dependent ROS contributes to increased fibronectin mRNA, phosphorylation and activation of FAK, and subsequent cell migration and invasion. This pathway can be inhibited with specific chemical inhibitors to TGF-β receptor-1 and SIS3, or overexpression of Nox4-DN can diminish TGF-β-induction of these events. Moreover, depletion of endogenous WT-p53 by siRNA increases Nox4 expression, whereas depleting endogenous mutant p53-R280K reduces Nox4 expression. Collectively, Nox4 is a mediator of pro-migratory events downstream of TGF-β and mutant p53, and thereby acts as an attractive target for managing or suppressing metastatic disease.
Acknowledgments
This work was supported by funds from the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (ZO1-AI-000614). We would like to thank Drs Lucy A Godley and Sharon H Jackson for their careful review of this manuscript, and Joseph Brzostowski for microscopy training and use of LIG core imaging facility.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, Bicciato S, Balmain A, Piccolo S. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137 (1:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106 (7:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28 (3:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65 (8:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87 (1:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Boivin B, Yang M, Tonks NK. Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Sci Signal. 2010;3 (137:pl2. doi: 10.1126/scisignal.3137pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol. 2010;21 (1:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53 (7:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau HE, Emerson SU, Korzeniowska A, Jendrysik MA, Leto TL. Hepatitis C virus (HCV) proteins induce NADPH oxidase 4 expression in a transforming growth factor beta-dependent manner: a new contributor to HCV-induced oxidative stress. J Virol. 2009;83 (24:12934–12946. doi: 10.1128/JVI.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannito S, Novo E, di Bonzo LV, Busletta C, Colombatto S, Parola M. Epithelial-mesenchymal transition: from molecular mechanisms, redox regulation to implications in human health and disease. Antioxid Redox Signal. 2010;12 (12:1383–1430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15 (3:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EC, Peshavariya HM, Liu GS, Jiang F, Lim SY, Dusting GJ. Nox4 modulates collagen production stimulated by transforming growth factor beta1 in vivo and in vitro. Biochem Biophys Res Commun. 2013;430 (3:918–925. doi: 10.1016/j.bbrc.2012.11.138. [DOI] [PubMed] [Google Scholar]
- Chen QK, Lee K, Radisky DC, Nelson CM. Extracellular matrix proteins regulate epithelial-mesenchymal transition in mammary epithelial cells. Differentiation. 2013;86 (3:126–132. doi: 10.1016/j.diff.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269 (1–2:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28 (9:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314 (1:143–152. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Coradini D, Fornili M, Ambrogi F, Boracchi P, Biganzoli E. TP53 mutation, epithelial-mesenchymal transition, and stemlike features in breast cancer subtypes. J Biomed Biotechnol. 2012;2012:254085. doi: 10.1155/2012/254085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 2003;113 (3:301–314. doi: 10.1016/s0092-8674(03)00308-8. [DOI] [PubMed] [Google Scholar]
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13 (2:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- Dupont S, Zacchigna L, Adorno M, Soligo S, Volpin D, Piccolo S, Cordenonsi M. Convergence of p53 and TGF-beta signaling networks. Cancer Lett. 2004;213 (2:129–138. doi: 10.1016/j.canlet.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285 (48:37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci USA. 2002;99 (6:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijhoff J, Dagnell M, Godfrey R, Östman A.2013Regulation of Protein Tyrosine Phosphatase Oxidation in Cell Adhesion and Migration Antioxid Redox Signale-pub ahead of print 10 March 2014; doi: 10.1089/ars.2013.5643 [DOI] [PubMed]
- Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97 (14:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Conway-Dorsey K, Edmiston SN, Tse CK, Lark AA, Livasy CA, Moore D, Millikan RC, Cance WG. FAK overexpression and p53 mutations are highly correlated in human breast cancer. Int J Cancer. 2009;125 (7:1735–1738. doi: 10.1002/ijc.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, Cance WG. p53 regulates FAK expression in human tumor cells. Mol Carcinogen. 2008;47 (5:373–382. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280 (47:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV, Suri RM, Miller JD. TGF-beta signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res. 2013;99 (1:175–184. doi: 10.1093/cvr/cvt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ.2008Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration Hypertension 52(1143–149.18474828 [Google Scholar]
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15 (9:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24 (4:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- Ho JS, Ma W, Mao DY, Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol. 2005;25 (17:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Ramachandrarao SP, Siva S, Valancius C, Zhu Y, Mahadev K, Toh I, Goldstein BJ, Woolkalis M, Sharma K. Reactive oxygen species production via NADPH oxidase mediates TGF-beta-induced cytoskeletal alterations in endothelial cells. Am J Physiol Renal Physiol. 2005;289 (4:F816–F825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Kleinhenz DJ, Lassegue B, Griendling KK, Dikalov S, Hart CM. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol. 2005;288 (4:C899–C905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Stehelin D. Down-regulation of fibronectin gene expression by the p53 tumor suppressor protein. Cell Growth Differ. 1996;7 (5:629–634. [PubMed] [Google Scholar]
- Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol. 2009;296 (3:L489–L499. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan-Sakin I, Tabach Y, Buganim Y, Molchadsky A, Solomon H, Madar S, Kamer I, Stambolsky P, Shelly A, Goldfinger N, Valsesia-Wittmann S, Puisieux A, Zundelevich A, Gal-Yam EN, Avivi C, Barshack I, Brait M, Sidransky D, Domany E, Rotter V. Mutant p53(R175H) upregulates Twist1 expression and promotes epithelial-mesenchymal transition in immortalized prostate cells. Cell Death Differ. 2011;18 (2:271–281. doi: 10.1038/cdd.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XT, Gao H, Stanbridge EJ. Mechanisms of differential activation of target gene promoters by p53 hinge domain mutants with impaired apoptotic function. J Biol Chem. 2001;276 (35:32990–33000. doi: 10.1074/jbc.M103681200. [DOI] [PubMed] [Google Scholar]
- Koziel R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, Jansen-Durr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J. 2013;452 (2:231–239. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107 (35:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101 (47:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Neish AS, Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123 (1:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hanze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal. 2008;10 (10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- Luo M, Guan JL. Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 2010;289 (2:127–139. doi: 10.1016/j.canlet.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105 (3:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24 (5:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal CC, Ganapathy S, Gorin Y, Mahadev K, Block K, Abboud HE, Harris SE, Ghosh-Choudhury G, Ghosh-Choudhury N. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem J. 2011;433 (2:393–402. doi: 10.1042/BJ20100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18 (1:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24 (12:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80 (2:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- Muller PA, Vousden KH, Norman JC. p53 and its mutants in tumor cell migration and invasion. J Cell Biol. 2011;192 (2:209–218. doi: 10.1083/jcb.201009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Ahn J, Walker KK, Hoffman WH, Evans RM, Levine AJ, George DL. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 1999;13 (19:2490–2501. doi: 10.1101/gad.13.19.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Park YY, Yoon G, Cho H, Lee JH. Co-treatment with hepatocyte growth factor and TGF-beta1 enhances migration of HaCaT cells through NADPH oxidase-dependent ROS generation. Exp Mol Med. 2010;42 (4:270–279. doi: 10.3858/emm.2010.42.4.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DD, Block K, Bhandhari B, Gorin Y, Abboud HE. IGF-I increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. Am J Physiol Cell Physiol. 2012;302 (1:C122–C130. doi: 10.1152/ajpcell.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harbor Perspect Biol. 2010;2 (2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2009;11 (4:747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peshavariya H, Dusting GJ, Jiang F, Halmos LR, Sobey CG, Drummond GR, Selemidis S. NADPH oxidase isoform selective regulation of endothelial cell proliferation and survival. Naunyn-Schmiedeberg Arch Pharmacol. 2009;380 (2:193–204. doi: 10.1007/s00210-009-0413-0. [DOI] [PubMed] [Google Scholar]
- Peshavariya H, Jiang F, Taylor CJ, Selemidis S, Chang CW, Dusting GJ. Translation-linked mRNA destabilization accompanying serum-induced Nox4 expression in human endothelial cells. Antioxid Redox Signal. 2009;11 (10:2399–2408. doi: 10.1089/ars.2009.2579. [DOI] [PubMed] [Google Scholar]
- Sadok A, Bourgarel-Rey V, Gattacceca F, Penel C, Lehmann M, Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochim Biophys Acta. 2008;1783 (1:23–33. doi: 10.1016/j.bbamcr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Sadok A, Pierres A, Dahan L, Prevot C, Lehmann M, Kovacic H. NADPH oxidase 1 controls the persistence of directed cell migration by a Rho-dependent switch of alpha2/alpha3 integrins. Mol Cell Biol. 2009;29 (14:3915–3928. doi: 10.1128/MCB.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, Huber H, Eferl R, Mikulits W, Fabregat I. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One. 2012;7 (9:e45285. doi: 10.1371/journal.pone.0045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14 (1:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692 (2–3:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27 (8:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Adachi Y, Mitsushita J, Kuwabara M, Nagasawa A, Harada S, Furuta S, Zhang Y, Seheli K, Miyazaki H, Kamata T. Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J Biol Chem. 2010;285 (7:4481–4488. doi: 10.1074/jbc.M109.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276 (2:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- Strano S, Dell'Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene. 2007;26 (15:2212–2219. doi: 10.1038/sj.onc.1210296. [DOI] [PubMed] [Google Scholar]
- Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290 (4:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292 (6:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu X, Eaton EN, Lane WS, Lodish HF, Weinberg RA. Interaction of the Ski oncoprotein with Smad3 regulates TGF-beta signaling. Mol Cell. 1999;4 (4:499–509. doi: 10.1016/s1097-2765(00)80201-4. [DOI] [PubMed] [Google Scholar]
- Termen S, Tan EJ, Heldin CH, Moustakas A. p53 regulates epithelial-mesenchymal transition induced by transforming growth factor beta. J Cell Physiol. 2013;228 (4:801–813. doi: 10.1002/jcp.24229. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279 (6:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278 (14:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139 (5:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tian M, Neil JR, Schiemann WP. Transforming growth factor-beta and the hallmarks of cancer. Cell Signal. 2011;23 (6:951–962. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16 (4:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303 (5659:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137 (3:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Walsh MF, Ampasala DR, Hatfield J, Vander Heide R, Suer S, Rishi AK, Basson MD. Transforming growth factor-beta stimulates intestinal epithelial focal adhesion kinase synthesis via Smad- and p38-dependent mechanisms. Am J Pathol. 2008;173 (2:385–399. doi: 10.2353/ajpath.2008.070729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyemi U, Lagente-Chevallier O, Boufraqech M, Prenois F, Courtin F, Caillou B, Talbot M, Dardalhon M, Al Ghuzlan A, Bidart JM, Schlumberger M, Dupuy C. ROS-generating NADPH oxidase NOX4 is a critical mediator in oncogenic H-Ras-induced DNA damage and subsequent senescence. Oncogene. 2011;31 (9:1117–1129. doi: 10.1038/onc.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Tsai WW, Schumacher MA, Barton MC. Chromatin-bound p53 anchors activated Smads and the mSin3A corepressor to confer transforming-growth-factor-beta-mediated transcription repression. Mol Cell Biol. 2008;28 (6:1988–1998. doi: 10.1128/MCB.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25 (4:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19 (2:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang Y, Ries W, Key L. Expression of Nox4 in osteoclasts. J Cell Biochem. 2004;92 (2:238–248. doi: 10.1002/jcb.20048. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28 (1–2:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]