Abstract
Background:
Amino-acid transporters are necessary for the tumour cell growth and survival, and have a crucial role in the development and invasiveness of cancer cells. But, it remains unclear about the prognostic significance of L-type amino-acid transporter 1 (LAT1), system ASC amino-acid transporter-2 (ASCT2), and xCT expression in patients with tongue cancer. We conducted the clinicopathological study to investigate the protein expression of these amino-acid transporters in tongue cancer.
Methods:
Eighty-five patients with surgically resected tongue cancer were evaluated. Tumour sections were stained by immunohistochemistry for LAT1, ASCT2, xCT, 4F2hc/CD98hc (4F2hc), Ki-67, and microvessel density (MVD) determined by CD34, and p53.
Results:
L-type amino-acid transporter 1 and 4F2hc were highly expressed in 61% (52 out of 85) and 45% (38 out of 47), respectively. ASC amino-acid transporter-2 and xCT were positively expressed in 59% (50 out of 85) and 21% (18 out of 85), respectively. The expression of both LAT1 and ASCT2 was significantly associated with disease staging, lymph-node metastasis, lymphatic permeation, 4F2hc expression and cell proliferation (Ki-67). xCT expression indicated a significant association with advanced stage and tumour factor. By univariate analysis, disease staging, lymphatic permeation, vascular invasion, LAT1, ASCT2, 4F2hc, and Ki-67 had a significant relationship with overall survival. Multivariate analysis confirmed that LAT1 was an independent prognostic factor for predicting poor prognosis.
Conclusions:
L-type amino-acid transporter 1 and ASCT2 can serve as a significant prognostic factor for predicting worse outcome after surgical treatment and may have an important role in the development and aggressiveness of tongue cancer.
Keywords: tongue cancer, LAT1, ASCT2, xCT, prognostic factor
Cancer of the tongue is a malignant tumour treated by surgery, and comprises ∼30% of all malignant tumours of the oral-pharyngeal lesion (Regezi et al, 2008). Disease staging remains the most important tool for the clinician in predicting disease outcome in patients with oral tongue squamous cell carcinoma (SQC) (Bello et al, 2010). However, it is not the only prognostic factor for predicting poor prognosis. Clarifying poor prognostic factors at the time of diagnosis is essential to enable identification of patients whose tumours have high risk if treatment failure. Nowadays, there has been no established biomarker that correlates with the prognosis and therapeutic response in patient with tongue cancer.
Amino-acid transporters are necessary for the tumour cell growth and proliferation, and the overexpression of amino-acid transporters has been described to have a crucial role in the survival and metastasis of cancer cells (Christensen, 1990; Kanai et al, 1998; Yanagida et al, 2001; Fuchs and Bode, 2006; Baek et al, 2012). Recent studies have described that L-type amino-acid transporter 1 (LAT1), system ASC amino-acid transporter-2 (ASCT2), and xCT were significantly linked to the carcinogenesis and tumour pathogenesis (Yanagida et al, 2001; Whitte et al, 2002; Li et al, 2003; Kaira et al, 2008, 2011, 2012; Baek et al, 2012). L-type amino-acid transporter 1 is one of the L-type amino-acid transporters, and transports large neutral amino acids such as leucine, isoleucine, valine, phenylalanine, tyrosine, tryptophan, methionine, and histidine and requires covalent association with the heavy chain of 4F2 cell-surface antigen (4F2hc/CD98hc) for its functional expression in plasma membrane (Kanai et al, 1998; Yanagida et al, 2001). It has been described to be highly expressed in proliferating tissues, many tumour cell lines and primary human neoplasms (Yanagida et al, 2001; Kaira et al, 2008, 2012). Expression of LAT1 has a close relationship with 4F2hc, cell proliferation, angiogenesis and cell-cycle regulator (Kaira et al, 2011). Recently, the expression of LAT1 has been described to be a significant factor indicating a poor outcome in human neoplasms (Kaira et al, 2008, 2012). However, it remains unclear about the pathological significance of LAT1 expression in patients with tongue cancer. ASC amino-acid transporter-2 is a Na+-dependent transporter responsible for transport of neutral amino acids including glutamine, leucine, and isoleucine, and is a major glutamine transporter in human hepatoma cells. Recently, ASCT2 has been described to be highly expressed in human neoplasms such as hepatocellular carcinoma, colorectal, and prostate cancer (Whitte et al, 2002; Li et al, 2003; Fuch et al, 2007). Researchers have documented that the protein level of ASCT2 was related to poor outcome and malignant aggressiveness in patients with colorectal and prostate cancer (Whitte et al, 2002; Li et al, 2003). System xc− is a cystine-glutamate exchanger transporter, composed of a light chain (xCT) and heavy chain (4F2hc) (Baek et al, 2012; Takeuchi et al, 2013). The expression of xCT has been reported to have a crucial role in the tumour progression and growth of cancer and glutathione-based drug resistance (Huang et al, 2005). Recent research also demonstrated that the overexpression of xCT could be a pathological marker for predicting poor outcome in patients with glioblastoma (Takeuchi et al, 2013). The study using human tissue specimens showed that xCT is highly expressed in patients with lung cancer and breast cancer (Baek et al, 2012).
But, it remains unclear whether the amino-acid transporters such as LAT1, ASCT2, and xCT have a significant relationship with the tumour progression and development of patients with tongue cancer. Therefore, we conducted the clinicopathological study evaluating the prognostic significance of these three amino-acid transporters (LAT1, ASCT2, and xCT) in patients with tongue cancer. In this study, therefore, the expression level of LAT1, ASCT2, and xCT proteins was assessed in the resected tissue specimen and correlated with outcome of patients. In addition, the expression of Ki-67 labelling index, cell-cycle regulator (p53) and angiogenic markers such as microvessel density (MVD) determined by CD34 were also examined by immunohistochemistry.
Materials and methods
Patients
Between November 2000 and January 2012, we analysed 94 consecutive patients with tongue cancer who underwent surgical resection at Gunma University Hospital. Nine patients who had a histological type other than SQC were excluded. Therefore, 85 patients were analysed in this study. The study was approved by the institutional review board.
The age of the patients ranged from 33 to 92 years, and the median age was 69 years. None of the patients had received neo-adjuvant chemotherapy. All surgical specimens were reviewed and classified according to the WHO classification by an experienced pathologist who was unaware of clinical or imaging findings. Pathological tumour-node-metastasis stages were established using the International System for Staging adopted by the American Joint Committee on Cancer and the Union Internationale Centre le Cancer. Histologically, all patients had histology of SQC, and 38, 12, 15, and 20 of the total patients had stage I, II, III, and IV tumours, respectively. Postoperative adjuvant chemotherapy with S-1 (Taiho Pharmaceutical Co., Ltd, Tokyo, Japan), oral administration of tegafur (a fluorouracil derivative drug) and docetaxel were administered to 9, 12, and 3 patients, respectively. The day of surgery was considered as the starting day for measuring postoperative survival. The follow-up duration ranged from 61 to 3452 days (median, 1033 days).
Immunohistochemical staining
The LAT1 expression was determined by immunohistochemical staining with a murine anti-human LAT1 monoclonal antibody 4A2 (provided by Dr H Endou (J-Pharma, Tokyo, Japan), 2 mg ml−1, dilution; 1 : 3200) (Sakata et al, 2009). The production and characterisation of the LAT1 antibody has previously been described (Kaira et al, 2008, 2012). The anti-CD98 antibody is an affinity-purified rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1 : 100 dilution) raised against a peptide mapping at the carboxy terminus of CD98 of human origin. CD98 clearly detects 4F2hc and this antigen is SLC3A2/4F2hc/CD98hc, therefore, we used this antibody. The detailed protocol for immunostaining was also published elsewhere (Kaira et al, 2008, 2012). The LAT1 and 4F2hc expression scores were assessed by the extent of staining as follows: 1, ⩽10% of tumour area stained; 2, 11–25% stained; 3, 26–50% stained; and 4, ≧51% stained. The tumours in which stained tumour cells were scored as 3 or 4 were defined as high expression.
Anti-ASCT2 and anti-xCT antibodies are affinity-purified rabbit polyclonal antibody (1 : 300 dilution) and affinity-purified murine polyclonal antibody (Santa Cruz Biotechnology, Inc., 1 : 200 dilution), respectively. An oligopeptide (RDSKGLAAAEPTAN) corresponding to amino-acid residues 7–20 of a rabbit polyclonal antibody against ASCT2 was synthesised (see Supplementary Figure S1). The N-terminal cysteine residue was used for conjugation with keyhole limpet haemocyanin. An anti-ASCT2 rabbit polyclonal antibody was produced as described elsewhere (Altman et al, 1984). The antiserum was affinity purified as described (Chairoungdua et al, 2001). The specificity of the antibody was confirmed as described in Supplementary Procedure. Briefly, HEK 293T cells were transfected with a plasmid encode ASCT2 or the parent plasmid. Crude membrane fractions were isolated, and then subjected to SDS–PAGE and western blotting as described in Khunweeraphong et al (2012). Immunohistochemical staining was performed on paraffin sections using a polymer peroxidase method (Histofine Simple Stain MAX PO (MULTI or G) kit; Nichirei Corporation, Tokyo, Japan). Briefly, deparaffinised and rehydrated sections were treated with 0.3% hydrogen peroxide in methanol for 30 min to block endogenous peroxidase activity. To expose antigens, sections were autoclaved in EDTA (pH 8.0) for 5 min and cooled for 30 min. After rinsing in phosphate-buffered saline (PBS), sections were incubated with affinity-purified anti-ASCT2 antibody (1 : 300) overnight. Thereafter, they were incubated with the Histofine Simple Stain MAX PO (MULTI or G) kit (Nichirei Corporation). The peroxidase reaction was performed using 0.02% 3,3′-diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide in 0.05 M tris–HCl buffer, pH 7.4. Negative control tissue sections were prepared by omitting the primary antibody. The expression of ASCT2 and xCT was considered as positive only if distinct membrane staining was present. The ASCT2 expression scores were assessed by the extent of staining as follows: 1, ⩽10% of tumour area stained; 2, 11–25% stained; 3, 26–50% stained; and 4, ≧51% stained. The tumours in which stained tumour cells were scored as > 2 were defined as positive expression.
For CD34, Ki-67, and p53, immunohistochemical staining was performed according to the procedures described in previous reports (Kaira et al, 2008, 2012). The following antibodies were used: murine monoclonal antibodies against CD34 (Nichirei, 1 : 800 dilution), Ki-67 (Dako, Glostrup, Denmark; 1 : 40 dilution), and p53 (D07; Dako, 1 : 50 dilution). The number of CD34-positive vessels was counted in four selected hot spots in a × 400 field (0.26 mm2 field area). The MVD was defined as the mean count of microvessels per 0.26 mm2 field area. The median numbers of CD34-positive vessels were evaluated, and the tumours in which stained tumour cells made up more than each median value were defined as high expression. For p53, microscopic examination for the nuclear reaction product was performed and scored. Based on a previous report (Kaira et al, 2008, 2012), p53 expression in >10% of tumour cells was defined as positive expression. For, Ki-67, a highly cellular area of the immunostained sections was evaluated. All epithelial cells with nuclear staining of any intensity were defined as high expression. Approximately 1000 nuclei were counted on each slide. Proliferative activity was assessed as the percentage of Ki-67-stained nuclei (Ki-67 labelling index) in the sample. The median value of the Ki-67 labelling index was evaluated, and the tumour cells with greater than the median value were defined as high expression. The sections were assessed using a light microscopy in a blinded manner by at least two of the authors.
Statistical analysis
Probability values of <0.05 indicated a statistically significant difference. Fisher's exact test was used to examine the association of two categorical variables. The correlation between different variables was analysed using the non-parametric Spearman's rank test. Follow-up for these 85 patients was conducted using the patient medical records. The Kaplan–Meier method was used to estimate survival as a function of time, and survival differences were analysed by the log-rank test. Overall survival (OS) was determined as the time from tumour resection to death from any cause. Progression-free survival (PFS) was defined as the time between tumour resection and the first disease progression or death. Multivariate analyses were performed using stepwise Cox proportional hazards model to identify independent prognostic factors. Statistical analysis was performed using JMP 8 (SAS, Institute Inc., Cary, NC, USA) for Windows.
Results
Immunohistochemical analysis
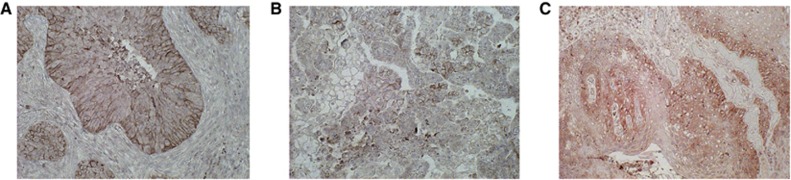
The immunohistochemical analysis of the biomarkers was performed on the 85 primary lesions with tongue cancer. Figure 1 shows the representative immunohistochemical staining of LAT1, ASCT2, and xCT expression. The immunostaining of LAT1, ASCT2, and xCT was detected in carcinoma cells in tumour tissues and localised predominantly on their plasma membrane. All positive cells revealed strong membranous immunostaining. In total patients, a high LAT1 and 4F2hc expression was recognised in 61% (52 out of 85) and 45% (38 out of 47), respectively (P=0.045). A positive ASCT2 and xCT expression was recognised in 59% (50 out of 85) and 21% (18 out of 85), respectively.
Figure 1.
Representative immunohistochemical staining of patient with tongue squamous cell carcinoma. Immunostaining of LAT1 (A), ASCT2 (B), and xCT (C) displays a membranous immunostaining pattern.
The median number of CD34 was 14 (range, 2–29), and the value of 14 was chosen as a cutoff point. The median value of the Ki-67 labelling index was 21% (range, 5–72), and the value of 21% was chosen as a cutoff point. The high expression of CD34 and Ki-67 was recognised in 52% (44 out of 85) and 51% (43 out of 85), respectively. Positive expression of p53 was recognised in 44% (37 out of 85).
Patient's demographics according to LAT1, ASCT2, and xCT expression status is listed in Table 1. High LAT1 expression was significantly associated with disease staging, lymph-node status, lymphatic permeation, vascular invasion, 4F2hc, Ki-67, and CD34. Positive ASCT2 expression yielded a significant relationship with disease staging, primary tumour status, lymph-node status, lymphatic permeation, 4F2hc, and Ki-67. Positive expression of xCT was significantly associated with disease staging and T factor.
Table 1. Patient's demographics according to LAT1, ASCT2, and xCT expression.
| |
|
LAT1 |
ASCT2 |
xCT |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Total (n=85) | High (n=52) | Low (n=33) | P-value | Positive (n=50) | Negative (n=35) | P-value | Positive (n=18) | Negative (n=67) | P-value |
|
Age | ||||||||||
| ⩽65 years/>65 years |
36/49 |
19/33 |
17/16 |
0.185 |
18/32 |
18/17 |
0.184 |
7/11 |
29/38 |
0.793 |
|
Sex | ||||||||||
| Male/Female |
56/29 |
34/18 |
22/11 |
>0.999 |
31/19 |
25/10 |
0.486 |
12/6 |
44/23 |
>0.999 |
|
Differentiation | ||||||||||
| WD or MD/PD |
73/11 |
43/9 |
30/3 |
0.353 |
42/10 |
31/4 |
0.753 |
14/4 |
59/8 |
0.271 |
|
Stage | ||||||||||
| I or II/III or IV |
50/35 |
26/26 |
24/9 |
0.044 |
26/24 |
30/5 |
0.001 |
7/11 |
49/18 |
0.011 |
|
Primary tumour status | ||||||||||
| T1-2/T3-4 |
72/13 |
42/10 |
30/3 |
0.236 |
40/10 |
34/1 |
0.023 |
11/7 |
63/4 |
0.001 |
|
Lymph-node status | ||||||||||
| N0/N1-2 |
31/54 |
27/ 25 |
4/29 |
<0.001 |
28/22 |
8/27 |
0.004 |
10/8 |
20/47 |
0.054 |
|
Lymphatic permeation | ||||||||||
| Positive/Negative |
37/48 |
30/22 |
7/26 |
<0.001 |
27/33 |
11/24 |
0.047 |
11/7 |
27/40 |
0.181 |
|
Vascular invasion | ||||||||||
| Positive/Negative |
26/59 |
22/30 |
4/29 |
0.003 |
20/30 |
7/28 |
0.061 |
6/12 |
21/46 |
>0.999 |
|
Resected status | ||||||||||
| Positive/Negative |
15/70 |
11/41 |
4/29 |
0.383 |
10/40 |
5/30 |
0.573 |
4/14 |
11/56 |
0.727 |
|
Adjuvant CTx | ||||||||||
| Yes/No |
24/61 |
19/33 |
5/28 |
0.047 |
17/33 |
7/28 |
0.221 |
7/11 |
17/50 |
0.376 |
|
4F2hc | ||||||||||
| High/Low |
38/47 |
33/19 |
5/28 |
<0.001 |
27/23 |
11/24 |
0.048 |
12/6 |
26/41 |
0.059 |
|
Ki-67 | ||||||||||
| High/Low |
43/42 |
33/19 |
10/23 |
0.003 |
29/21 |
11/24 |
0.026 |
11 /7 |
29/38 |
0.195 |
|
CD34 | ||||||||||
| High/Low |
44/41 |
33/19 |
11/22 |
0.008 |
26/24 |
16/19 |
0.661 |
10/8 |
32/35 |
0.604 |
|
p53 | ||||||||||
| Positive/Negative | 37/48 | 25/27 | 12/21 | 0.370 | 21/29 | 17/18 | 0.658 | 8/10 | 30/37 | >0.999 |
Abbreviations: ASCT2=ASC amino-acid transporter 2; CTx=chemotherapy; LAT1=L-type amino-acid transporter 1; MD=moderate differentiated; PD=poorly differentiated; WD=well differentiated.
The bold entries show a statistically significant difference.
Correlation between LAT1 expression and different variables
Using Spearman's rank correlation, LAT1 had a statistically significant correlation with ASCT2 (r=0.608, P<0.001), xCT (r=0.409, P<0.001), 4F2hc (r=0.496, P<0.001), Ki-67(r=0.473, P<0.001), and CD34 (r=0.468, P<0.001). ASC amino-acid transporter-2 yielded a significant correlation with 4F2hc (r=0.221, P=0.041) and Ki-67 (r=0.417, P<0.001); and a significant correlation was observed between xCT and 4F2hc (r=0.251, P=0.019) (Table 2).
Table 2. Correlation between LAT1, ASCT2, and xCT, and other biomarkers.
| Biomarkers | Spearman γ | 95% CI | P-value |
|---|---|---|---|
|
LAT1 | |||
| ASCT2 | 0.608 | 0.449–0.730 | <0.001 |
| xCT | 0.409 | 0.209–0.576 | <0.001 |
| 4F2hc | 0.496 | 0.338–0.627 | <0.001 |
| CD34 | 0.468 | 0.298–0.609 | <0.001 |
| Ki-67 |
0.473 |
0.311–0.608 |
<0.001 |
|
ASCT2 | |||
| 4F2hc | 0.221 | 0.026–0.421 | 0.041 |
| CD34 | 0.127 | −0.094–0.337 | 0.244 |
| Ki-67 |
0.417 |
0.217–0.583 |
<0.001 |
|
xCT | |||
| 4F2hc | 0.251 | 0.035–0.447 | 0.019 |
| CD34 | 0.029 | −0.191–0.247 | 0.786 |
| Ki-67 | 0.177 | −0.043–0.382 | 0.105 |
Abbreviations: ASCT2=ASC amino-acid transporter 2; 95% CI=95% confidence interval; LAT1=L-type amino-acid transporter 1.
The bold entries show a statistically significant difference.
Different variables survival analysis
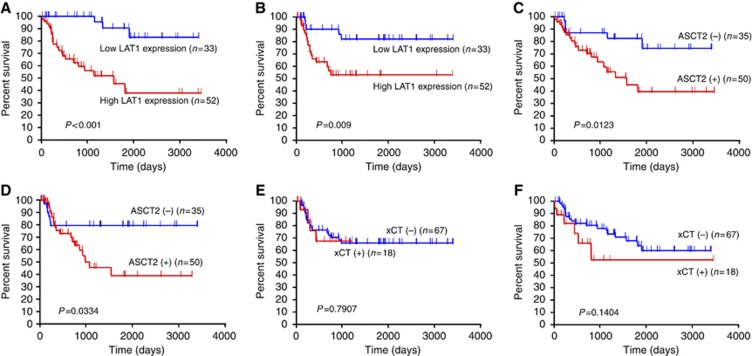
The 5-year survival rate and median survival time for all patients were 31% and 18 months, respectively. Tables 3 and 4 show the univariate and multivariate analysis in OS and PFS. By univariate analysis, disease staging, lymphatic permeation, vascular invasion, LAT1, ASCT2, 4F2hc, and Ki-67 had a significant relationship with OS, and tumour differentiation, lymphatic permeation, LAT1, ASCT2, 4F2hc, and Ki-67 were significantly associated with PFS. Figure 2 shows the Kaplan–Meier survival curve in patients with tongue cancer according to the expression status of LAT1, ASCT2, and xCT. Multivariate analysis confirmed that LAT1 was an independent prognostic factor for predicting poor PFS and OS.
Table 3. Univariate analysis in overall survival and progression-free survival.
| |
Overall survival |
Progression-free survival |
||
|---|---|---|---|---|
| Variables | 5-year survival rate (%) | P-value | 5-year survival rate (%) | P-value |
|
Age | ||||
| ⩽65 years/>65 years |
64.8/56.3 |
0.383 |
61.8/68.3 |
0.504 |
|
Sex | ||||
| Male/Female |
58.9/71.2 |
0.847 |
62.3/72.4 |
0.585 |
|
Differentiation | ||||
| WD or MD/PD |
64.6/43.6 |
0.090 |
40.0/37.8 |
0.037 |
|
Stage | ||||
| I or II/III or IV |
73.4/34.2 |
0.003 |
72.1/46.9 |
0.103 |
|
Primary tumour status | ||||
| T1-2/T3-4 |
65.6/23.1 |
0.112 |
66.9/36.4 |
0.785 |
|
Lymphatic permeation | ||||
| Positive/Negative |
39.6/74.3 |
0.007 |
43.7/77.7 |
0.004 |
|
Vascular invasion | ||||
| Positive/Negative |
32.3/72.7 |
0.001 |
50.5/70.6 |
0.140 |
|
Resected status | ||||
| Positive/Negative |
54.5/63.0 |
0.806 |
59.3/66.8 |
0.715 |
|
Adjuvant chemotherapy | ||||
| Yes/No |
40.7/71.1 |
0.217 |
42.3/73.3 |
0.058 |
|
LAT1 | ||||
| High/Low |
37.8/90.1 |
<0.001 |
53.0/81.8 |
0.009 |
|
ASCT2 | ||||
| Positive/Negative |
39.4/82.3 |
0.012 |
39.2/79.5 |
0.033 |
|
xCT | ||||
| Positive/Negative |
52.5/73.5 |
0.140 |
67.5/65.9 |
0.791 |
|
4F2hc | ||||
| High/Low |
41.9/75.8 |
0.012 |
47.9/79.0 |
0.023 |
|
Ki-67 | ||||
| High/Low |
46.8/73.3 |
0.036 |
48.1/81.2 |
0.001 |
|
CD34 | ||||
| High/Low |
48.9/75.3 |
0.182 |
68.7/72.1 |
0.617 |
|
p53 | ||||
| Positive/Negative | 49.8/71.6 | 0.111 | 60.2/70.4 | 0.542 |
Abbreviations: ASCT2=ASC amino-acid transporter 2; LAT1=L-type amino-acid transporter1; MD=moderate differentiated; PD=poorly differentiated; WD=well differentiated.
The bold entries show a statistically significant difference.
Table 4. Multivariate analysis in overall survival and progression-free survival.
| |
Overall survival |
Progression-free survival |
||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|
Age | ||||
| ⩽65 years/>65 years |
0.796 (0.328–1.878) |
0.603 |
0.801 (0.509–1.241) |
0.321 |
|
Sex | ||||
| Male/Female |
0.981 (0.336–2.244) |
0.701 |
1.047 (0.352–2.798) |
0.928 |
|
Stage | ||||
| I or II/III or IV |
2.204 (0.898–5.487) |
0.084 |
1.365 (0.509–3.653) |
0.532 |
|
LAT1 | ||||
| High/Low |
6.167 (2.044–32.44) |
0.001 |
4.100 (1.263–15.35) |
0.018 |
|
ASCT2 | ||||
| Positive/Negative | 1.289 (0.404–3.594) | 0.649 | 1.367 (0.407–4.299) | 0.603 |
Abbreviations: ASCT2=ASC amino-acid transporter 2; 95% CI=95% confidence interval; HR=hazard ratio; LAT1=L-type amino-acid transporter1. The bold entries show a statistically significant difference.
Figure 2.
Kaplan–Meier analysis of overall survival (OS) and progression-free survival (PFS) according to LAT1, ASCT2, and xCT expression. A statistically significant difference in OS and PFS was observed between the patients with high LAT1 and those with low LAT1 tumour expression (OS, P<0.001(A); PFS, P=0.009 (B)), and between the patients with positive and negative ASCT2 tumour expression (OS, P=0.012 (C); PFS, P=0.033 (D)). No statistically significant difference in OS (E) and PFS (F) was observed between the patients with positive xCT and those with negative xCT tumour expression.
Discussion
This is a first study to evaluate the clinical significance of LAT1, ASCT2, and xCT expression in patients with tongue cancer. Of these three amino-acid transporters, the expression of LAT1 was confirmed to be a significant independent factor for predicting poor prognosis in tongue cancer by multivariate analysis. However, we also found that the positive expression of ASCT2 yielded a significant relationship with worse prognosis after surgical treatment, but not xCT. Our study showed that the expression of LAT1 and ASCT2 was significantly associated with disease staging, lymph-node metastasis, tumour invasiveness, 4F2hc expression, and cell proliferation (Ki-67). 4F2hc has an important role in the functional expression of LAT1, ASCT2, and xCT; therefore, 4F2hc significantly correlated with the expression of these amino-acid transporters. In our study, the percentage of patients who received adjuvant chemotherapy was significantly higher in patients with high LAT1 expression than in those with low LAT1, but adjuvant chemotherapy did not affect the outcome after surgical treatment. Our study indicated that the prognosis of patients with high LAT1 expression was worse despite of adjuvant chemotherapy. Therefore, our results suggest that the prognostic significance of LAT1 and ASCT2 expression was not affected by adjuvant chemotherapy. The prognostic significance of LAT1 expression has been extensively evaluated in various types of human neoplasms; however, little is known about the clinicopathological significance of ASCT2 and xCT (Fuchs and Bode, 2006). To our knowledge, there has been no report comparing the clinical significance of three amino-acid transporters of LAT1, ASCT2, and xCT in human tissue specimen. Our data suggest that the expression level of these amino-acid transporters has a crucial role in the development and carcinogenesis of tumour cells, and it may be an important clinical marker of therapy for patients with tongue cancer.
L-type amino-acid transporter 1 is highly expressed in cancer cells in general; however, LAT1 expression in SQC was markedly higher than that of adenocarcinoma (AC) (Kaira et al, 2008, 2012; Sakata et al, 2009; Ichinoe et al, 2011; Furuya et al, 2012; Nobusawa et al, 2013). For example, the percentage of high LAT1 expression in patients with AC showed 29% in pulmonary AC (Kaira et al, 2008), 22% in prostate cancer (Sakata et al, 2009), 43% in breast cancer (Furuya et al, 2012), 43% in gastric cancer (Ichinoe et al, 2011), and 53% in pancreatic AC (Kaira et al, 2012), whereas patients with SQC revealed 91% in pulmonary SQC (Kaira et al, 2008), 56% in oral SQC (Nobusawa et al, 2013), and 61% in tongue cancer (the present study). But, it remains unknown why the extent of LAT1 expression in cancer cells is different between SQC and AC.
As described by previous studies (Kaira et al, 2008, 2012; Sakata et al, 2009; Ichinoe et al, 2011; Furuya et al, 2012; Nobusawa et al, 2013), the results of our study indicated that LAT1 expression has a close relationship with advanced stage, lymph-node metastasis, cell proliferation, angiogenesis, and survival in tongue cancer. A recent report documented that LAT1 provides the essential amino acids that signal to enhance growth of cancer cells via the mammalian target of rapamycin (mTOR)-stimulated translation (Fuchs and Bode, 2006). Likewise, mTOR is also regulated by amino-acid transporter gene expression and trafficking to the plasma membrane in response to growth signals. In in vitro study, the inhibition of LAT1 has been described to reduce the level of phosphorylation of mTOR, p70 ribosomal S6 kinase (p70SK1), and 4E-binding protein-1 (4E-BP1), suggesting that the activation of mTOR signalling pathway is associated with LAT1 expression (Liu et al, 2004; Yamauchi et al, 2009; Imai et al, 2010). Moreover, several authors have described that the inhibition of LAT1 leads to apoptosis by inducing intracellular depletion of amino acids required for the growth of cancers, and induces cell-cycle arrest at G1 phase (Liu et al, 2004; Kim et al, 2010), These investigation have suggest that the inhibition of LAT1 could be an effective therapeutic target for human neoplasms.
ASC amino-acid transporter-2 is highly expressed in various types of cancer cells that require glutamine for their growth and survival (Fuchs and Bode, 2006). An experimental study has described that the inhibition of ASCT2 reduces the availability of glutamine and other amino acids transported by ASCT2, and this could inhibit the survival of cancer cells depending on increased glutamine metabolism (Oppedisano et al, 2012). Therefore, ASCT2 could be the potential target for anticancer therapeutics as well. Clinically, only two reports showed aggressiveness biological behaviour of ASCT2 expression using tumour tissue specimen (Whitte et al, 2002; Li et al, 2003). Although LAT1 enhances tumour cell growth via the mTOR signalling pathway, whereas ASCT2 keeps the cytoplasmic amino-acid pool necessary to drive LAT1 function and fuels energy via delivery of glutamine (Xu and Hemler, 2005; Fuchs and Bode, 2006; Nicklin et al, 2009). Therefore, the expression levels of ASCT2 and LAT1 are coordinately rose in human cancer and these two obligate amino-acid exchangers are closely related to the cellular growth and survival linked to the mTOR signalling pathway (Xu and Hemler, 2005; Nicklin et al, 2009). Nicklin et al (2009) has described that L-glutamine transporter regulates mTOR and autophagy to coordinate cell growth and proliferation. Our study indicated that ASCT2 expression was significantly associated with tumour aggressiveness, cell proliferation, advanced staging, metastasis, and worse prognosis in patients with tongue cancer. But, the positive expression of ASCT2 was not confirmed to be an independent prognostic factor for poor outcome in tongue cancer by multivariate analysis.
In the present study, the expression of xCT indicated any significant association with advanced stage and primary tumour factor. However, we could not find a significant relationship between xCT expression with tumour cell proliferation, angiogenesis, or survival in tongue cancer. It is expected to examine whether the expression of xCT protein has any relationship with the clinical significance of tumour cells with chemotherapy or radiotherapy resistance. Further study is warranted to investigate the xCT expression using tumour specimens resistant to anticancer therapy.
Limitations of the current study must be addressed. One limitation is that the sample size in this study was small, which may bias our results. Another limitation is that it remains unclear about the optimal cutoff points for the expression level of LAT1, ASCT2, and xCT. Previous studies revealed that there was a strong correlation between the two transporters (LAT1 and ASCT2) and their obligate chaperone (Xu and Hemler, 2005; Fuchs and Bode, 2006; Nicklin et al, 2009). However, the present study shows the relatively low percentage correlation between LAT1 and ASCT2 expression. These disparities may be due to the qualities of these antibodies and the evaluating system to measure their expression level. This is one of the limitations of immunohistochemical studies. Further study is warranted to evaluate a large number of patients with tongue cancer and to investigate the appropriate cutoff level of these amino-acid transporters.
In conclusion, the high expression of LAT1 can serve as an independent prognostic factor for predicting worse outcome after surgical treatment and may have an important role in the metastasis, cell proliferation, angiogenesis, and invasiveness of tongue cancer. The expression of ASCT2 is also closely associated with tumour growth and proliferation, and could be an indicator for predicting poor prognosis as well. The inhibition of LAT1 and ASCT2 may, therefore, be a potential target for anticancer therapy in tongue cancer.
Acknowledgments
This work was supported in part by Grant 23591750 (KK) and Grant 23592523 (KC) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and the National Hospital Organization Policy Based Medical Services. Also supported by Advanced research for medical products Mining Programme of the National Institute of Biomedical Innovation (NIBIO).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Altman A, Cardenas JM, Houghten RA, Dixon FJ, Theofilopoulos AN. Antibodies of predetermined specificity against chemically synthesized peptides of human interleukin 2. Proc Natl Acad Sci. 1984;81:2176–2180. doi: 10.1073/pnas.81.7.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek S, Choi CM, Ahn SH, Lee JW, Gong G, Ryu JS, Oh SJ, Bacher-Stier C, Fels L, Koglin N, Hultsch C, Schatz CA, Dinkelborg LM, Mittra ES, Gambhir SS, Moon DH. Exploratory clinical trial of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate for imaging xC- transporter using positron emission tomography in patients with non-small cell lung or breast cancer. Clin Cancer Res. 2012;18:5427–5437. doi: 10.1158/1078-0432.CCR-12-0214. [DOI] [PubMed] [Google Scholar]
- Bello IO, Soini Y, Salo T. Prognostic evaluation oral tongue cancer: Means, markers and perspectives (I) Oral Oncol. 2010;46:630–635. doi: 10.1016/j.oraloncology.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Chairoungdua A, Kanai Y, Matsuo H, Inatomi J, Kim DK, Endou H. Identification and characterization of a novel member of the heterodimeric amino acid transporter family presumed to be associated with an unknown heavy chain. J Biol Chem. 2001;276:49390–49399. doi: 10.1074/jbc.M107517200. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Fuch BC, Finger RE, Onan MC, Bode BP. ASCT2 silencing regulates mammalian target of rapamycin growth and survival signaling in human hepatoma cells. Am J Physiol Cell Physiol. 2007;293:C55–C63. doi: 10.1152/ajpcell.00330.2006. [DOI] [PubMed] [Google Scholar]
- Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime. Semin Cancer Biol. 2006;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382–389. doi: 10.1111/j.1349-7006.2011.02151.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Dai Z, Barbacioru C, Sadee W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005;65:7446–7454. doi: 10.1158/0008-5472.CAN-04-4267. [DOI] [PubMed] [Google Scholar]
- Ichinoe M, Mikami T, Yoshida T, Igawa I, Tsuruta T, Nakada N, Anzai N, Suzuki Y, Endou H, Okayasu I. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int. 2011;61:281–289. doi: 10.1111/j.1440-1827.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, Ishizuka T, Nagamori S, Promchan K, Nakajima T, Yamamoto N, Mori M, Kanai Y. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–4828. [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br J Cancer. 2008;98:742–748. doi: 10.1038/sj.bjc.6604235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, Murakami H, Shukuya T, Kenmotsu H, Naito T, Kanai Y, Endo M, Kondo H, Nakajima T, Yamamoto N. LAT1 expression is closely associated with hypoxic markers and mTOR in resected non-small cell lung cancer. Am J Transl Res. 2011;3:468–478. [PMC free article] [PubMed] [Google Scholar]
- Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Segawa A, Furuya M, Mori M, Oyama T, Takeyoshi I. Prognostic significance of L-type amino acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer. 2012;107:632–638. doi: 10.1038/bjc.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Khunweeraphong N, Nagamori S, Wiriyasermkul P, Nishinaka Y, Wongthai P, Ohgaki R, Tanaka H, Tominaga H, Sakurai H, Kanai Y. L-glutamate enhances methylmercury toxicity by synergistically increasing oxidative stress. J Pharmacol Sci. 2012;119:368–380. doi: 10.1254/jphs.08118fp. [DOI] [PubMed] [Google Scholar]
- Kim CS, Moon IS, Park JH, Shin WC, Chun HS, Lee SY, Kook JK, Kim HJ, Park JC, Endou H, Kanai Y, Lee BK, Kim do K. Inhibition of L-type amino acid transporter modulates the expression of cell cycle regulatory factors in KB oral cancer cells. Biol Pharm Bull. 2010;33:1117–1121. doi: 10.1248/bpb.33.1117. [DOI] [PubMed] [Google Scholar]
- Li R, Younes M, Frolov A, Wheeler TM, Scardino P, Ohori M, Ayala G. Expression of neutral amino acid transporter ASCT2 in human prostate. Anticancer Res. 2003;23:3413–3418. [PubMed] [Google Scholar]
- Liu XM, Reyna SV, Ensenat D, Peyton KJ, Wang H, Schafer AI, Durante W. Platelet-derived growth factor stimulates LAT1 gene expression in vascular smooth muscle: role in cell growth. FASEB J. 2004;18:768–770. doi: 10.1096/fj.03-0886fje. [DOI] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobusawa A, Kim M, Kaira K, Miyashita G, Negishi A, Oriuchi N, Higuchi T, Tsushima Y, Kanai Y, Yokoo S, Oyama T. Diagnostic usefulness of 18F-FAMT PET and L-type amino acid transporter 1 (LAT1) expression in oral squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2013;40 (11:1692–1700. doi: 10.1007/s00259-013-2477-9. [DOI] [PubMed] [Google Scholar]
- Oppedisano F, Catto M, Koutentis PA, Nicolotti O, Pochini L, Koyioni M, Introcaso A, Michaelidou SS, Carotti A, Indiveri C. Inactivation of the glutamine/amino acid transporter ASCT2 by 1,2,3-dithiazoles: proteoliposomes as a tool to gain insights in the molecular mechanism of action and of antitumor activity. Toxicol Appl Pharmacol. 2012;93:93–102. doi: 10.1016/j.taap.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Regezi JA, Sciubba JJ, Jordan RCK.2008Oral Pathology: Clinical, Pathologic Correlation5th edn.Saunders Elsevier: St Louis, USA [Google Scholar]
- Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Wada K, Toyooka T, Shinomiya N, Shimazaki H, Nakanishi K, Nagatani K, Otani N, Osada H, Uozumi Y, Matsuo H, Nawashiro H. Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastomas. Neurosurgery. 2013;72:33–41. doi: 10.1227/NEU.0b013e318276b2de. [DOI] [PubMed] [Google Scholar]
- Whitte D, Ali N, Carlson N, Younes M. Overexpression of the neutral amino acid transporter ASCT2 in human colorectal adenocarcinoma. Anticancer Res. 2002;22:2555–2557. [PubMed] [Google Scholar]
- Xu D, Hemler ME. Metabolic activation-related CD147-CD98 complex. Mol Cell Proteomics. 2005;4:1061–1071. doi: 10.1074/mcp.M400207-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Sakurai H, Kimura T, Wiriyasermkul P, Nagamori S, Kanai Y, Kohno N. System L amino acid transporter inhibitor enhances anti-tumor activity of cisplatin in a head and neck squamous cell carcinoma cell line. Cancer Lett. 2009;276:95–101. doi: 10.1016/j.canlet.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto K, Takeda E, Goya T, Endou H. Human L-type amino-acid transporter 1 (LAT 1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.