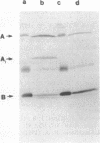
Abstract
A number of ToxR-regulated genes that encode products required for the biogenesis or function of the toxin-coregulated colonization pilus (TCP) of Vibrio cholerae have been identified previously by TnphoA fusions. In this study we have examined the role of the product of one of these genes, tcpG, to which a fusion results in a piliated cell lacking all of the in vivo and in vitro functions associated with TCP. Our results show that TcpG is not an ancillary pilus adhesin component as suggested by the mutant phenotype but instead is a 24-kDa periplasmic protein that shares active-site homology with several different bacterial thioredoxins and protein disulfide isomerase, as well as overall homology with the disulfide bond-forming DsbA periplasmic oxidoreductase protein of E. coli. Corresponding activity can be demonstrated in vitro for TcpG-enriched fractions from a wild-type strain but is absent in a similarly fractionated tcpG-phoA mutant. The phenotype conferred by a tcpG mutation was found to be pleiotropic in nature, also affecting the extracellular secretion of cholera toxin A subunit and a major protease. This suggests a general role for TcpG in allowing a group of virulence-associated (and perhaps other) proteins that contain disulfide bonds to assume a secretion or functionally competent state.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell J. C., McGovern K., Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991 Nov 1;67(3):581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- CRICK F. H. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Sastry P. A., Hodges R. S., Lee K. K., Paranchych W., Irvin R. T. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect Immun. 1990 Jan;58(1):124–130. doi: 10.1128/iai.58.1.124-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hirst T. R., Holmgren J. Transient entry of enterotoxin subunits into the periplasm occurs during their secretion from Vibrio cholerae. J Bacteriol. 1987 Mar;169(3):1037–1045. doi: 10.1128/jb.169.3.1037-1045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Sanchez J., Kaper J. B., Hardy S. J., Holmgren J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7752–7756. doi: 10.1073/pnas.81.24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J Bacteriol. 1991 Jun;173(11):3311–3317. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Doig P., Lee K. K., Sastry P. A., Paranchych W., Todd T., Hodges R. S. Characterization of the Pseudomonas aeruginosa pilus adhesin: confirmation that the pilin structural protein subunit contains a human epithelial cell-binding domain. Infect Immun. 1989 Dec;57(12):3720–3726. doi: 10.1128/iai.57.12.3720-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. I. New method of assay and the mechanism of ADP-ribosyl transfer. J Biol Chem. 1979 Jul 10;254(13):5849–5854. [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Simple method for purifying choleragenoid, the natural toxoid of Vibrio cholerae. Infect Immun. 1977 Jun;16(3):789–795. doi: 10.1128/iai.16.3.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M., Hogenkamp H. P. Purification, characterization, and amino acid sequence of thioredoxin from Corynebacterium nephridii. J Biol Chem. 1981 Sep 10;256(17):9174–9182. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Mekalanos J. J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988 Nov;56(11):2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Seyer J. M., Kovari I., Sumrada R. A., Taylor R. K. Localization of protective epitopes within the pilin subunit of the Vibrio cholerae toxin-coregulated pilus. Infect Immun. 1991 Jan;59(1):114–118. doi: 10.1128/iai.59.1.114-118.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R., Shaw C., Peterson K., Spears P., Mekalanos J. Safe, live Vibrio cholerae vaccines? Vaccine. 1988 Apr;6(2):151–154. doi: 10.1016/s0264-410x(88)80019-7. [DOI] [PubMed] [Google Scholar]
- Thayer M. M., Flaherty K. M., McKay D. B. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-A resolution. J Biol Chem. 1991 Feb 15;266(5):2864–2871. doi: 10.2210/pdb1ezm/pdb. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K. F., Wyman A. R., Botstein D. Host/vector interactions which affect the viability of recombinant phage lambda clones. Gene. 1986;49(2):253–262. doi: 10.1016/0378-1119(86)90286-6. [DOI] [PubMed] [Google Scholar]