Abstract
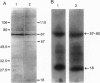
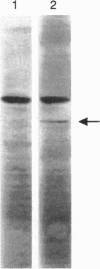
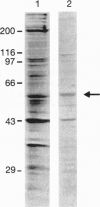
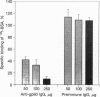
Albumin-binding proteins identified in vascular endothelial cells have been postulated to contribute to the transport of albumin via a process involving transcytosis. In the present study, we have purified and characterized a 57- to 60-kDa (gp60) putative albumin-binding protein from bovine pulmonary microvessel endothelial cells. The endothelial cell membranes were isolated from cultured cells by differential centrifugation and solubilized with sodium cholate and urea. The solubilized extract was concentrated after dialysis by ethanol precipitation and reextracted with Triton X-100, and the resulting extract was subjected to DEAE-cellulose column chromatography. Proteins eluted from this column were further separated using preparative sodium dodecyl sulfate/polyacrylamide gel electrophoresis and used for immunizing rabbits. Fluorescence-activated cell sorter analysis using the anti-gp60 antibodies demonstrated the expression of gp60 on the endothelial cell surface. Affinity-purified anti-gp60 antibodies inhibited approximately 90% of the specific binding of 125I-labeled albumin to bovine pulmonary microvessel endothelial cell surface. The anti-gp60 antibodies reacted with gp60 from bovine pulmonary artery, bovine pulmonary microvessel, human umbilical vein, and rat lung endothelial cell membranes. Bovine anti-gp60 antibodies also reacted with bovine secreted protein, acidic and rich in cysteine (SPARC). However, bovine SPARC NH2-terminal sequence (1-56 residues) antibodies did not react with gp60, indicating that the endothelial cell-surface-associated albumin-binding protein gp60 was different from the secreted albumin-binding protein SPARC. We conclude that the endothelial cell-surface-associated gp60 mediates the specific binding of native albumin to endothelial cells and thus may regulate the uptake of albumin and its transcytosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Del Vecchio P. J., Siflinger-Birnboim A., Belloni P. N., Holleran L. A., Lum H., Malik A. B. Culture and characterization of pulmonary microvascular endothelial cells. In Vitro Cell Dev Biol. 1992 Nov-Dec;28A(11-12):711–715. doi: 10.1007/BF02631058. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Lindner W., Young M. F., Termine J. D. Synthetic peptide antisera: their production and use in the cloning of matrix proteins. Connect Tissue Res. 1989;21(1-4):43–50. doi: 10.3109/03008208909049994. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin-mediated transport of rose bengal by perfused rat liver. Kinetics of the reaction at the cell surface. J Clin Invest. 1983 Nov;72(5):1764–1771. doi: 10.1172/JCI111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinea N., Eskenasy M., Simionescu M., Simionescu N. Endothelial albumin binding proteins are membrane-associated components exposed on the cell surface. J Biol Chem. 1989 Mar 25;264(9):4755–4758. [PubMed] [Google Scholar]
- Ghinea N., Fixman A., Alexandru D., Popov D., Hasu M., Ghitescu L., Eskenasy M., Simionescu M., Simionescu N. Identification of albumin-binding proteins in capillary endothelial cells. J Cell Biol. 1988 Jul;107(1):231–239. doi: 10.1083/jcb.107.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Takata K., Morino Y. Characterization of a membrane-associated receptor from rat sinusoidal liver cells that binds formaldehyde-treated serum albumin. J Biol Chem. 1985 Jan 10;260(1):475–481. [PubMed] [Google Scholar]
- Horiuchi S., Takata K., Morino Y. Purification of a receptor for formaldehyde-treated serum albumin from rat liver. J Biol Chem. 1985 Jan 10;260(1):482–488. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J., Livesey G., Williams K. E. Effects of microbial proteinase inhibitors on the degradation of endogenous and internalized proteins by rat yolk sacs. Biochem J. 1981 Apr 15;196(1):41–48. doi: 10.1042/bj1960041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makni H., Malter J. S., Reed J. C., Nobuhiko S., Lang G., Kioussis D., Trinchieri G., Kamoun M. Reconstitution of an active surface CD2 by DNA transfer in CD2-CD3+ Jurkat cells facilitates CD3-T cell receptor-mediated IL-2 production. J Immunol. 1991 Apr 15;146(8):2522–2529. [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. Carrier-mediated transport of thyroid hormones through the rat blood-brain barrier: primary role of albumin-bound hormone. Endocrinology. 1979 Sep;105(3):605–612. doi: 10.1210/endo-105-3-605. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Pratten M. K., Williams K. E., Lloyd J. B. A quantitative study of pinocytosis and intracellular proteolysis in rat peritoneal macrophages. Biochem J. 1977 Dec 15;168(3):365–372. doi: 10.1042/bj1680365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu D., Simionescu M., Simionescu N., Palade G. E. Binding and transcytosis of glycoalbumin by the microvascular endothelium of the murine myocardium: evidence that glycoalbumin behaves as a bifunctional ligand. J Cell Biol. 1988 Nov;107(5):1729–1738. doi: 10.1083/jcb.107.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P. N., Mego J. L. Renal plasma membrane receptors for certain modified serum albumins. Evidence for participation of a heparin receptor. Biochem J. 1986 Nov 1;239(3):537–543. doi: 10.1042/bj2390537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage H., Johnson C., Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984 Mar 25;259(6):3993–4007. [PubMed] [Google Scholar]
- Sage H., Vernon R. B., Funk S. E., Everitt E. A., Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J Cell Biol. 1989 Jul;109(1):341–356. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Bravo J. High affinity binding, endocytosis, and degradation of conformationally modified albumins. Potential role of gp30 and gp18 as novel scavenger receptors. J Biol Chem. 1993 Apr 5;268(10):7562–7570. [PubMed] [Google Scholar]
- Schnitzer J. E., Carley W. W., Palade G. E. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6773–6777. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Oh P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J Biol Chem. 1994 Feb 25;269(8):6072–6082. [PubMed] [Google Scholar]
- Schnitzer J. E., Oh P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am J Physiol. 1992 Dec;263(6 Pt 2):H1872–H1879. doi: 10.1152/ajpheart.1992.263.6.H1872. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E., Sung A., Horvat R., Bravo J. Preferential interaction of albumin-binding proteins, gp30 and gp18, with conformationally modified albumins. Presence in many cells and tissues with a possible role in catabolism. J Biol Chem. 1992 Dec 5;267(34):24544–24553. [PubMed] [Google Scholar]
- Schnitzer J. E., Ulmer J. B., Palade G. E. A major endothelial plasmalemmal sialoglycoprotein, gp60, is immunologically related to glycophorin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6843–6847. doi: 10.1073/pnas.87.17.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siflinger-Birnboim A., Schnitzer J., Lum H., Blumenstock F. A., Shen C. P., Del Vecchio P. J., Malik A. B. Lectin binding to gp60 decreases specific albumin binding and transport in pulmonary artery endothelial monolayers. J Cell Physiol. 1991 Dec;149(3):575–584. doi: 10.1002/jcp.1041490329. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C., Lum H., Andersen T. T., Fenton J. W., 2nd, Malik A. B. Thrombin receptor 14-amino acid peptide binds to endothelial cells and stimulates calcium transients. Am J Physiol. 1992 Nov;263(5 Pt 1):L595–L601. doi: 10.1152/ajplung.1992.263.5.L595. [DOI] [PubMed] [Google Scholar]
- Yost J. C., Sage E. H. Specific interaction of SPARC with endothelial cells is mediated through a carboxyl-terminal sequence containing a calcium-binding EF hand. J Biol Chem. 1993 Dec 5;268(34):25790–25796. [PubMed] [Google Scholar]