Abstract
Aim:
To investigate the prognostic significance of certain clinical and pathological factors of renal cell cancer.
Materials and Methods:
One hundred and fourteen patients who underwent radical nephrectomy between 1996 and 2011 in our hospital were examined. Parameters including age, gender, mode of presentation, hematological and pathological parameters were evaluated for their role as predictors of disease-free and overall survival.
Results:
Median follow-up was 69 months. Predominant histological type, pathological stage, and nuclear grade were clear cell carcinoma, pT1, and Fuhrman II, respectively. Five-year overall and disease-free survival were 86% and 82%, respectively. Only nuclear grade (P = 0.02) and preoperative anemia (P < 0.01) were correlated with overall survival, while pathological stage, nuclear grade, anemia, and neutrophil-to-lymphocyte ratio of 2.7 or greater were associated with disease-free survival (P = 0.02, P = 0.038, P < 0.01, P = 0.049, respectively). In the multivariate setting, anemia (P = 0.04) and pathological stage (P = 0.026) were the only independent statistically significant predictors of disease-free survival, while anemia (P = 0.018) and neutrophil to lymphocyte ratio (P = 0.034) were the only factors correlated with overall survival.
Conclusions:
Due to the wide application of various imaging studies, patients with kidney cancer are diagnosed more often with localized disease and favorable pathological features. Fuhrman nuclear grade, pathological stage, preoperative anemia, and neutrophil to lymphocyte ratio are strongly associated with survival. In localized disease, such information could be used to guide the intensity of follow-up and identify high-risk patients who can be targeted for adjuvant therapy trials.
Keywords: Nephrectomy, prognosis, renal cell carcinoma, survival
INTRODUCTION
Renal cell carcinoma (RCC) accounts for approximately 3% of adult malignancies and 90-95% of neoplasms arising from the kidney. It ranks 14th on the list of most common malignancies worldwide.[1] The highest incidences occurred in North America, Australia/New Zealand, and Europe, with lower rates in Africa, Asia, and the Pacific.[2] In men, the mortality rate per 100,000 population fell from 4.8 in 1990-1994 to 4.1 in 2000-2004; in women, the rate fell from 2.1 to 1.8.[3] Several prognostic models have been created in order to stratify patients in risk groups.[4] Laboratory abnormalities, including anemia, hypercalcemia, liver dysfunction, neutrophilia, neutrophil to lymphocyte ratio (NLR) of 2.7 or greater, thrombocytosis, and elevated markers of inflammation, have all been acknowledged as predictors of poor survival in RCC.[5,6] Pathologic features, such as nuclear grade, tumor-node-metastasis (TNM) stage,[7] and histologic subtype, have been assessed as potential prognostic factors. Currently, the choice of the more appropriate algorithm or nomogram is an unresolved question.[8]
In this study, we retrospectively analyzed the clinico-pathological factors of patients with non-metastatic renal cell carcinoma treated with radical nephrectomy. Additionally, the relationship between survival and each variable was recorded.
MATERIALS AND METHODS
We retrospectively reviewed all patients with renal cell carcinoma who underwent nephrectomy in our hospital during the period 1996-2011. Recorded clinical features included age, gender, and mode of presentation. Routine laboratory variables were measured from preoperative blood samples, including hemoglobin, neutrophil count, lymphocyte and platelet count, serum sodium, alkaline phosphatase, and calcium. Neutrophil to lymphocyte ratio was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. Anemia was defined as hemoglobin <13.5 g/dL in males and <12.0 g/dL in females. Pathologic features assessed included histologic subtype, TNM stage, Fuhrman nuclear grade, tumor size, and presence or absence of sarcomatoid differentiation. Overall survival (OS) and disease-free survival (DFS) were recorded and correlated with the above clinical and pathological parameters.
No patients received new adjuvant therapy preoperatively and/or synchronic postoperative adjuvant therapy. All patients had negative margins. Lymphadenectomy was restricted to staging purposes with dissection of palpable and enlarged lymph nodes. Exclusion criteria were the following: Pathologically-confirmed urothelial carcinoma, specimens with tissue unavailable for accurate evaluation, patients with lymph node or distant metastases, and patients treated with partial nephrectomy.
The prognosis of these patients was determined from information from hospital charts and telephone follow-up. The data obtained were recorded on a standard research form and filled in a database. All patients were followed up every 6 months in the first three years after surgery and every year thereafter by physical examination, blood chemistry analysis, chest X-ray, and abdominal enhanced computer tomography (CT).
Regarding statistical analysis, univariate and multivariate Cox proportional hazards models were used to assess the predictive ability of the hematologic, biochemical, and pathological baseline characteristics on DFS and OS. Cox models aiming at prediction should be used with a minimum of 10 events per predictor variable (EPV). However, Vittinghoff and McCulloch demonstrated that this rule of thumb is too conservative in analyzes of causal influences based on observational data, and control of confounding may require adjustment for more covariates than the rule of 10 EPV allows.[9] DFS was defined as time to the date of progression of disease and/or to the date of death from disease while OS was the time from date of nephrectomy to the date of death from any cause. The Kaplan-Meier technique was used to evaluate OS and DFS, and the log rank test was used to compare survival curves with P < 0.05 as the significance cutoff.
RESULTS
Between 1996 and 2011, 114 consecutive patients with renal cell carcinoma were referred to our hospital and treated with curative intent by radical nephrectomy. Clinico-pathological characteristics of the patients are summarized in Table 1. There were 80 (70.1%) men and 34 (29.9%) women with a median age of 64 years old. Right kidney was most often (56.9%) affected. Half of the patients presented with a mass identified incidentally in ultrasound or computer tomography examination. Gross hematuria and flank pain were the first symptom in 21.1% and 7.8% of the patients, respectively. Median sodium level was 142.
Table 1.
Clinico-pathological characteristics of the patients
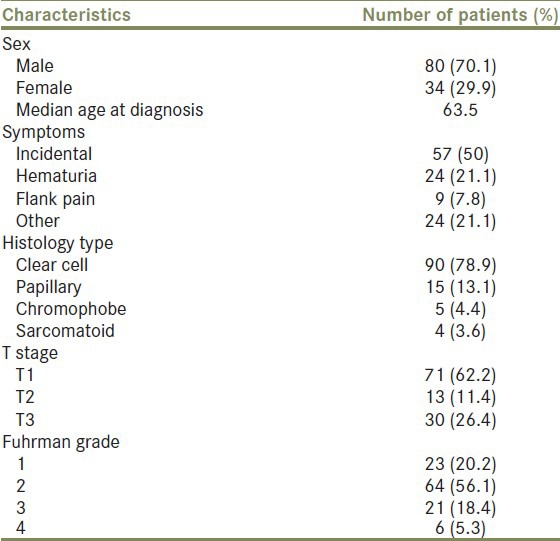
Histological findings confirmed renal cell carcinoma in 90 (78.9%) patients, while 15 (13.1%) had papillary tumor and 5 (4.4%) had chromophobe tumor. One group of 4 patients (3.6%) presented sarcomatoid features. When our patients were stratified according to pathological stage, there were 71 (62.2%) with stage pT1, 13 (11.4%) with stage pT2, and 30 (26.4%) with stage pT3. Regarding Fuhrman grade, 23 patients (20.2%) were classified as grade I, 64 (56.1%) as grade II, 21 (18.4%) as grade III, and 6 (5.3%) as grade IV.
Survival data existed for 103 patients. At the time of data analysis, median length of follow-up from nephrectomy was 69 months (range, 1-179 months). During this time, 14 patients (13.6%) died, 10 (9.7%) of whom died from RCC and 4 (3.9%) died from other causes. A total of 13 (12.6%) patients had metastatic disease. The site of metastasis was lung in 7 cases, liver in 2 cases, and other in 4. Overall survival rates from nephrectomy for all patients were 93% for 1 year, 88% for 3 years, and 85% for 5 years. Disease-free survival rates at 1, 3, and 5 years were 94%, 88%, and 82%, respectively.
Fuhrman nuclear grade and preoperative anemia [Figure 1] were the only factors significantly associated with overall survival (P = 0.02 and P < 0.01, respectively). The differences in OS were not significant between histological subtypes (P = 0.14) and TNM pathological stage (P = 0.17). Additionally, overall survival was not associated with age (P = 0.68), gender (P = 0.93), mode of presentation (P = 0.18), thrombocytosis (P = 0.37), NLR ≥2.7 (P = 0.12), sodium levels below median (P = 0.09) hypercalcemia (P = 0.53), and abnormal alkaline phosphate levels (P = 0.59) [Table 2].
Figure 1.
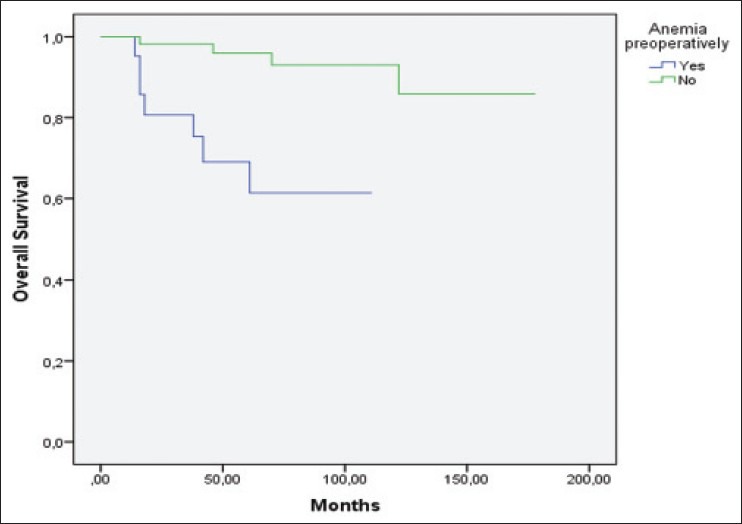
Patients with preoperative anemia had a worse overall survival rate. (Kaplan-Meier log rank test, P = 0.000)
Table 2.
Univariate analysis of prognostic factors of OS and DFS in RCC
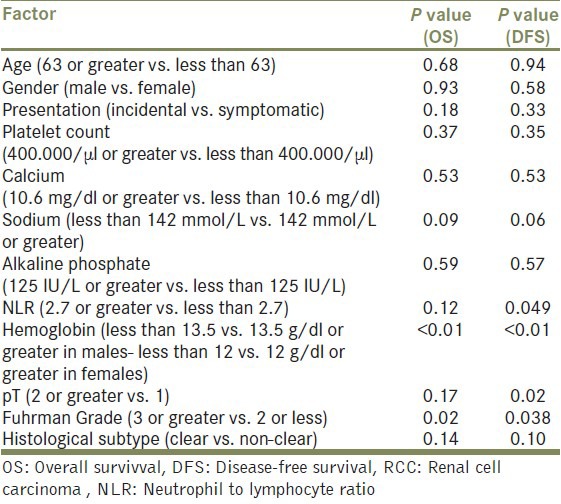
Pathological stage (P = 0.02), Fuhrman grade (P = 0.038), preoperatively anemia (P < 0.01) and neutrophil to lymphocyte ratio ≥2.7 (P = 0.049) [Figure 2] were the only factors predictive of disease-free survival. Histological subtypes (P = 0.10), age (P = 0.94), gender (P = 0.58), mode of presentation (P = 0.33), thrombocytosis (P = 0.35), sodium levels below median (P = 0.06), hypercalcemia (P = 0.53) and abnormal alkaline phosphate levels (P = 0.57) had no significant impact on disease-free survival [Table 2].
Figure 2.
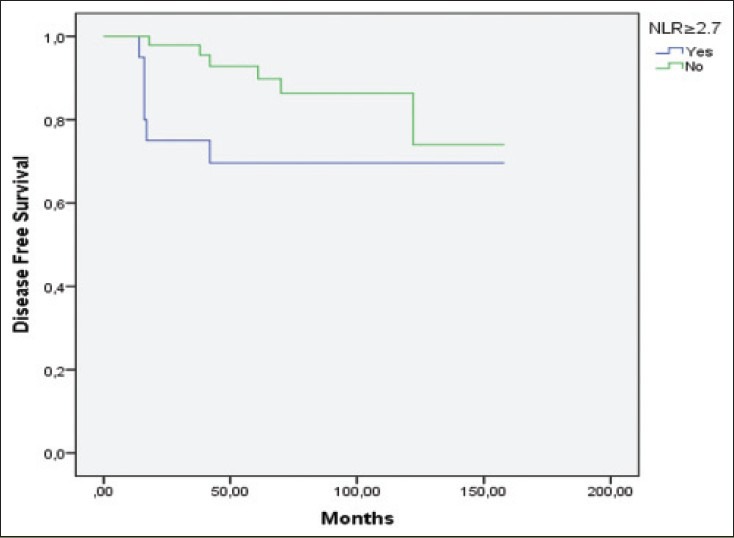
Patients with preoperative NLR ≥2.7 had a worse disease-free survival rate. (Kaplan-Meier log rank test, P = 0.049)
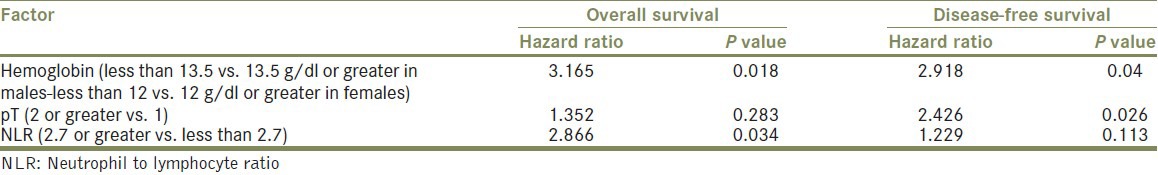
The following variables were considered in the multivariate model building analysis: Gender, age, Fuhrman nuclear grade, pathological stage, histological subtype, hemoglobin, NLR, platelet number, and sodium concentration. Preoperative calcium and alkaline phosphate levels were not included because of missing data. Among all patients with RCC, the only factors which found to be independent statistically significant predictors of OS in the multivariate setting was preoperative anemia (P = 0.018) and NLR ≥ 2.7 (P = 0.034). On multivariate analysis of DFS, only pathological stage (P = 0.026) and preoperative anemia (P = 0.04) proved to be independent significant predictive factors [Table 3].
Table 3.
Multivariate analysis with cox regression model for risk factors predictive for overall survival and disease-free survival
DISCUSSION
The incidence of RCC has clearly risen over the past 20 years, largely due to the widespread utilization of non-invasive imaging modalities such as ultrasonography, CT scan, and MRI. There is a stage migration to smaller localized renal tumors and better disease-specific survival.[10] Surgery remains the only curative therapy despite the introduction of a number of new promising treatment options such as nephron-sparing surgery and thermal ablation.[11]
Classical prognostic factors for non-metastatic RCC include anatomical, histological, clinical, and molecular features. Kattan et al. were the first authors to develop a nomogram to predict the probability of RCC recurrence after nephrectomy.[12] Currently, the most commonly used prognostic models for localized RCC are the University of California Los Angeles integrated staging system (UISS) and the Stage, Size, Grade, and Necrosis (SSIGN) developed at the Mayo Clinic. UISS predicts patient survival by integrating the tumor-node-metastasis (TNM) stage, Fuhrman's grade, and Eastern Cooperative Oncology Group (ECOG) performance status (PS), while SSIGN calculates prognostic score according to stage, size, grade, and necrosis (SSIGN).[13,14] The use of prognostic indicators might play a crucial role in predicting outcome and adopting new adjuvant treatments to the needs of individual patients. Patient profiling and assigning into risk categories is an important concept as it allows prediction of tumor behavior and, therefore, patient prognosis.[15] Additionally, it allows the selection of the most suitable therapeutic option for each of them.[16]
As shown in the results, our patients had an excellent prognosis with 5 year OS and DFS of 85% and 82%, respectively. In the present study, we found that ≈74% of RCCs were pathologically localized at the moment of the initial diagnosis. On univariate analysis, Fuhrman nuclear grade and preoperative anemia were independent predictor of OS and DFS, while pathological stage and neutrophil to lymphocyte ratio ≥2.7 were independent factor of DFS. Notably, histological type of the primary tumor failed to be an independent predictor of OS and DFS. Concerning the other prognostic factors, we found that age, mode of presentation, thrombocytosis, sodium levels below median, hypercalcemia, and abnormal alkaline phosphate levels were not associated with OS and DFS.
Only the pathological stage and preoperative anemia remained significantly associated with disease-free survival upon multivariate analysis. Overall survival remained associated with anemia and neutrophil to lymphocyte ratio. Several of our findings confirm previous associations.[17,18] Most authors agree that TNM stage and Fuhrman nuclear grade are the strongest independent prognostic factors for localized RCC.[19] With regard to tumor grade, Fuhrman nuclear grade is widely applied in RCC of all histological subtypes, although little evidence indicates that it has prognostic use for tumor types other than clear cell RCC.[20] Preoperative anemia (hemoglobin <13.5 g/dL in males and <12.0 g/dL in females) is established adverse prognostic factor, while recent studies confirm that pre- and post-treatment neutrophil to lymphocyte ratio is a significant prognostic factor for recurrence in patients with clear cell carcinoma.[21,22]
There are ongoing studies in non-metastatic RCC aiming to investigate the use of targeted agents in the adjuvant, postoperative setting in the context of surgical treatment. The S-TRAC study assesses disease-free survival in high-risk patients (according to the UISS staging system) receiving a nephrectomy prior to randomization to either sunitinib or placebo treatment for 1 year.[23] The PROTECT trial evaluates the efficacy and safety of pazopanib in patients with T2-T4 clinical stage.[24] In the ASSURE study, patients with stage II–IV disease are stratified and randomized to treatment with sunitinib, sorafenib as adjuvant therapy following nephrectomy.[25]
Approximately 20-30% of patients diagnosed with kidney cancer present with metastatic disease, and a similar percentage of patients with initially localized disease experience a relapse and develop metastatic disease.[26] The introduction of vascular endothelial growth factor (VEGF)-pathway inhibitors (e.g., sunitinib, sorafenib, pazopanib, and bevacizumab) and mTOR inhibitors (e.g., everolimus and temsirolimus) has substantially improved the outcomes of patients with metastatic RCC.[27] These agents have largely replaced cytokines (immunotherapy) in treatment-naive patients.[28] Despite new promising therapies, metastatic RCC is one of the therapy-resistant malignancies. Therefore, methods to predict which patients are likely to develop metastases are needed, and it is also important to identify those that respond to various treatments.[29]
The limitations to the present study are inherent to its retrospective nature and the relatively small number of patients. We also could not study some preoperative biological prognostic factors (such as C-reactive protein, Lactate dehydrogenase, Erythrocyte Sedimentation Rate, eGFR) and patient's performance status because of the lack of data. The potential inter-observer variability in the determination of the histological variables may represent limitations in the interpretation of the results obtained in the study. Prognostic factors that can risk stratify patients, predictive biomarkers that can help individualize treatment selection and predict a patient's response to therapy, facilitate the better understanding and treatment of the disease.[30] Despite their adequate prognostic ability, none of the established prognostic models is 100% accurate. In consequence, the search for more accurate markers continues. Molecular events that can unveil the biologic heterogeneity underlying the varied clinical behavior of RCC may help improve individualized prognostication and risk-stratified clinical decision making.[31] Novel prognostic factors and more up-to-date models are urgently needed for patients with localized and metastatic RCC, especially in the era of targeted therapies.[32]
CONCLUSION
Our findings confirm the potential role of histologic features and hematological parameters as predictive tools of RCC. Prognostic models should widely be used in the clinical practice to counsel patients, plan surveillance protocols, and select appropriate candidates for inclusion in adjuvant treatment protocols. Further improvements in our ability to predict RCC prognosis will rely on the integration of molecular and genetic markers in the currently established models.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Weikert S, Ljungberg B. Contemporary epidemiology of renal cell carcinoma: Perspectives of primary prevention. World J Urol. 2010;28:247–52. doi: 10.1007/s00345-010-0555-1. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Levi F, Ferlay J, Galeone C, Lucchini F, Negri E, Boyle P, et al. The changing pattern of kidney cancer incidence and mortality in Europe. BJU Int. 2008;101:949–58. doi: 10.1111/j.1464-410X.2008.07451.x. [DOI] [PubMed] [Google Scholar]
- 4.Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski R. Prognostic Factors for Survival in Metastatic Renal Cell Carcinoma. Cancer. 2009;115:2273–81. doi: 10.1002/cncr.24226. [DOI] [PubMed] [Google Scholar]
- 6.Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010;184:873–8. doi: 10.1016/j.juro.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Byrd DR, Comptom CC, Fritz AG, Greene FL, Trotti A. 7th ed. New York: Springer Verlag; 2010. AJCC Staging Manual. [Google Scholar]
- 8.Ficarra V, Novara G, Galfano A, Brunelli M, Cavalleri S, Martignoni G, et al. The ‘Stage, Size, Grade and Necrosis’ score is more accurate than the University of California Los Angeles Integrated Staging System for predicting cancer-specific survival in patients with clear cell renal cell carcinoma. BJU Int. 2009;103:165–70. doi: 10.1111/j.1464-410X.2008.07901.x. [DOI] [PubMed] [Google Scholar]
- 9.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–8. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 10.Fan L, Lianfang D, Jinfang X, Yijin S, Ying W. Diagnostic efficacy of contrast-enhanced ultrasonography in solid renal parenchymal lesions with maximum diameters of 5 cm. J Ultrasound Med. 2008;27:875–85. doi: 10.7863/jum.2008.27.6.875. [DOI] [PubMed] [Google Scholar]
- 11.Miller DC, Ruterbusch J, Colt JS, Davis FG, Linehan WM, Chow WH, et al. Contemporary clinical epidemiology of renal cell carcinoma: Insight from a population based case-control study. J Urol. 2010;184:2254–8. doi: 10.1016/j.juro.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–7. [PubMed] [Google Scholar]
- 13.Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 14.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J Urol. 2002;168:2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 15.Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. The role of molecular markers in the staging of renal cell carcinoma. BJU Int. 2007;99:1208–11. doi: 10.1111/j.1464-410X.2007.06812.x. [DOI] [PubMed] [Google Scholar]
- 16.Brassart E, Lebdai S, Berger J, Traore S, Bernhard JC, Fardoun T, et al. Overall mortality after radical nephrectomy in patients aged over 80 years with renal cancer: A retrospective study on preoperative prognostic factors. Int J Urol. 2012;19:626–32. doi: 10.1111/j.1442-2042.2012.03006.x. [DOI] [PubMed] [Google Scholar]
- 17.Sun M, Shariat SF, Karakiewicz PI. Factors affecting outcome in renal cell carcinoma. Curr Opin Urol. 2010;20:355–60. doi: 10.1097/MOU.0b013e32833c7b19. [DOI] [PubMed] [Google Scholar]
- 18.Flanigan RC, Polcari AJ, Hugen CM. Prognostic variables and nomograms for renal cell carcinoma. Int J Urol. 2011;18:20–31. doi: 10.1111/j.1442-2042.2010.02642.x. [DOI] [PubMed] [Google Scholar]
- 19.Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol. 2010;28:319–27. doi: 10.1007/s00345-010-0540-8. [DOI] [PubMed] [Google Scholar]
- 20.Sika-Paotonu D, Bethwaite PB, McCredie MRE, William Jordan T, Delahunt B. Nucleolar grade but not Fuhrman grade is applicable to papillary renal cell carcinoma. Am J Surg Pathol. 2006;30:1091–6. doi: 10.1097/01.pas.0000209833.69972.2b. [DOI] [PubMed] [Google Scholar]
- 21.Magera JS, Jr, Leibovich BC, Lohse CM, Sengupta S, Cheville JC, Kwon ED, et al. Association of abnormal preoperative laboratory values with survival after radical nephrectomy for clinically confined clear cell renal cell carcinoma. Urology. 2008;71:278–82. doi: 10.1016/j.urology.2007.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187:411–7. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov. Sunitinib treatment of renal adjuvant cancer (S.TRAC): A randomized double blind phase 3 study of adjuvant sunitinib VS. placebo in subjects at high risk of recurrent RCC. 2012. [Last accessed on 2013 Jan 26]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00375674?term=NCT00375674andrank=1 .
- 24.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov. ASSURE: Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma. 2010. [Last accessed on 2013 Jan 26]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00326898?term=NCT00326898andrank=1 .
- 26.Bex A, Gore M, Mulders P, Sternberg CN. Recent advances in the treatment of advanced renal cell carcinoma: Towards multidisciplinary personalized care. BJU Int. 2012;110:1289–300. doi: 10.1111/j.1464-410X.2012.11100.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee JL, Park I, Park K, Park S, Ahn Y, Ahn JH, et al. Efficacy and safety of vascular endothelial growth factor receptor tyrosine kinase inhibitors in patients with metastatic renal cell carcinoma and poor risk features. J Cancer Res Clin Oncol. 2012;138:687–93. doi: 10.1007/s00432-012-1148-8. [DOI] [PubMed] [Google Scholar]
- 28.Posadas EM, Figlin RA. Systemic therapy in renal cell carcinoma: Advancing paradigms. Oncology (Williston Park) 2012;26:290–301. [PubMed] [Google Scholar]
- 29.Ljungberg B. Prognostic factors in renal cell carcinoma. Scand J Surg. 2004;93:118–25. doi: 10.1177/145749690409300206. [DOI] [PubMed] [Google Scholar]
- 30.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16(Suppl 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, Oudard S, et al. Prognostic factors and predictive models in renal cell carcinoma: A contemporary Review. Eur Urol. 2011;60:644–61. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 32.Sun M, Shariat SF, Karakiewicz PI. Factors affecting outcome in renal cell carcinoma. Curr Opin Urol. 2010;20:355–60. doi: 10.1097/MOU.0b013e32833c7b19. [DOI] [PubMed] [Google Scholar]


