Abstract
Background:
The purpose of this study was to evaluate the feasibility of a new shortened 3-week treatment schedule of carbon ion radiotherapy (CIRT) for prostate cancer.
Methods:
Beginning in May 2010, patients with T1b–T3bN0M0, histologically proven prostate adenocarcinoma were enrolled in the phase II trial of CIRT. Patients received 51.6 GyE in 12 fractions over 3 weeks (protocol 1002). The primary end point was defined as the incidence of late adverse events that were evaluated based on the Common Terminology Criteria for Adverse Events version 4.0. Biochemical failure was determined using the Phoenix definition (nadir +2.0 ng ml−1).
Results:
Forty-six patients were enrolled, and all patients were included in the analysis. The number of low-, intermediate-, and high-risk patients was 12 (26%), 9 (20%), and 25 (54%), respectively. The median follow-up period of surviving patients was 32.3 months. Two patients had intercurrent death without recurrence, and the remaining 44 patients were alive at the time of this analysis. In the analysis of late toxicities, grade 1 (G1) rectal haemorrhage was observed in 3 (7%) patients. The incidence of G1 haematuria was observed in 6 (13%) patients, and G1 urinary frequency was observed in 17 (37%) patients. No ⩾G2 late toxicities were observed. In the analysis of acute toxicities, 2 (4%) patients showed G2 urinary frequency, and no other G2 acute toxicities were observed.
Conclusions:
The new shortened CIRT schedule over 3 weeks was considered as feasible. The analysis of long-term outcome is warranted.
Keywords: prostate cancer, carbon ion radiotherapy, phase I/II clinical trial, radiotherapy
The morbidity in prostate cancer is the second highest in the world, and the mortality in prostate cancer is the fifth highest in the world (Ferlay et al, 2012). There are many treatment modalities for prostate cancer including radical prostatectomy (Roehl et al, 2004), laparoscopic surgery (Touijer et al, 2009), three-dimensional (3D)-conformal radiotherapy (CRT) by photons (Zelefsky et al, 2008), intensity-modulated radiotherapy (IMRT) (Cahlon et al 2008), low-dose rate (LDR) brachytherapy (Peinemann et al, 2011), high-dose rate brachytherapy (Masson et al, 2012), proton beam therapy (Zietman et al, 2010), carbon ion radiotherapy (CIRT) (Ishikawa et al, 2006), hormone therapy (Souhami et al, 2009), and robot-assisted laparoscopic prostatectomy (Menon et al, 2010). A high dose of 78–80 Gy is generally used in 3D-CRT or IMRT for prostate cancer, and definitive radiotherapy using photons or proton beam requires ∼8 weeks (Zelefsky et al, 2008; Cahlon et al 2008; Zietman et al, 2010). This treatment duration appears to be one of the disadvantages for patients, and some patients choose radical prostatectomy or LDR brachytherapy because of the treatment duration. Although treatment duration for radiotherapy tends to increase with the increase in the total irradiation dose, because of the biological characteristics of photons and proton beams, it is not easy to shorten the treatment duration by increasing the dose/fraction (Furusawa et al, 2000). Recent studies have reported stereotactic radiotherapy (SRT) for prostate cancer using 35–50 Gy/5 fractions (Boike et al, 2011; King et al, 2013). However, the efficacy and the safety (in higher dose) have not yet been established.
Carbon ion radiotherapy for prostate cancer has been in use since 1995, and an initial clinical trial was initiated using a schedule of 20 fractions over 5 weeks (Akakura et al, 2004). The clinical trial of CIRT for prostate cancer was initiated with a shorter duration than that typically used with photons. The efficacy and the feasibility were established with that schedule (Tsuji et al, 2005; Ishikawa et al, 2006). A new treatment schedule of 16 fractions over 4 weeks was initiated in 2003. This treatment schedule has also shown favourable outcomes and feasibility despite approximately half the duration compared with that of radiotherapy by photons (Okada et al, 2012). The possibility of shortening the treatment duration is one of the advantages of CIRT; however, it is also one of the challenges of CIRT (Tsujii et al, 2004). The purpose of this study (protocol 1002) was to evaluate the feasibility of a clinical trial using a new shortened treatment schedule of 12 fractions over 3 weeks.
Materials and methods
The primary end point was defined as the incidence of late adverse events. Late adverse events were defined as adverse events occurring in more than 6 months after the start of radiotherapy. Adverse events were evaluated based on the Common Terminology Criteria for Adverse Events version 4.0 (National Cancer Institute, 2009). The secondary end points were (1) biochemical failure-free survival, (2) overall/cause-specific survival, and (3) quality of life. Biochemical failure was determined using the Phoenix definition (nadir +2.0 ng ml−1) (Roach et al, 2006). Biochemical failure-free survival, cause-specific survival, and overall survival were calculated from the day CIRT was started. Risk categories of prostate cancer were defined as follows: low-risk group, initial prostate-specific antigen (PSA) <20 ng ml−1 and T1b–T2bN0M0 and Gleason score ⩽6; intermediate-risk group, initial PSA <20 ng ml−1 and/or T2cN0M0 and/or Gleason score=7; and high-risk group, initial PSA ⩾20 ng ml−1 or T3a/3bN0M0 or Gleason score ⩾8. The T stage was determined based on TNM classification 7th edition (Sobin et al, 2009). Clinical stage was determined by digital rectal examination, magnetic resonance imaging, computed tomography, bone scintigraphy, and other diagnostic images. Adverse effects were evaluated by interview of symptoms from patients, urine analysis, stool analysis, cystoscopy, and colonoscopy.
Inclusion and exclusion criteria
Patients who met all the following conditions were included: histologically diagnosed prostate adenocarcinoma, without any previous surgery or radiotherapy for prostate cancer, and T1bN0M0 to T3bN0M0. In addition, the following patients were included in this trial (excluded from another CIRT trial 9904): (1) low-risk patients who underwent hormonal therapy, (2) intermediate/high-risk patients who refused hormonal therapy, and (3) intermediate/high-risk patients who received longer than 6 months of hormonal therapy. Exclusion criteria included the following: (1) having a history of pelvic irradiation; (2) having prognosis for survival of < 6 months; (3) performance status of 3 or 4; (4) receiving other treatments for prostate cancer, with the exception of hormonal therapy; (5) having uncontrolled malignancy(ies) other than prostate cancer; (6) having uncontrolled infectious disease near the irradiation area; (7) medical/psychological/other reasons; and (8) showing PSA elevation during prior hormonal therapy. Patients who met the above inclusion criteria and who had provided informed consent were enrolled in the trial. All enrolled patients were examined before CIRT by the institutional review board including external committee members. The target number of patients was 45 and the registration period was 2 years. Once the target number of patients was reached, the clinical trial was closed. Analysis of the primary end point was performed at least 2 years after registration of the last patient. After CIRT, all patients are followed by measuring serum PSA level every 3 months.
Carbon ion radiotherapy
Clinical target volume (CTV) was defined as the whole prostate and proximal one third of seminal vesicles (gross tumour volume was not contoured). However, all seminal vesicles were included in the CTV in T3b cases. Planning target volume (PTV) was defined as the CTV with 10 mm margins in the anterior and lateral directions, and with 5 mm margins in the superior, inferior, and posterior directions. The prophylactic area (pelvic lymph nodes) was not included in the PTV. Rectum was delineated as organ at risk from 10 mm above the upper margin of the PTV to 10 mm below the lower margin of the PTV. The prescribed dose was 51.2 GyE/12 fr., and >95% of the prescription dose were planned to be irradiated to the CTV. The recommended (not restricted) dose constraints for rectum are V53GyE (rectal volume to be irradiated 53 GyE) ⩽0%, V50GyE ⩽7%, and V40GyE ⩽16%.Other dose constraints were not defined. The two-fields technique (opposing lateral fields) was routinely used for the CIRT planning. Compensators were individually made for each port, and irradiation fields were shaped using multi-leaf collimators. Purgatives or enema was used so that patients have bowel movements at least once a day. All patients were treated with a resinous shell and an image-guided irradiation system under shallow natural breathing, and irradiation was performed 4 days per week. The total irradiation dose was calculated on the basis of the past irradiation schedules. The past protocols of CIRT (63.0 GyE in 20 fractions in protocol 9904[2] and 57.6 GyE in 16 fractions in protocol 9904[3]) showed favourable outcomes with limited toxicity; the total dose of this clinical trial was estimated to be equivalent to the biological effect observed in the past protocols (Okada et al, 2012). Assuming the alpha–beta ratio of prostate cancer is 1.5–3.0 (Akimoto et al, 2005; Fowler, 2005; Khoo, 2005; Pollack et al, 2006), biologically effective dose when α/β=1.5 (BED1.5) of 63.0 GyE/20 fr. was calculated as 195.3, BED1.5 of 57.6 GyE/16 fr. was 195.8, and BED3.0 of 63.0 GyE/20 fr. was 129.2, and biologically effective dose when α/β=3.0 (BED3.0) of 57.6 GyE/16 fr. was 126.7. Similarly, BED1.5 and BED3.0 of 51.6 GyE/12 fr. were calculated as 199.5 and 125.6, respectively.
Statistical analysis
Time to event was calculated from the first day of CIRT to the date of the event. The follow-up period was calculated from the first date of CIRT to the date of last follow-up. The Kaplan–Meier method was used for survival analyses.
Results
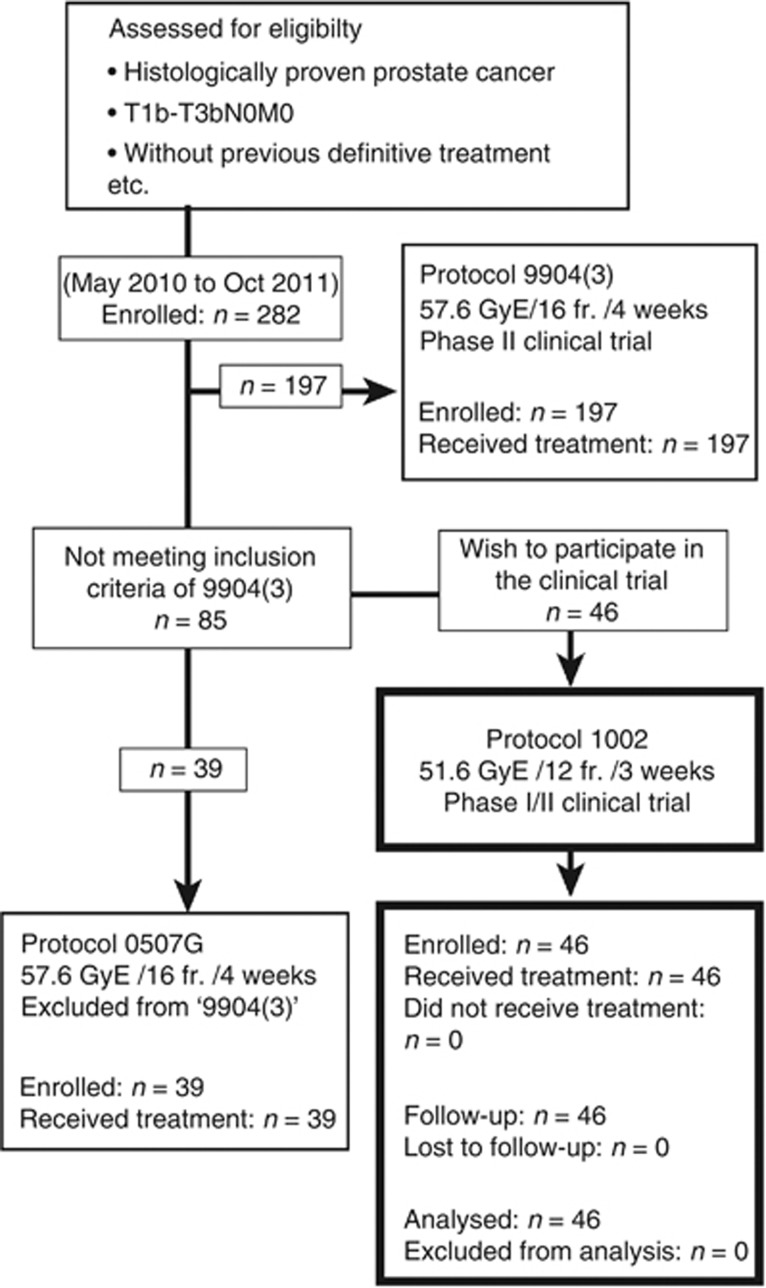
The phase I/II clinical trial of CIRT in 12 fractions for prostate cancer (protocol 1002) was approved by the research ethics review board of our institution on May 2010, and then the clinical trial was open for enrollment. Although the scheduled registration period was 2 years, registration was closed on October 2011 because the number of patients reached 46. Between May 2010 and October 2011, 282 prostate cancer patients were enrolled in CIRT protocols. Of the 282 patients, 197 patients were eligible for the protocol 9904(3) phase II clinical trial. In all, 46 of 85 patients who did not meet the eligibility criteria for protocol 9904(3), but wished to participate in clinical trial (protocol 1002) were enrolled in this clinical trial. The remaining 39 patients who did not meet the eligibility criteria for protocol 9904(3) and who did not wish to participate in the protocol 1002 trial were treated in the protocol 0507 G trial with the same dose fractionation as 9904(3) (Figure 1).
Figure 1.
Chart of the trial. The patients who did not meet the inclusion criteria of 9904(3) (CIRT over 4 weeks) and who wished to participate were enrolled in the study (1002).
The characteristics of 46 patients are shown in Table 1. According to the risk classification, patients with T1c–T2b, T2c, and T3a/b were 25, 6, and 15, respectively. The number of patients with initial PSA <20 ng ml−1 and ⩾20 ng ml−1 were 34 and 12, respectively. The number of patients with Gleason score ⩽6, =7, and ⩾8 was 12, 15, and 19, respectively. Finally, 12 patients (26%) were classified as low risk, 9 patients (20%) were classified as intermediate risk, and 25 (54%) patients were classified as high risk. Forty-five patients underwent hormonal therapy, and one of the forty-five patients underwent castration due to liver function failure after LH-RH analogue injection. One patient who was intermediate risk did not undergo hormonal therapy because he refused hormonal therapy.
Table 1. The characteristics of 46 patients.
| Enrolled patients |
|
46 |
| Gender |
Male |
46 (100%) |
| Age (years) |
Median |
66 (range 54–83) |
| Stage (UICC2009) | T1c 17 | T1 17 (37%) |
| T2a 5, T2b 3, T2c 6 | T2 14 (30%) | |
| |
T3a 13, T3b 2 |
T3 15 (33%) |
| Initial PSA (ng ml−1) |
Median |
11.1 (range 3.559–242.7) |
| Gleason score | 3+3=6 | 12 (26%) |
| 3+4=7 | 6 (13%) | |
| 4+3=7 | 9 (20%) | |
| 4+4=8 | 8 (17%) | |
| |
4+5=9 |
11 (24%) |
| Risk | Low | 12 (26%) |
| Intermediate | 9 (20%) | |
| |
High |
25 (54%) |
| ADT | With ADT | 45 (98%) |
| Castration | (1) (2%) | |
| |
Without ADT |
1 (denied) (2%) |
| Median (range) duration of ADT by risk (months) | Low risk | 7.7 (5.5–25.2) |
| Intermediate risk | 10.0 (7.3–28.3) | |
| High risk | 26.7 (9.7–52.9)a |
Abbreviations: ADT=androgen deprivation therapy; PSA=prostate-specific antigen.
One patient who underwent castration was excluded (duration of ADT: 3.5 months).
All 46 enrolled patients completed the scheduled irradiation. The median overall treatment time (OTT) of CIRT was 20 days (range, 16–21 days), and there were no treatment interruptions or treatment delays. The median follow-up period of surviving patients from the start of CIRT was 32.3 months (range, 23.5–38.9 months), and the median follow-up period of surviving patients from the start of treatment including hormonal therapy was 40.0 months. Two deaths were observed during the follow-up period. There were no deaths due to disease and no treatment-related deaths. The two deaths were due to intercurrent disease. One of the intercurrent deaths was in a 64-year-old patient who had hypertension, hyperlipidaemia, diabetes mellitus, hyperuricemia, cardiovascular disease, and an abdominal aortic aneurysm (AAA). This patient suddenly died from AAA rupture 4 months after CIRT without elevation of PSA. No relationships were found among CIRT, hormonal therapy, and the death; therefore, this case was determined to be intercurrent death without recurrence. A 78-year-old patient who had been diagnosed with interstitial pneumonitis (IP) before treatment for prostate cancer also died. Although IP was stable during the treatment for prostate cancer, the patient died from exacerbation of IP and pneumothorax 24 months after the start of hormonal therapy and 17 months after CIRT, without elevation of PSA. No relationships were found between hormonal therapy, CIRT, and exacerbation of IP; therefore, this case was also determined as intercurrent death without recurrence.
The acute and late toxicities observed with CIRT are shown in Table 2. The most frequent acute toxicity, grade 1 (G1) urinary frequency was observed in 34 out of 46 (74%) cases. Grade 2 (G2) urinary frequency was observed in 2 out of 46 (4%) cases. One of the patients with G2 urinary frequency had been diagnosed with benign prostatic hypertrophy before being diagnosed with prostate cancer, and the patient had pollakiuria before CIRT. The urine sample of the other patient tested positive for bacteria; therefore, G2 urinary frequency was considered to be due to CIRT and a complication of urinary infection. No ⩾G2 toxicities were observed. The most frequent late toxicity observed was G1 urinary frequency in 17 out of 46 (37%) cases. The second most frequent late toxicity was G1 haematuria observed in 6 out of 46 (13%) cases. The median time to occurrence of G1 haematuria was 20.7 months (range, 3.5–34.4 months). Grade 1 rectal haemorrhage was observed in 3 (7%) patients and G1 proctitis was observed in 1 (2%) patient. No G2 late toxicities were observed during the follow-up period.
Table 2. Follow-up data and toxicity.
| Follow-up period of surviving patients | Median (months) (range) | 32.3 (23.5–38.9) |
|---|---|---|
| Survival | Alive | 44 (96%) |
| |
Dead |
2 (4%) |
| Cause of death | Intercurrent death | 2 (100%) |
| Death from primary disease | 0 (0%) | |
| Treatment-related death | 0 (0%) |
| |
Grade |
|
||
|---|---|---|---|---|
| Toxicity | G0 | G1 | G2 | Total |
|
Acute | ||||
| Skin (radiation dermatitis) | 44 (96%) | 2 (4%) | 0 (0%) | 46 |
| Rectum (haemorrhage) | 46 (100%) | 0 (0%) | 0 (0%) | 46 |
| GU (urinary frequency) |
10 (22%) |
34 (74%) |
2 (4%) |
46 |
|
Acute toxicity by risk | ||||
| Low | 1 (2%) | 11 (24%) | 0 (0%) | 12 |
| Intermediate | 1 (2%) | 6 (13%) | 2 (4%) | 9 |
| High |
7 (15%) |
18 (39%) |
0 (0%) |
25 |
|
Late | ||||
| Skin (radiation dermatitis) | 46 (100%) | 0 (0%) | 0 (0%) | 46 |
| Rectum (haemorrhage) | 43 (94%) | 3 (7%) | 0 (0%) | 46 |
| Rectum (proctitis) | 45 (98%) | 1 (2%) | 0 (0%) | 46 |
| GU (haematuria) | 40 (87%) | 6 (13%) | 0 (0%) | 46 |
| GU (urinary frequency) | 29 (63%) | 17 (37%) | 0 (0%) | 46 |
| GU (urinary stricture) |
46 (100%) |
0 (0%) |
0 (0%) |
46 |
|
Late toxicity by risk | ||||
| Low | 5 (11%) | 7 (15%) | 0 (0%) | 12 |
| Intermediate | 4 (9%) | 5 (11%) | 0 (0%) | 9 |
| High | 16 (35%) | 9 (19%) | 0 (0%) | 25 |
Abbreviation: GU=genitourinary.
Acute grade 2 urinary frequency was seen in two patients (4%). There were no grade 2 or more toxicities in the late toxicity.
Treatment outcomes are shown in Table 3. There were no biochemical failures (PSA nadir +2.0) during the follow-up period. Almost all patients showed a good response to the hormonal therapy, and the PSA nadir was <0.1 ng ml−1 in 43 out of 46 (94%) patients. Two (4%) patients had a PSA nadir in the range of 0.1–1.0 ng ml−1, and 1 patient who refused to undergo hormonal therapy had a PSA nadir of 1.91 ng ml−1. At the last follow-up date, 26 (57%) patients had rising PSA (⩾0.1 ng ml−1) after cessation of hormonal therapy, and the remaining 20 patients, including 5 patients undergoing hormonal therapy. One patient who underwent castration did not show rising PSA. None of the patients showed rising PSA during the hormonal therapy.
Table 3. Biochemical failure and the changes of serum PSA level.
| Biochemical failure |
Failure (−) |
46 (100%) |
| (PSA nadir +2.0) |
Failure (+) |
0 (0%) |
| PSA nadir |
Median |
0.01 ng ml−1 |
| |
<0.01 |
20 (44%) |
| |
⩽0.01 to <0.1 |
23 (50%) |
| |
⩽0.1 to <1.0 |
2 (4%) |
| |
⩽1.0 to <2.0 |
1 (2%) |
| |
<2.0 |
0 (0%) |
| PSA value at latest follow-up date |
<0.01 |
7 (15%) |
| |
⩽0.01 to <0.1 |
12 (26%) |
| |
⩽0.1 to <1.0 |
23 (50%) |
| |
⩽1.0 to <2.0 |
4 (9%) |
| |
<2.0 |
0 (0%) |
| PSA rise ⩾0.1 |
+ |
26 (57%) |
| |
− |
20 (43%) |
| Local recurrence |
|
0 (0%) |
| Distant metastasis | 0 (0%) |
Abbreviation: PSA=prostate-specific antigen.
Although almost all patients show PSA rise after termination of ADT, none of the patients showed biochemical failure.
Discussion
Gastrointestinal adverse events
In definitive radiotherapy for prostate cancer, late adverse effects tend to be more problematic than acute adverse effects. The rate of ⩾G2 rectal toxicity after radiotherapy has been reported as 5.1% in the analysis of patients who enrolled in the RTOG 75-06 and 77-06 trials (Pilepich et al, 1984). However, a lower total dose of radiation of ∼65 Gy was used in those trials, so their results cannot be compared with recent data. The results of a randomised controlled trial by Pollack et al (2002) suggested that the rates of ⩾G2 rectal toxicity were 12% in patients who received 70 Gy/35 fr. and 26% in patients who received 78 Gy/39 fr., and the rate of rectal toxicity was significantly higher in the high-dose group. Those results may be higher than those of recent studies because the trial was performed from 1993 to 1998, before image-guided radiotherapy (IGRT) or IMRT had been developed. Vargas et al (2005) analysed 331 patients treated with a median dose of 79.7 Gy by 3D-CRT and reported that the rate of ⩾G2 rectal toxicity was 20% and the rate of G3 rectal toxicity was 4% at 3 years. Rectal toxicity has improved compared with the report of Pollack et al., despite using a higher total dose of radiation, seemingly due to development of radiotherapy techniques. In the analysis of 743 patients treated with 75.6–81.0 Gy by 3D-CRT at Memorial Sloan-Kettering Cancer Center, it was reported that the incidence of ⩾G2 rectal toxicity was 15% (Zelefsky et al, 1999). Although the incidence of rectal toxicity is approximately twice as high as that in the lower dose group (64.8–70.2 Gy), the rate of rectal toxicity has been improving. In a subsequent trial at the same institution that compared 3D-CRT and IMRT using a total dose of 81 Gy, the rate of rectal toxicity of IMRT was significantly lower than that of 3D-CRT (2% vs 14%) (Zelefsky et al, 2001). A similar result has been reported in the study on GI toxicity comparing 79.2 Gy by IMRT and 79.2 Gy by 3D-CRT (⩾G2 GI toxicity at 3 years; 15% vs 22%, P=0.039) (Michalski et al, 2013). Although dose escalation is inevitable from the viewpoint of treatment outcomes for prostate cancer, IMRT or IGRT allows for a high dose of radiation with feasible toxicity.
In the present phase I/II study of hypofractionated CIRT, the incidence of G1 late rectal toxicity was 7% (3 out of 46) and that of G2 late rectal toxicity was 0%. There were no acute rectal toxicities. The dose distribution of CIRT using Bragg-peak is better than that of photons, and the dose distribution of CIRT with less beam scattering is also better than that of protons. The ability to easily decrease the irradiated rectal volume is considered to be one of the reasons for lower rectal toxicity.
Genitourinary adverse events
Frequent GU adverse events include haematuria, urinary stricture, and urinary frequency. The incidence of ⩾G2 late GU toxicity was ∼5% in the RTOG 75-06 and 77-06 trials (Pilepich et al, 1984). In the abovementioned phase III clinical trial reported by Pollack et al (2002), the rates of G2 and G3 late GU toxicities in the 151 patients in the 78 Gy group were 10% and 3%, respectively, whereas the rates of G2 and G3 late GU toxicities in the 150 patients in 70 Gy group were 7% and 1%, respectively. The GU toxicity tended to increase according to the irradiation dose, but there were no significant differences. The study at the Memorial Sloan-Kettering Cancer Center reported that the rates of ⩾G2 late GU toxicity of the high-dose group (75.6–81.0 Gy) at 3 and 5 years were 10% and 15%, respectively (Zelefsky et al, 1999). In the following study comparing IMRT and 3D-CRT in 1100 patients, the rates of G2 and G3 late GU toxicities were 13% and 1.5%, respectively, in the group who received ⩾75.6 Gy (Zelefsky et al, 2001). Unlike the rates of rectal toxicity, there was no significant difference between 3D-CRT group and IMRT group in the late GU toxicity (P=0.32). In a recent study, ⩾G2 GU toxicity at 2 years of 3D-CRT group (76 Gy) and IMRT group (78 Gy) were 40% and 30%, respectively (P=0.011) (Sveistrup et al, 2014). Although significant difference was shown in GU toxicity in that study, the difference is smaller than that of rectal toxicity. In another study on SRT for prostate cancer, a total of two patients (7%) show grade 3 genitourinary (GU) toxicity in the arm of 47.5 and 50 Gy in five fractions (Boike et al, 2011). Further, because the median follow-up time of 47.5-Gy group and 50-Gy group is 18 and 12 months, respectively, the incidence of late adverse effect may increase in the future. It is believed that sufficient data are needed when performing the short-term irradiation by X-rays.
In the present study of CIRT, the rate of ⩾G2 late GU toxicity was 0% (Table 2). Grade 1 haematuria was observed in 6 (13%) patients and G1 urinary frequency was observed in 17 (37%) patients. None of these patients required any treatment, and symptoms were improved with observation. No other ⩾G2 late toxicities were found. However, GU late toxicity sometimes occurs ⩾36 months after radiotherapy; therefore, the follow-up period of the present study was not sufficient to observe all late GU adverse events (Zelefsky et al, 1999). However, because the long-term incidence of late GU toxicity can be predicted from the incidence at 2–3 years (Zelefsky et al, 1999), the rate of late GU toxicity of this phase I/II CIRT study is highly favourable.
Biochemical failure and metastases
There were no biochemical failures or distant metastases as of the last follow-up date. The PSA nadir of almost all patients (94%) showed good response to the treatment (<0.1 ng ml−1), which may be due to the fact that all but one patient received hormonal therapy. Hormonal therapy to the low-risk patients may be overtreatment, but this trial was designed to be able to include these patients. Twenty-six of forty-six (57%) patients showed rising PSA ⩾0.1 ng ml−1 after cessation of hormonal therapy, but there were no patients with rising PSA >2.0 ng ml−1 above the PSA nadir. Several studies have reported on PSA bouncing after radiotherapy or PSA rising after hormonal therapy (Crook et al, 2007; Pinkawa et al, 2007). It has been reported that long-term PSA rising after hormonal therapy is a concern for the recovery of serum testosterone level (Pickles et al, 2002). After completion of CIRT, the PSA level was not so different from that in patients treated with radiotherapy by photons and hormonal therapy. Long-term outcomes will be reported in the future.
Treatment period
The standard total dose of definitive radiotherapy for prostate cancer by photons is considered to be ⩾70 Gy. In the EORTC (European Organisation for Research and Treatment of Cancer) 22 863 trial, 70 Gy/35 fr. over 7 weeks was used (Bolla et al, 2010). After which, the total dose was increased to 75.6–81.0 Gy for intermediate- or high-risk patients. These treatments require 8.5–9 weeks to complete the irradiation (Zelefsky et al, 2001). In proton therapy as well as photon radiotherapy, 7.5 weeks are required for the completion of the treatment of 74 GyE, and >8 weeks are required for the dose-escalated treatment using 82 GyE (Slater et al, 1998; Coen et al, 2011). Although there are few studies that evaluated OTT of radiotherapy and prognosis in the treatment of prostate cancer, a significant relationship between OTT of radiotherapy and recurrence rate has been reported in a recent study (D'Ambrosio et al, 2008). Although there were no significant differences between the short-OTT group and the long-OTT group in the patients who received >74 Gy, patients in the long-OTT group tended to have a higher rate of biochemical failure than the patients in the short-OTT group (Liauw and Liauw, 2011). From these studies, it can be said that dose escalation contributes to the improvement of local control; however, extension of the treatment period due to the increase in dose is considered to be unfavourable for local control. Recently, SRT for prostate cancer has been suggested as short-term external body radiotherapy (Madsen et al, 2007). A study concluded that SRT could be completed with a schedule of five fractions with tolerable toxicity. However, the biological treatment effects and adverse effects can be different from conventional dose/fraction because dose/fraction has a large impact on biological effect in radiotherapy by photons. But the long-term follow-up results of SRT have not been reported.
Although the median follow-up period was 31 months, the results of the present study demonstrated feasibility and local control at this time point. More than 1600 prostate cancer patients have been treated with CIRT in the >18 years it has been available. It has been reported that the long-term outcome of CIRT was very favourable even in high-risk prostate cancer (Tsuji et al, 2005). Compared with other EBRTs, with the exception of SRT, CIRT for prostate cancer has steadily shortened the treatment time while maintaining good local controllability. The biological characteristics of CIRT are considered to be one of the advantages of this modality. The biological effect of the carbon ion beam is less susceptible to dose/fraction than that of photons (Kanai et al, 1999). However, ∼2 months of hospital visits and admissions for prostate cancer patients is one of the disadvantages. Shortening the treatment period is favourable not only for treatment outcomes but also for quality of life of the patients.
Conclusions
The results of the phase I/II clinical trial shortening the treatment period of CIRT for prostate cancer have been reported. The treatment period of irradiation has been shortened from 4 weeks to 3 weeks without occurring ⩾G2 adverse events with a median follow-up of 32 months. Long-term outcomes (e.g., biochemical failure-free survival and local control rate) of the 3-week treatment period are required.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Akakura K, Tsujii H, Morita S, Tsuji H, Yagishita T, Isaka S, Ito H, Akaza H, Hata M, Fujime M, Harada M, Shimazaki J, Working Group for Genitourinary Tumors 2004Phase I/II clinical trials of carbon ion therapy for prostate cancer Prostate 58(3252–258.Erratum in: Prostate 2004;61(1):103. [DOI] [PubMed] [Google Scholar]
- Akimoto T, Ito K, Saitoh J, Noda SE, Harashima K, Sakurai H, Nakayama Y, Yamamoto T, Suzuki K, Nakano T, Niibe H. Acute genitourinary toxicity after high-dose-rate (HDR) brachytherapy combined with hypofractionated external-beam radiation therapy for localized prostate cancer: correlation between the urethral dose in HDR brachytherapy and the severity of acute genitourinary toxicity. Int J Radiat Oncol Biol Phys. 2005;63 (2:463–471. doi: 10.1016/j.ijrobp.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Boike TP, Lotan Y, Cho LC, Brindle J, DeRose P, Xie XJ, Yan J, Foster R, Pistenmaa D, Perkins A, Cooley S, Timmerman R. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol. 2011;29 (15:2020–2026. doi: 10.1200/JCO.2010.31.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Billiet I, Torecilla JL, Pfeffer R, Cutajar CL, Van der Kwast T, Collette L. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11 (11:1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- Cahlon O, Zelefsky MJ, Shippy A, Chan H, Fuks Z, Yamada Y, Hunt M, Greenstein S, Amols H. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71 (2:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Crook J, Gillan C, Yeung I, Austen L, McLean M, Lockwood G. PSA kinetics and PSA bounce following permanent seed prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2007;69 (2:426–433. doi: 10.1016/j.ijrobp.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Coen JJ, Bae K, Zietman AL, Patel B, Shipley WU, Slater JD, Rossi CJ. Acute and late toxicity after dose escalation to 82 GyE using conformal proton radiation for localized prostate cancer: initial report of American College of Radiology Phase II study 03-12. Int J Radiat Oncol Biol Phys. 2011;81 (4:1005–1009. doi: 10.1016/j.ijrobp.2010.06.047. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio DJ, Li T, Horwitz EM, Chen DY, Pollack A, Buyyounouski MK. Does treatment duration affect outcome after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72 (5:1402–1407. doi: 10.1016/j.ijrobp.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, GLOBOCAN 2012. v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. International Agency for Research on Cancer: Lyon, France; 2013. Available from: http://globocan.iarc.fr accessed on day/month/year.
- Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44 (3:265–276. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi-Kasai K, Ohara H, Yatagai F, Kanai T, Ando K. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat Res. 2000;154 (5:485–496. doi: 10.1667/0033-7587(2000)154[0485:ioaahc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Tsuji H, Kamada T, Yanagi T, Mizoe JE, Kanai T, Morita S, Wakatsuki M, Shimazaki J, Tsujii H, Working Group for Genitourinary Tumors Carbon ion radiation therapy for prostate cancer: results of a prospective phase II study. Radiother Oncol. 2006;81 (1:57–64. doi: 10.1016/j.radonc.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Tsuji H, Kamada T, Hirasawa N, Yanagi T, Mizoe JE, Akakura K, Suzuki H, Shimazaki J, Tsujii H. Risk factors of late rectal bleeding after carbon ion therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66 (4:1084–1091. doi: 10.1016/j.ijrobp.2006.06.056. [DOI] [PubMed] [Google Scholar]
- Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka T, Furusawa Y, Ando K, Suzuki M, Soga F, Kawachi K. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44 (1:201–210. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- Khoo VS. Radiotherapeutic techniques for prostate cancer, dose escalation and brachytherapy. Clin Oncol (R Coll Radiol) 2005;17 (7:560–571. doi: 10.1016/j.clon.2005.07.006. [DOI] [PubMed] [Google Scholar]
- King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, Meier R, Wang J, Kupelian P, Steinberg M, Katz A. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109 (2:217–221. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Liauw SL, Liauw SH. Prolongation of total treatment time because of infrequently missed days of treatment is not associated with inferior biochemical outcome after dose-escalated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81 (3:751–757. doi: 10.1016/j.ijrobp.2010.06.054. [DOI] [PubMed] [Google Scholar]
- Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67 (4:1099–1105. doi: 10.1016/j.ijrobp.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Masson S, Persad R, Bahl A. HDR brachytherapy in the management of high-risk prostate cancer. Adv Urol. 2012;2012:980841. doi: 10.1155/2012/980841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, Rogers CG, Sammon J, Siddiqui SA, Diaz M. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol. 2010;58 (6:838–846. doi: 10.1016/j.eururo.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Michalski JM, Yan Y, Watkins-Bruner D, Bosch WR, Winter K, Galvin JM, Bahary JP, Morton GC, Parliament MB, Sandler HM. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87 (5:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute 2009. Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. May 29, NIH publication # 09-7473.
- Okada T, Tsuji H, Kamada T, Akakura K, Suzuki H, Shimazaki J, Tsujii H, Working Group for Genitourinary Tumors Carbon ion radiotherapy in advanced hypofractionated regimens for prostate cancer: from 20 to 16 fractions. Int J Radiat Oncol Biol Phys. 2012;84 (4:968–972. doi: 10.1016/j.ijrobp.2012.01.072. [DOI] [PubMed] [Google Scholar]
- Peinemann F, Grouven U, Hemkens LG, Bartel C, Borchers H, Pinkawa M, Heidenreich A, Sauerland S. Low-dose rate brachytherapy for men with localized prostate cancer. Cochrane Database Syst Rev. 2011. p. CD008871. [DOI] [PubMed]
- Pickles T, Agranovich A, Berthelet E, Duncan GG, Keyes M, Kwan W, McKenzie MR, Morris WJ, British Columbia Cancer Agency, Prostate Cohort Outcomes Initiative Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer. 2002;94 (2:362–367. doi: 10.1002/cncr.10219. [DOI] [PubMed] [Google Scholar]
- Pilepich MV, Krall J, George FW, Asbell SO, Plenk HD, Johnson RJ, Stetz J, Zinninger M, Walz BJ. Treatment-related morbidity in phase III RTOG studies of extended-field irradiation for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1984;10 (10:1861–1867. doi: 10.1016/0360-3016(84)90263-3. [DOI] [PubMed] [Google Scholar]
- Pinkawa M, Fischedick K, Piroth MD, Gagel B, Borchers H, Jakse G, Eble MJ. Prostate-specific antigen kinetics after brachytherapy or external beam radiotherapy and neoadjuvant hormonal therapy. Urology. 2007;69 (1:129–133. doi: 10.1016/j.urology.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA, Rosen I. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53 (5:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Konski AA, Movsas B, Greenberg RE, Uzzo RG, Ma CM, McNeeley SW, Buyyounouski MK, Price RA., Jr Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64 (2:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach M, 3rd, Hanks G, Thames H, Jr, Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65 (4:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172 (3:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- Slater JD, Yonemoto LT, Rossi CJ, Jr, Reyes-Molyneux NJ, Bush DA, Antoine JE, Loredo LN, Schulte RW, Teichman SL, Slater JM. Conformal proton therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 1998;42 (2:299–304. doi: 10.1016/s0360-3016(98)00225-9. [DOI] [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz M, Wittekind C, International Union against Cancer (UICC) 2009TNM Classification of Malignant Tumours7th edn.Wiley-Blackwell: Hoboken, NJ [Google Scholar]
- Souhami L, Bae K, Pilepich M, Sandler H. Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: a secondary analysis of RTOG 85-31. J Clin Oncol. 2009;27 (13:2137–2143. doi: 10.1200/JCO.2008.17.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveistrup J, Af Rosenschöld PM, Deasy JO, Oh JH, Pommer T, Petersen PM, Engelholm SA. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol. 2014;9 (1:44. doi: 10.1186/1748-717X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touijer K, Secin FP, Cronin AM, Katz D, Bianco F, Vora K, Reuter V, Vickers AJ, Guillonneau B. Oncologic outcome after laparoscopic radical prostatectomy: 10 years of experience. Eur Urol. 2009;55 (5:1014–1019. doi: 10.1016/j.eururo.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Yanagi T, Ishikawa H, Kamada T, Mizoe JE, Kanai T, Morita S, Tsujii H, Working Group for Genitourinary Tumors Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63 (4:1153–1160. doi: 10.1016/j.ijrobp.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Tsujii H, Mizoe JE, Kamada T, Baba M, Kato S, Kato H, Tsuji H, Yamada S, Yasuda S, Ohno T, Yanagi T, Hasegawa A, Sugawara T, Ezawa H, Kandatsu S, Yoshikawa K, Kishimoto R, Miyamoto T. Overview of clinical experiences on carbon ion radiotherapy at NIRS. Radiother Oncol. 2004;73 (Suppl 2:S41–S49. doi: 10.1016/s0167-8140(04)80012-4. [DOI] [PubMed] [Google Scholar]
- Vargas C, Martinez A, Kestin LL, Yan D, Grills I, Brabbins DS, Lockman DM, Liang J, Gustafson GS, Chen PY, Vicini FA, Wong JW. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62 (5:1297–1308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, Venkatramen ES, Leibel SA. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85 (11:2460–2468. doi: 10.1002/(sici)1097-0142(19990601)85:11<2460::aid-cncr23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, Reuter VE, Venkatraman ES, Leibel SA.2001High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer J Urol 166(3876–881.Erratum in: J Urol166(5):1839. [PubMed] [Google Scholar]
- Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, Park J, Shippy A. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys. 2008;71 (4:1028–1033. doi: 10.1016/j.ijrobp.2007.11.066. [DOI] [PubMed] [Google Scholar]
- Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, Bush DA, Lunt M, Spiegel DY, Skowronski R, Jabola BR, Rossi CJ. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28 (7:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]