A brief history of the beginnings of telomere research
Origins
The appreciation that there might be something special about chromosome ends began in the 1930’s through 2 independent observations. Herman Muller, working with fruit flies, noticed that X-rays caused chromosome breakages and that the broken ends subsequently fused with each other (1). He noticed that the real ends of chromosomes never took part in these fusion events and concluded chromosome ends were sealed in some way. Barbara McClintock at about the same time, observed in maize that dicentric chromosomes, chromosomes with two centromeres, broke at mitosis and that the broken ends fused with each other (2). Again the natural chromosome ends were not involved. She also noticed that the fusion events did not happen in embryonic cells – the broken ends here were “healed” somehow.
In 1961 Leonard Hayflick found that he could grow normal human diploid cells but they would only divide a limited number of times before they stopped dividing, a state he attributed to cellular senescence (3). A decade later Jim Watson worked out that there was a problem with replicating linear DNA at the ends. The problem is caused by the fact that DNA polymerase uses an RNA primer and only synthesizes DNA in the 5′ to 3′ direction. That is not a problem for the strand being built that ends at the 3′, the leading strand, but the other one, that begins at the 3′ end, the lagging strand, cannot start at the beginning because the RNA primer has to anneal with the other strand. Watson called this the end replication problem Watson, 1972 #23}. The same problem occurred to a Russian scientist Olovnikov (4, 5), reputedly inspired by looking at the train tracks in a Moscow underground station, but he realized that this meant that the chromosomes would get shorter with each replication cycle and proposed that this might be the reason for the replicative senescence described by Hayflick.
Discovery
Elizabeth Blackburn arrived in Joe Gall’s lab in 1975, fresh from her PhD in Fred Sanger’s lab in England where techniques of DNA sequencing were being developed. Gall was interested in how some organisms produce extra copies of ribosomal RNA (rRNA) genes. In frogs rRNA genes are amplified as circular molecules. He found the same thing in the ciliate protozoan Tetrahymena – here some molecules were circular and some linear. Ciliates like Tetrahymena contain a micronucleus which contains germ line DNA and gives rise to a macronucleus which contains thousands of DNA molecules, amplified and rearranged from the micronuclear genome which are templates for transcription. Blackburn decided to sequence the ends of these molecules to test the hypothesis that the linear to circular switching involved duplicated sequences at the ends as it does in Phage λ. When the sequence of the ends of the linear rDNA molecules was determined, not so easy in those days, she found tandem repeats of 6 mers, 5′TTGGGG3′n (6). She found the same sequence at the ends of other DNA molecules in the macronucleus (the structures at the ends of chromosomes in the micronucleus were basically similar when determined later (7)). Similar repeats were found at the ends of rDNA repeats in slime moulds. The repeats were added to the ends and there was no common sequence to which they were added.
The questions now were whether the structure of these telomeres is related to their function and did similar structures exist in other organisms. Jack Szostak was working on recombination in yeast and found that if plasmids were cut with a restriction enzyme, converting them from a circular to a linear form, they became reactive and unstable. When he made a linearized plasmid and attached Tetrahymena telomere sequences to the end he obtained many stable linear plasmids, showing that telomeres were functionally conserved (8). He went on to clone yeast telomeres by selecting for sequences that would stabilize linear plasmids (Figure 1). Yeast telomeres consisted of tandem repeats of TG1-3. The telomeres were heterogenous in length, leading to the hypothesis of a terminal transferase activity to compensate for erosion at the ends caused by incomplete replication (9).
Figure 1. Conservation of telomeres.
When circular DNA from yeast is cut with a restriction enzyme, the linearized DNA becomes very unstable. However, if telomeres from Tetrahymena are added, the linearized plasmid becomes stable (10). This demonstrates that telomeres are very conserved between species. In order to clone the yeast telomeres Szostak cut the end of the linearize DNA and added different yeast sequences to select for those that stabilzed it (8).
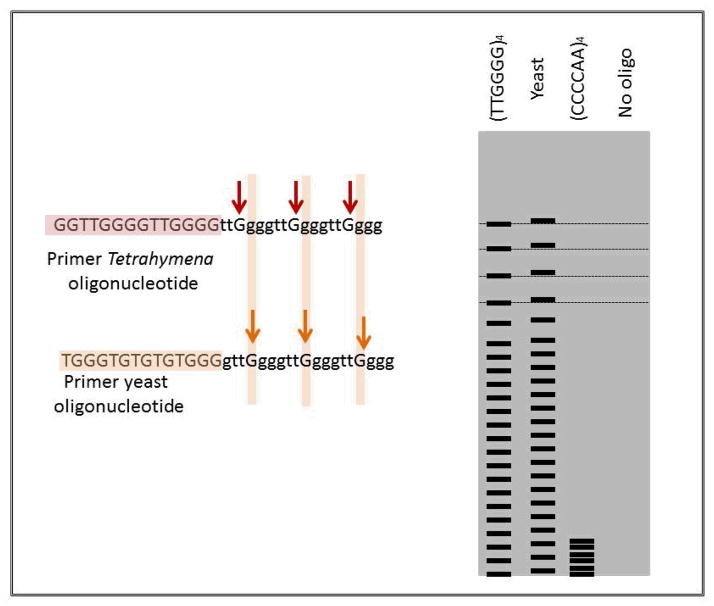
In 1984 Carol Greider started as a PhD student in Blackburn’s lab and took on the project of identifying the enzyme responsible for synthesizing the telomeric repeats. She added restriction fragments to Tetrahymena extracts hoping they might be extended by the hypothetical enzyme. At first this didn’t work but when she added the synthetic oligonucleotide (5′TTGGGG3′)4 she got a ladder of repeats with 6 base periodicity, showing the proposed enzyme activity (Figure 2). Oligonucleotides with sequences unrelated to telomere were not extended. When she tried to extend a 24 mer with the yeast derived telomere sequence 5′TGGG3′ at the end she got a repeating pattern that was shifted up by one base, showing a G must be added before the addition will work (10). The enzyme was called telomere terminal transferase and later became known as telomerase.
Figure 2. Identification of telomerase.
As shown in the gel diagram, the oligonucleotide (TTGGGG)4, the telomere sequence for Tetrahymena, was extended by telomerase in a cell free extract (10). The extension of yeast telomeres with the sequence TGGG is shifted one base showing that a G must be added before extension takes place. Telomerase activity is blocked by the complimentary sequence of Tetrahymena telomeres CCCCAA.
Greider went on to show that telomerase activity was sensitive to RNA and began to try and identify and purify an RNA component. She eventually succeeded in her own laboratory in Cold Spring Harbor by obtaining partial sequence of an RNA that co-purified with telomerase and using it to clone the gene. The RNA contains the sequence 5′CAACCCCAA3′, complimentary to the Tetrahymena telomere repeat TTGGGG and oligonucleotides that blocked this putative template blocked enzyme activity, showing a requirement for an RNA template (11). In Blackburn’s lab mutants were made in the template sequence in the Tetrahymena telomerase RNA, which was then overexpressed in vivo. Telomeres in these cells contained the mutant repeat sequence, showing that the 5′CAACCCCAA3′ was the true template. All 3 mutants made either lost the mutant genes or became senescent – pointing to a new function for telomeres (12).
Towards translation – Senescence, cancer and inherited disease
In 1989 Szostak’s lab isolated telomerase mutants in yeast. One, in a gene called EST1, led to shortening telomeres and showed a delayed senescence phenotype, many generations of normal growth, then an increase in chromosome loss and reduction in growth potential (13). One of the RNA mutants studied in Blackburn’s lab had a similar phenotype. This mutant blocked telomere addition – the cells grew for 20–25 divisions and then stopped dividing (senesced). These experiments showed that functional telomerase is necessary for indefinite replication of yeast and Tetrahymena. EST1 turned out not to be the gene for the catalytic subunit of telomerase, which was cloned in 1997 and called EST2(14).
Knowing the structure of telomere DNA and telomerase enabled the hypothesis, that telomeres may be responsible for the Hayflick limit, to be examined. Harley and Greider showed that telomeres shorten in human fibroblasts, cells that do not express telomerase, with increasing numbers of cell divisions (15). When the cells are expressing telomerase this shortening does not take place (16). Expressing telomerase in human cells extends their lifespan in terms of the number of divisions they can undergo before they senesce, establishing short telomeres as a cause of cellular senescence (17).
It was realized in the 1990s that while telomerase is not expressed in most somatic cells high levels of telomerase are expressed in most cancer cells (18), where they are required to prevent telomere attrition and consequent replicative senescence and enable proliferation and immortality of the cells. The exceptions to this are tumor cells that use an alternative method of telomere lengthening (ALT) whereby telomeres are lengthened by a mechanism involving homologous recombination between telomeres, underscoring the importance of telomere maintenance in cancer (19). Telomeres play another role in cancer formation. When telomeres become critically short, which may be due to a mutation affecting telomeres or to the effects of aging or chronic inflammation, somatic cells undergo a cell cycle arrest triggered by pathways involving p53 activation. These cells often die through apoptosis but in rare cases, due to a somatic mutation in a gene encoding a product involved in the control of the cell cycle, or to the activation of telomerase, such cells can begin to divide. With short telomeres dividing cells undergo end-to end telomeric fusions which can lead to chromosomal breakage at mitosis and this breakage/fusion cycle leads to genomic instability and the opportunity for carcinogenic mutational events (20, 21). Thus while lack of telomerase and short telomeres can initiate tumorigenesis, telomerase is required for tumor growth (Figure 3).
Figure 3. Dynamics of telomere length in germ, stem, somatic and tumor cells.
Telomere length in germ cells is stable, while it decreases slightly in stem cells with time. Telomerase is expressed in both cell types. However, telomerase is not expressed in normal somatic cells, and telomere length decreases with each replication cycle. When telomeres get critically short, normal cells go into senescence. However, mutations can occur that enable the cells to continue dividing and telomeres get even shorter. They enter a crisis. Crisis is characterized by cell death and concomitant cytogenetic abnormalities produced by chromosome fusion/breakage cycles. Telomeric crisis produces significant chromosomal instability, a hallmark of human cancer, and may increase the occurrence of genetic alterations that would favor neoplastic transformation. If the telomerase enzyme is activated, these cells can grow to form a tumor (15–18).
The importance of telomere maintenance in inherited disease was realized when the gene causing a rare bone marrow failure syndrome, X-linked dyskeratosis congenita, turned out to be DKC1, encoding dyskerin (22). Subsequently it was discovered that dyskerin was part of the telomerase complex (23) and that autosomal forms of DC could be caused by mutations in the core components of telomerase TERC (24) and TERT (25). As explained in the article by Grammatges and Bertush (26) a feature of the autosomal forms of the disease is that they show anticipation, with the disease becoming more severe, and the age of onset lower, in succeeding generations (27) This is almost certainly because children of affected parents inherit shortened telomeres as well as the mutation, so with each generation life starts with shorter telomeres than the last one. While later generations may show the severe bone marrow failure associated with dyskeratosis congenita, earlier generations may be less severely affected, developing aplastic anemia as adults or in middle age and contracting pulmonary fibrosis, osteoporosis or liver cirrhosis later in life (28). It is interesting that with these mutations that affect telomeres the clinical picture changes with increasing severity with the phenotype being influenced by the time point when telomeres become critically short.
Translational aspects of telomerase and telomere research
From the discussion above telomere maintenance is important to human health in 3 ways, which we will discuss separately. The first concerns telomere shortening in normal aging, and a consideration of factors, including life quality or stress, that can increase or decrease telomere length. The second is the effect of inherited mutations in genes encoding telomere and telomerase components and the third is the role of telomeres and telomerase in cancer.
Diseases of stress and aging
Since telomere length decreases with cell division, and since telomere length gets shorter as we age, it is popular to assume that telomere length influences aging, but proving this connection will require large longitudinal studies, because the natural variation in telomere length is so great, and because the phenomenon may operate in only certain cells or tissues. Nevertheless a connection between telomere length and aging is suggested by a number of observations. A pioneering study by Cawthon was the first to show that telomere length measured in blood cells is related to mortality, people with shorter telomeres being more likely to succumb to cardiovascular problems and infectious diseases (29). Since then short telomeres have been found to be associated with a number of conditions including increased susceptibility to cancer. It is never perfectly clear in these studies whether short telomeres increase the likelihood of disease or the disease leads to increased telomere shortening (30). Large prospective studies are needed to answer this crucial question.
Over the past decade there has been a deluge of publications showing the factors known to be associated with poor health and early mortality are also correlated with telomere length. Thus people living with chronic stress, like mothers with a disabled child (31), or those caring for a relative with Alzheimers disease (32) have significantly shorter telomeres, measured in blood leukocytes, than a carefully selected control population. Smoking (33), obesity (34) and occupational exposure to pollutants (35) are associated with short telomere lengths and early mortality while telomeres are longer in those with a healthy diet (36) and plenty of aerobic exercise (37). A diet rich in antioxidants was shown to be associated with longer telomeres in a study where patients with coronary heart disease were followed for 5 years and with longer telomeres (38) and a decreased rate of breast cancer in another study (39). Recently evidence has been accumulating that stress early in life, in childhood or even in utero, which can lead to poor health in later life, is associated with short telomeres in adulthood (40).
Translation of this information into clinical practice can be attempted by persuading people to live a more healthy life style. If the mechanism whereby stress leads to telomere shortening was established new therapeutic approaches could be developed. The major culprits are thought to be the immune system and oxidative stress (41, 42). Short telomeres in immunological cells may lead to poor immune surveillance and increased disease susceptibility while increased oxidative stress increase the rate of damage of telomere DNA. Life stress may lead to oxidative stress by chronic activation of the autonomic or neuroendocrine system (43). Telomeres, which are rich in guanine residues, may be particularly sensitive to ROS because guanine can be oxidized to 8 hydroxyguanine which is unstable.
The issues of connecting telomere length with disease and the difficulties and problems are comprehensively discussed, with respect to cardiovascular disease in the paper by Nilsson et al (44). An interesting new connection between telomeres and heart disease has recently been made by Mourkioti and her colleagues (45). These workers found that mdx mice, which contain a mutation in dystrophin that causes Duchene muscular dystrophy in humans, did not show the DMD phenotype. When they bred the mutation into mice with short telomeres however the mice developed severe cardiac defects, a characteristic feature of DMD, which were accompanied by telomere erosion specifically in cardiomyocytes. Cardiac function was improved in these mice by antioxidant treatment. Interestingly short telomeres in cardiomyocytes were also observed in human samples from DMD patients. In this model at least it seems that oxidative stress and telomere shortening in a specific cell type are conspiring to induce cardiac failure. Again the work suggests that treatment with antioxidants could be explored as a beneficial therapy in DMD.
Rare and not so rare inherited diseases
Dyskeratosis congenita is a rare bone marrow failure syndrome caused by mutations in genes that affect telomere maintenance (26). At the moment (Figure 4) these genes comprise DKC1(22), NOP10(46), NHP2(47), TERT (25, 48) and hTR (24), which are all part of the telomerase complex, TINF2 (49, 50) which encodes a component of shelterin, TCAB1 (51) which is involved in assembly of telomerase and its localization in the Cajal body and RTEL1 (48, 52) which is important in telomere replication. Some cases are also caused by mutations in CTC1(53, 54) which is essential for DNA replication, and it is likely that replication at the telomere may be particularly sensitive to CTC1 mutations. CTC1 is also part of the CST complex which is involved in regulating telomerase activity at the telomere (55). The effect of all these mutations is to lead to an increased rate of telomere shortening and the eventual failure to maintain tissues that depend on stem cells for renewal, particularly blood cells. Mutations in the telomerase core components, TERT and TERC, show genetic anticipation whereby telomeres get shorter with each successive generation and cause more severe disease with earlier age of onset (27, 56). A consequence of this is that parents and grandparents of some DC patients may carry DC causing mutations in TERT and TERC but be asymptomatic or have benign mild anemia. Examination of DC pedigrees revealed distinct patterns of disease in these early generations. The prevalence of aplastic anemia, pulmonary fibrosis, liver cirrhosis, osteoporosis and malignancy, particularly AML, MDS and epithelial cancers of the gastrointestinal tract, was significantly higher than expected (26, 28, 57–59). This observation led to investigations of patients with some of these conditions, previously regarded as idiopathic, for the presence of telomerase mutations (25, 60, 61). It is now apparent that about 4% of cases of aplastic anemia are due to TERC and 4% due to TERT mutations. In addition about 10% of cases of pulmonary fibrosis are due to telomerase mutations, mainly TERT but also some TERC mutations. The effect of telomere defects in blood and lung disease are discussed in more detail in the articles by Gramatges and Bertusch (26) and by Gansner (62) respectively in this volume.
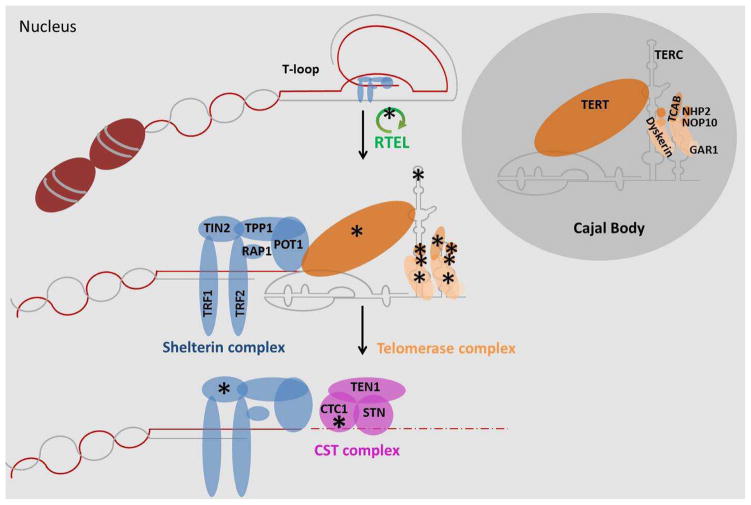
Figure 4. Telomerase and telomeres.
Telomeres are nucleoprotein complexes at the end of chromosomes that protect and stabilise them. The T-loop conformation of the telomeres are resolved by the protein RTEL and others before replication. Then, members of the shelterin complex recruit the telomerase complex to the telomere region. Here, the telomerase works uses its integral RNA as a template to elongate telomeres. The CST complex binds to the extended telomeres and supresses telomerase access (55). The CST complex also promotes fill-in synthesis of the C-strand stimulating DNA polymerase α-primase. * mutations in the genes encoding these proteins (or RNA in the case of TERC) have been linked to Dyskeratosis congenita (26).
In terms of translational research into inherited diseases the most important advance so far is the understanding of the genetic basis of dyskeratosis congenita and the realization that some more common conditions are sometimes due to telomerase mutations. This knowledge greatly improves the speed and accuracy with which DC patients can be diagnosed. This is important since the major childhood forms of DC can be treated with bone marrow transplant but patients are very sensitive to the normal conditioning regimens. With a diagnosis of DC modified regimens are used (63, 64). Rapid genetic diagnosis also enables prenatal diagnosis to be offered in pregnancies known to be at risk. In addition in young adults, where DC is associated with an increased risk of malignancy, tumor surveillance is implemented as part of the patient care (65). Knowledge of the genetics is of course a prerequisite for the development of new and more effective treatments.
For the later onset conditions such as aplastic anemia and pulmonary fibrosis, can anything be gained by knowing that a patient is susceptible to develop these conditions later in life? Such mutation carriers should certainly be advised not to smoke to decrease the chance of developing PF. Trials are needed to explore whether antioxidant or anti-inflammatory treatment from an early age might delay the appearance of telomere associated diseases, since they are associated with an increase in the levels of cellular reactive oxygen species (ROS) (66, 67).
Cancer
Paradoxically, short telomeres can be responsible for initiating a string of events that lead to cancer while the ability to maintain telomeres above a critical length is an important feature of a malignant cell. As telomeres become critically short they can no longer be protected from degradation or from the cells own DNA damage response mechanisms and they trigger a cell cycle arrest mediated by a signaling pathway involving p53. Rarely such cells might acquire a mutation, for example in p53 or another gene involved in mediating the cell cycle arrest and cells with short telomeres can divide. Under these circumstance telomeres fuse together and such fused chromosomes break apart at mitosis, leading to repeated breakage/fusion cycles and genomic instability that favors the generation of malignant cells (68). If telomerase is upregulated, or the ALT mechanism activated, these cells, harboring mutations and genomic rearrangements can then proliferate forming a tumor. As we learn more about the causes of short telomeres the incidence of cancer caused in this way may be decreased, perhaps by lifestyle changes as discussed above or through the development of specific drugs, for example, that affect generation of ROS.
While most adult cells do not express telomerase cancer cells need telomerase to maintain their telomeres through multiple rounds of cell division as they proliferate (18). A crucial step in carcinogenesis is therefore the switching on of telomerase, or in some cases the activation of the ALT mechanism, by which telomeres are maintained by a method involving homologous recombination (19). About 90% of tumors express telomerase. The mechanisms by which telomerase is switched include gene amplification of the TERT (69, 70) gene or activation of the c-myc oncogene which promotes transcription of TERT (71, 72). However it has recently been discovered that, in many cases, expression of TERT is due to specific mutations in the promoter of the TERT gene itself (73). These mutations occur in either of two specific hot spots and, in each case, create a consensus recognition site for transcription factors called ETS/TCF. They were initially discovered in melanoma (73, 74) but have now been found in a significant proportion of many cancers including gliomas, thyroid, bladder and hapatocarcinomas (75). In many cases an increase in the TERT transcript associated with these mutations has been demonstrated experimentally. In the case of hepatocellular carcinoma that arises from preneoplastic cirrhotic lesions TERT promoter mutations are the first genetic events that occur, suggesting that short telomeres may have been central to the mechanism leading to transformation (76).
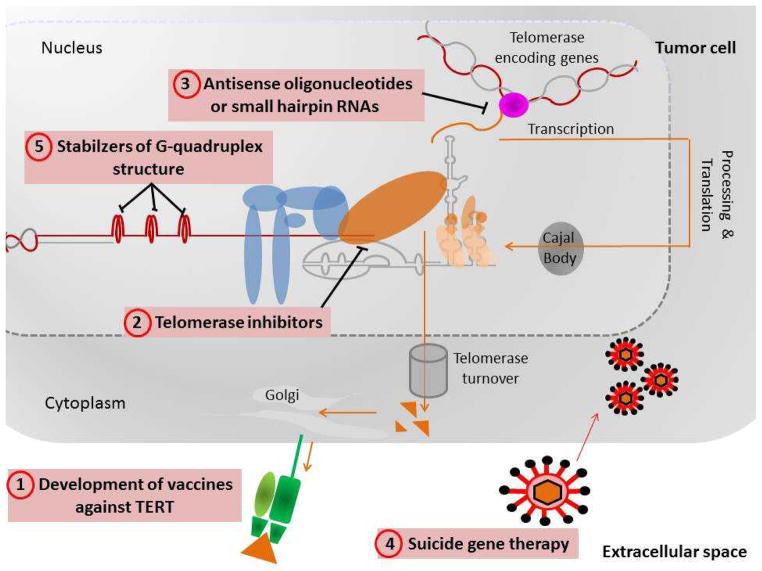
The fact that many cancers appear to depend on the expression of telomerase for their continued proliferation has stimulated the development of new therapeutic approaches (77)(Figure 5). Different ways of exploiting this to treat cancer are:-
Figure 5. Telomere based therapeutic approaches proposed to treat cancer.
These possible routes to cancer therapy are based on the observation that telomerase is expressed in cancer cells but not in other somatic cells (77).
Development of a vaccine against TERT. Proliferating cancer cells, since they express TERT, should present some TERT peptides in the context of HLA1 molecules. After vaccination the patient may mount an immune response against the tumor cells which will be killed by cytotoxic T cells. Clinical trials for combinations of a TERT based vaccine and chemotherapy are ongoing, some are at Phase III.
Telomerase inhibitors. Small molecules that inhibit telomerase could lead to failure of the cancer cells to maintain their telomeres and they would then senesce and die.
Antisense oligonucleotides or small interfering RNAs may be used to inhibit production of telomerase. A compound known as GRN163L, also known as imetelstat, a lipid-modified 13-mer oligonucleotide complementary to the TERC template region is currently in clinical trials.
Suicide gene therapy. If a construct in which a toxin or a lethal virus was expressed from the TERT promoter, was transfected into the cancer cells it may kill them, whereas other cells, not expressing TERT, would survive.
Stabilizers of G-quadruplex structure at telomeres. Telomeric DNA, in common with other G rich single stranded DNA can exist as a 4 stranded structure called a G-quadruplex. When in this conformation telomeres cannot be extended by telomerase and would shorten and cause senescence.
These methods all rely on killing cells expressing telomerase. Although most cancer cells do express telomerase so do other cells in the body, particularly tissue stem cells. It is possible however, that cancer cells, which tend to have short telomeres, may be more sensitive to telomerase inhibition than stem cells. All of these methods have been developed to inhibit telomerase based telomere maintenance. If inhibiting telomerase were to select for cells that used the ALT mechanism of telomere maintenance then these cells would be able to proliferate despite telomerase inhibition. This might be a particular problem in certain cancers, like the esophageal cancer discussed in the article in this volume by Pal et al., (78) (79) where both telomerase and homologous recombination based methods of telomere maintenance seem to operate in the same cells. Another problem with manipulating telomeres is the fact that while longer telomeres seem to promote healthy aging telomerase activity promotes tumor growth, and short telomeres, while inhibiting the growth of tumors, can also promote the early steps in tumor formation.
Despite these problems telomerase vaccines (80), oligogonucleotides that inhibit telomerase (81, 82) and G-quadruplex stabilizing ligands (83, 84) have shown promising results in pre-clinical studies. As more is learnt about the basic biology of telomeres and telomerase, and as more soluble compounds with greater ability to reach and enter cells are developed telomerase inhibition remains a promising route to cancer therapy.
Conclusions
Spectacular discoveries in basic biology have greatly increased our understanding of how cells maintain the integrity of the genetic material in higher organisms by maintaining special structures, telomeres, at the ends of chromosomes. If this maintenance fails it can lead to premature aging, devastating inherited diseases, or cancer. Research is now proceeding to intervene in these conditions by using our knowledge to devise methods of manipulating telomeres. This is going to be particularly difficult since short telomeres lead to accelerated aging while long telomeres favor the development of malignancy. Nevertheless these are very important problems in health and biology and some early results are promising.
Acknowledgments
We would like to thank Monica Bessler for her insightful comments on the manuscript and Foteini Mourkioti for sending us her manuscript before publication. PJM is supported in part by Grant R01 106695 from the NCI/NIH.
References
- 1.Muller HJ. The remaking of chromosomes. Collecting Net. 1938;8:182–95. [Google Scholar]
- 2.McClintock B. The Behavior in Successive Nuclear Divisions of a Chromosome Broken at Meiosis. Proc Natl Acad Sci U S A. 1939;25:405–16. doi: 10.1073/pnas.25.8.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 4.Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201:1496–9. [PubMed] [Google Scholar]
- 5.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–90. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120:33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 7.Kirk KE, Blackburn EH. An unusual sequence arrangement in the telomeres of the germ-line micronucleus in Tetrahymena thermophila. Genes Dev. 1995;9:59–71. doi: 10.1101/gad.9.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–55. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 9.Shampay J, Szostak JW, Blackburn EH. DNA sequences of telomeres maintained in yeast. Nature. 1984;310:154–7. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- 10.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 11.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–7. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 12.Yu GL, Bradley JD, Attardi LD, Blackburn EH. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344:126–32. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- 13.Lundblad V, Blackburn EH. RNA-dependent polymerase motifs in EST1: tentative identification of a protein component of an essential yeast telomerase. Cell. 1990;60:529–30. doi: 10.1016/0092-8674(90)90653-v. [DOI] [PubMed] [Google Scholar]
- 14.Counter CM, Meyerson M, Eaton EN, Weinberg RA. The catalytic subunit of yeast telomerase. Proc Natl Acad Sci U S A. 1997;94:9202–7. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 16.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–9. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 18.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 19.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–4. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 20.Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–5. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 21.Healy KC. Telomere dynamics and telomerase activation in tumor progression: prospects for prognosis and therapy. Oncol Res. 1995;7:121–30. [PubMed] [Google Scholar]
- 22.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–8. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–5. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 24.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–5. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–24. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 26.Gramatges MM, Bertuch AA. Short telomeres: from dyskeratosis congenita to sporadic aplastic anemia and malignancy. Transl Res. 2013 doi: 10.1016/j.trsl.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36:447–9. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 28.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 30.Hornsby PJ. Short telomeres: cause or consequence of aging? Aging Cell. 2006;5:577–8. doi: 10.1111/j.1474-9726.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 31.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179:4249–54. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Parks CG, DeRoo LA, Chen H, Taylor JA, Cawthon RM, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18:816–20. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Lin S, Funk WE, Hou L. Environmental and occupational exposure to chemicals and telomere length in human studies. Occup Environ Med. 2013 doi: 10.1136/oemed-2012-101350. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, et al. Healthy lifestyle and leukocyte telomere length in U.S. women. PLoS One. 2012;7:e38374. doi: 10.1371/journal.pone.0038374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Ko JH, Lee DC, Lim I, Bang H. Habitual physical exercise has beneficial effects on telomere length in postmenopausal women. Menopause. 2012;19:1109–15. doi: 10.1097/gme.0b013e3182503e97. [DOI] [PubMed] [Google Scholar]
- 38.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–7. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen J, Gammon MD, Terry MB, Wang Q, Bradshaw P, Teitelbaum SL, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer. 2009;124:1637–43. doi: 10.1002/ijc.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masi S, Salpea KD, Li K, Parkar M, Nibali L, Donos N, et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med. 2011;50:730–5. doi: 10.1016/j.freeradbiomed.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsson PM, Tufvesson H, Leosdottir M, Melander O. Telomeres and cardiovascular disease risk: an update 2013. Transl Res. 2013 doi: 10.1016/j.trsl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Mourkioti F, Kustan J, Kraft P, Day JW, Zhao MM, Kost-Alimova M, et al. Role of telomere dysfunction in cardiac failure in Duchenne muscular dystrophy. Nat Cell Biol. 2013 doi: 10.1038/ncb2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–29. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vulliamy T, Beswick R, Kirwan M, Marrone A, Digweed M, Walne A, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105:8073–8. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol Dis. 2005;34:257–63. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–9. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25:11–6. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, et al. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum Genet. 2013;132:473–80. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keller RB, Gagne KE, Usmani GN, Asdourian GK, Williams DA, Hofmann I, et al. CTC1 Mutations in a patient with dyskeratosis congenita. Pediatr Blood Cancer. 2012;59:311–4. doi: 10.1002/pbc.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walne AJ, Bhagat T, Kirwan M, Gitiaux C, Desguerre I, Leonard N, et al. Mutations in the telomere capping complex in bone marrow failure and related syndromes. Haematologica. 2013;98:334–8. doi: 10.3324/haematol.2012.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen LY, Redon S, Lingner J. The human CST complex is a terminator of telomerase activity. Nature. 2012;488:540–4. doi: 10.1038/nature11269. [DOI] [PubMed] [Google Scholar]
- 56.Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–4. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–57. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–7. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, Pons V, et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS One. 2009;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 61.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gansner JM, Rosas IO. Telomeres in lung disease. Transl Res. 2013 doi: 10.1016/j.trsl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Vuong LG, Hemmati PG, Neuburger S, Terwey TH, Vulliamy T, Dokal I, et al. Reduced-intensity conditioning using fludarabine and antithymocyte globulin alone allows stable engraftment in a patient with dyskeratosis congenita. Acta Haematol. 2010;124:200–3. doi: 10.1159/000318721. [DOI] [PubMed] [Google Scholar]
- 64.Dietz AC, Orchard PJ, Baker KS, Giller RH, Savage SA, Alter BP, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2011;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savage SA, Alter BP. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009;23:215–31. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci. 2004;117:2417–26. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 67.Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42:1039–42. doi: 10.1016/j.exger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992;27:383–9. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- 69.Capezzone M, Cantara S, Marchisotta S, Filetti S, De Santi MM, Rossi B, et al. Short telomeres, telomerase reverse transcriptase gene amplification, and increased telomerase activity in the blood of familial papillary thyroid cancer patients. J Clin Endocrinol Metab. 2008;93:3950–7. doi: 10.1210/jc.2008-0372. [DOI] [PubMed] [Google Scholar]
- 70.Zhu CQ, Cutz JC, Liu N, Lau D, Shepherd FA, Squire JA, et al. Amplification of telomerase (hTERT) gene is a poor prognostic marker in non-small-cell lung cancer. Br J Cancer. 2006;94:1452–9. doi: 10.1038/sj.bjc.6603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenberg RA, O’Hagan RC, Deng H, Xiao Q, Hann SR, Adams RR, et al. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene. 1999;18:1219–26. doi: 10.1038/sj.onc.1202669. [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev. 1998;12:1769–74. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–61. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 74.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–9. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 76.Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 2013;4:2218. doi: 10.1038/ncomms3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mocellin S, Pooley KA, Nitti D. Telomerase and the search for the end of cancer. Trends Mol Med. 2013;19:125–33. doi: 10.1016/j.molmed.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Pal J, Munshi NC, Shammas MA. Biology of Telomeres: Importance in Etiology of Esophageal Cancer and as Therapeutic Target. Translational Research. 2013 doi: 10.1016/j.trsl.2013.09.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu R, Pal J, Buon L, Nanjappa P, Shi J, Fulciniti M, et al. Targeting homologous recombination and telomerase in Barrett’s adenocarcinoma: impact on telomere maintenance, genomic instability and tumor growth. Oncogene. 2013 doi: 10.1038/onc.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu JP, Chen W, Schwarer AP, Li H. Telomerase in cancer immunotherapy. Biochim Biophys Acta. 2010;1805:35–42. doi: 10.1016/j.bbcan.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Joseph I, Tressler R, Bassett E, Harley C, Buseman CM, Pattamatta P, et al. The telomerase inhibitor imetelstat depletes cancer stem cells in breast and pancreatic cancer cell lines. Cancer Res. 2010;70:9494–504. doi: 10.1158/0008-5472.CAN-10-0233. [DOI] [PubMed] [Google Scholar]
- 82.Marian CO, Cho SK, McEllin BM, Maher EA, Hatanpaa KJ, Madden CJ, et al. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin Cancer Res. 2010;16:154–63. doi: 10.1158/1078-0432.CCR-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burger AM, Dai F, Schultes CM, Reszka AP, Moore MJ, Double JA, et al. The G-quadruplex-interactive molecule BRACO-19 inhibits tumor growth, consistent with telomere targeting and interference with telomerase function. Cancer Res. 2005;65:1489–96. doi: 10.1158/0008-5472.CAN-04-2910. [DOI] [PubMed] [Google Scholar]
- 84.Miyazaki T, Pan Y, Joshi K, Purohit D, Hu B, Demir H, et al. Telomestatin impairs glioma stem cell survival and growth through the disruption of telomeric G-quadruplex and inhibition of the proto-oncogene, c-Myb. Clin Cancer Res. 2012;18:1268–80. doi: 10.1158/1078-0432.CCR-11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]