Abstract
Objectives:
Since its introduction, there have been many refinements in the technique and implementation of robotic-assisted radical prostatectomy (RARP). However, it is unclear whether operative outcomes are influenced by surgical case order. We evaluated the effect of case order on perioperative outcomes for RARP within a large health maintenance organization.
Materials and Methods:
We conducted a retrospective review of RARP cases performed at our institution from September 2008 to December 2010 using a single robotic platform. Case order was determined from surgical schedules each day and surgeries were grouped into 1st, 2nd and 3rd round cases. Fourth round cases (n = 1) were excluded from analysis. We compared clinicopathological variables including operative time, estimated blood loss (EBL), surgical margin rates and complication rates between groups.
Results:
Of the 1018 RARP cases in this cohort, 476 (47%) were performed as 1st round cases, 398 (39%) 2nd round cases and 144 (14%) 3rd round cases by a total of 18 surgeons. Mean operative time was shorter as cases were performed later in the day (213 min vs. 209 min vs. 180 min, P < 0.0001) and similarly, EBL also decreased with surgical order (136 mL vs. 134 mL vs. 103 mL, P = 0.01). Transfusion rates, surgical margin rates and complication rates did not significantly differ between groups. Patients undergoing RARP later in the day were much more likely to have a hospital stay of 2 or more days than earlier cases (10% vs. 11% vs. 32%, P = 0.01).
Conclusions:
Surgical case order may influence perioperative outcomes for RARP with decreased operative times and increased length of hospital stay associated with later cases. These findings indicate that select perioperative factors may improve with ascending case order as the surgical team “warms up” during the day. In addition, 3rd round cases can increase hospital costs associated with increased lengths of hospital stay. Knowledge of these differences may assist in surgical planning to improve outcomes and limit costs.
Keywords: Case order, cost, morbidity, robotic radical prostatectomy
INTRODUCTION
Prostate cancer is the most common malignancy diagnosed among men in the United States.[1] Robotic-assisted surgery has become widely adopted for the treatment of various urologic malignancies. In the U.S., robotic-assisted radical prostatectomy (RARP) is now the most common treatment for localized prostate cancer.[2]
Some of the purported benefits of robotic-assistance include improved visualization, repeatable dexterous movements and favorable ergonomics for the surgeon. Reports from large series have demonstrated low complication rates, decreased estimated blood loss (EBL) and shorter lengths of hospital stay in comparison to open radical retropubic prostatectomy.[3,4,5] Furthermore, increased case volumes and surgeon experience have also been shown to improve perioperative outcomes with RARP.[6,7,8,9]
With dedicated robotic teams and refinements in technique, many high volume centers are capable of performing 3 or more robotic cases per room each day. In addition, there has been considerable recent interest in the development of surgical simulators in order to improve a surgeon's performance.[10,11,12,13,14,15] It is hypothesized that “warming up” with surgical simulation before a case may also improve surgical outcomes with RARP. Studies have demonstrated that practice before surgery may not only improve a surgeon's sensorimotor coordination, but also cognitive processes including attention, intellectual arousal and working memory.[16] However, to date there are few data regarding these effects in clinical practice and even fewer evaluating the effect of surgical case order on perioperative outcomes and cost from RARP. In the current study, we viewed our center's experience to examine whether case order influences perioperative outcomes for RARP.
MATERIALS AND METHODS
The study cohort consisted of 1,018 patients who were treated with RARP at our institution between September 2008 and December 2010 using a single da Vinci robot (Intuitive Surgical, Sunnyvale, CA). Case order was determined from electronic surgical schedules each day. Surgical cases were then divided into groups according to case order into 1st, 2nd and 3rd round cases on each operating day. 4th round cases (n = 1) were excluded from analysis and any aborted surgeries were also excluded (n = 2).
At our institution, robotic urologic surgery was implemented within the Kaiser Permanente Southern California region in 2008 with the purchase of a single da Vinci 4S robot at one medical center. All cases were performed in a similar manner as previously described using a standard 6-port transperitoneal approach.[17] Operating teams consisted of two surgeons per case, a scrub nurse, surgical technician and an Anesthesiologist and/or certified nurse anesthetist. For each surgical case, one surgeon operated as the console surgeon and another assisted at the patient's bedside. Age, body mass index, American Society of Anesthesiologists score, serum prostate-specific antigen, Gleason score and clinical stage were recorded for each patient. Perioperative details of total operative time, EBL, transfusion rate, pelvic lymph node dissection rate, pathologic stage, prostatic weight, positive margin rate, intraoperative and post-operative complications and length of hospital stay were also noted. Comparisons of groups were made using the Chi-square test for categorical measures and Wilcoxon's rank-sum test for continuous measures. All tests were two-sided, with a P value <0.05 taken to be statistically significant.
RESULTS
A total of 1018 RARPs were performed in the study cohort with 476 (47%) 1st round cases, 398 (39%) 2nd round cases and 144 (14%) 3rd round cases by a total of 18 surgeons. Clinical characteristics were similar between men undergoing surgery with respect to case order [Table 1].
Table 1.
Patient demographics
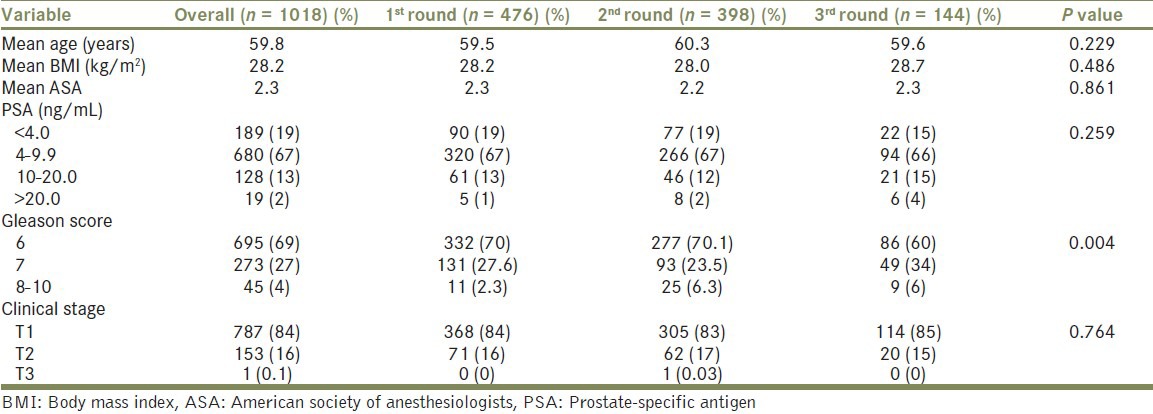
Table 2 presents the perioperative results stratified by case order. Overall mean operative time was shorter as cases were performed later in the day (213 min vs. 209 min vs. 180 min, P < 0.001). This effect was also seen with RARP and pelvic lymphadenectomy (PLND) (233 min vs. 215 min vs. 189 min, P < 0.001). A larger proportion of patients underwent PLND in 3rd round cases (12% vs. 17% vs. 18%, P = 0.045), which may be related to patients with a higher proportion of Gleason 7-10 disease undergoing surgery as 3rd round surgeries [Table 1] (P = 0.004). In addition, EBL significantly decreased with surgical order with 3rd round cases having the least blood loss (136 mL vs. 134 mL vs. 103 mL, P = 0.01). However, transfusion rates, surgical margin rates and complication rates did not significantly differ between the groups.
Table 2.
Perioperative details
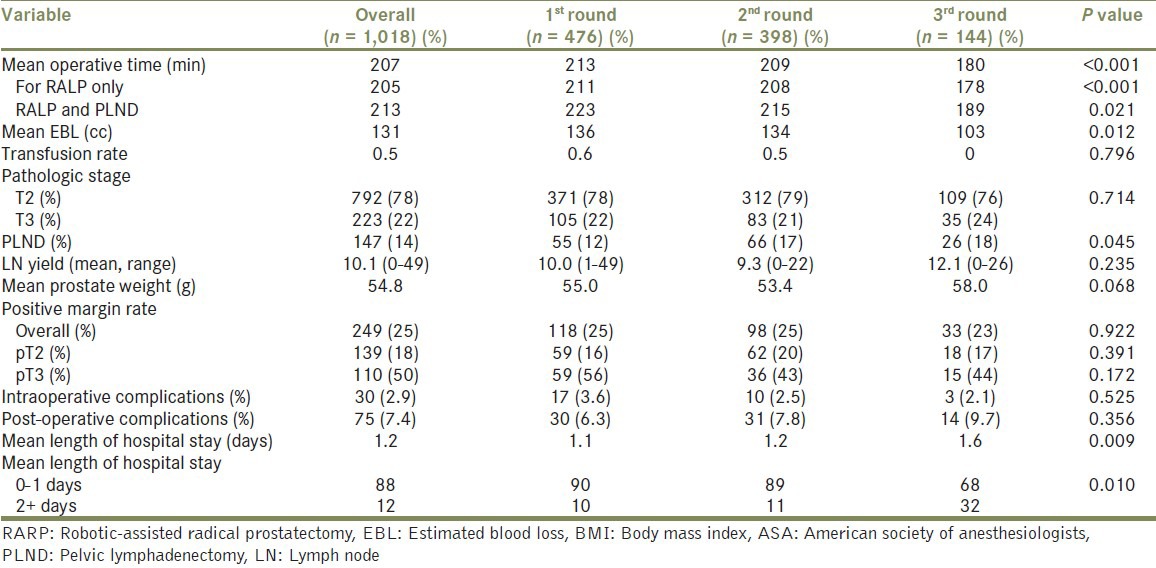
Patients undergoing RARP later in the day were found to have a longer mean length of hospital stay (1.1 vs. 1.2 vs. 1.6 days, P = 0.009) and 3rd round case patients were more likely to have a longer hospital stay of 2 or more days than earlier cases (10% vs. 11% vs. 32%, P = 0.01).
DISCUSSION
In this study, we found that increasing case order was associated with decreased operative times, decreased EBL and longer lengths of hospital stay for patients undergoing RARP. With similar patient characteristics between groups, our results illustrate differences in perioperative outcomes with intraday case repetition. Significantly decreased mean operative times were also noted for RARP with PLND for 3rd round cases with a difference of 34 min in comparison to 1st round cases. This may be a real-life example of the old adage that “practice makes perfect” in surgical training. These results may be especially important for the training and scheduling of minimally invasive surgical cases. Repetitive experience in surgery has been shown to improve results as surgeons with higher case volumes often have better outcomes.[7,18,19] However, to our knowledge the effects of surgical repetition within an operative day have not been evaluated in this manner.
Several studies have shown that increased surgeon experience and case volume are associated with decreased operative times and improved surgical outcomes for RARP.[6,7,18,19] Decreased operative times with increased case volume over time often indicate that surgeons or surgical teams are overcoming their learning curves. However, decreased operative time with successive cases within a single day may indicate that a surgeon or surgical team is actually “warming up” and improving performance, irrespective of their learning curve. Alternatively, one could hypothesize that cases later in the day would be associated with increased fatigue, which may also result in shorter operating times with negative operative outcomes; however, in the current study we did not find increased complications or EBL associated with later surgical case order.
These data also suggest that the beneficial effects of “warming up” before a sports event by athletes may be applicable to surgical practice. The advantages of improving psychomotor skills and cognitive performance before physical sports are also indispensable to improving surgical performance and reducing errors. A study by Kahol et al., demonstrated that pre-operative warm up exercises for 15-20 min with simple surgical tasks lead to a substantial increase in surgical skills proficiency during follow-up among groups with differing experience levels.[16] Importantly, there was also significant improvement in the performance of fatigued groups. Such data have spurred the development of surgical simulators to help trainees practice and refine surgical procedures.[11,12,20]
In the current study, EBL also decreased with surgical cases performed later in the day. Despite this finding, transfusion rates as well as intraoperative and post-operative complication rates did not differ between surgical groups. Positive surgical margin rates are also frequently assessed as surrogate measures for surgical performance and have been linked to a learning curve and experience with RARP.[8] There was a slight trend towards a lower positive surgical margin rate for patients undergoing 3rd round cases (3rd round: 23% vs. 1st/2nd round: 25%/25%); however, these differences did not reach statistical significance.
One purported advantage of RARP is that patients often have shorter periods of convalescence and may be discharged from hospital earlier.[4] In addition, institutions performing RARP may often perform higher surgical volumes in order to offset the associated increased cost of robotic surgery. In our study, patients undergoing 3rd round RARP surgery were much more likely to have longer lengths of hospital stay. This finding is unlikely to be related to surgical repetition, but instead more attributable to the later start times of 3rd round cases. On average, these patients had a 32% chance of staying in the hospital for 2 or more days in comparison to 10% of 1st round patients and 11% of 2nd round patients (P = 0.01). This finding may indicate a point of diminishing returns as increasing case volume with 3rd round cases may lengthen patients’ hospital stays, thus potentially increasing hospital costs.
Shorter operative times and longer lengths of hospital stays associated with 3rd round RARP cases are especially poignant due to the recent focus on limiting medical costs.[21,22,23] Several studies comparing costs of RARP to other modalities of radical prostatectomy demonstrated that the robotic approach is more expensive.[24,25,26] In a large comparison study by Bolenz et al., RARP exceeded the median costs of laparoscopic prostatectomy by over $1000 and cost of over $2000 more than the open retropubic approach.[24] Results from their study were calculated using estimated operating room costs of $772/h and estimated hospital costs of approximately $500/night. From our results, the reduced mean operative time for a 3rd round case of 33 min would translate to a cost saving of $429 dollars in decreased operative time (calculated at $13/min[24]); however, the benefit of reduced operating room time and costs would be diminished by the added expense of an extra hospital stay of $500 dollars/day for select patients. Thus, the additional expenditures of 3rd round cases should be considered during surgical planning.
There are several potential limitations of our study. This was a retrospective analysis with considerable heterogeneity in the experience of the surgeon cohort. Furthermore, we assessed the surgical team as a whole and not the performance of individual surgeons as the operating or “console surgeon” who often differed with each case. Lastly, we did not investigate other meaningful post-operative outcomes such as potency, continence and biochemical recurrence rates, which could add further information to our results.
Despite these limitations, the study has several important advantages. Our study analyzed data generated from a controlled setting as only one robotic platform at a single medical center with a dedicated nursing team was used for all surgical cases. Thus, we were able to minimize the potential for bias by limiting environmental factors associated with our cohort. To our knowledge, this is the first study to assess the impact of surgical case order on perioperative outcomes for patients undergoing RARP. Future studies may help elucidate if these effects translate to other significant clinical outcomes.
CONCLUSIONS
The current study demonstrates that surgical case order may influence perioperative outcomes for RARP including decreased operative times and blood loss and increased length of hospital stay for later cases. Taken together, these findings suggest that surgical teams may “warm up” with repetitive practice during the day. Knowledge of these differences may assist in surgical scheduling and supports the use of pre-operative surgical simulation to improve surgical outcomes.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Eastham JA, Savage C, Maschino AC, Laudone VP, Dechet CB, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–92. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farnham SB, Webster TM, Herrell SD, Smith JA., Jr Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67:360–3. doi: 10.1016/j.urology.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Hu JC, Gu X, Lipsitz SR, Barry MJ, D’Amico AV, Weinberg AC, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–64. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 5.Lowrance WT, Elkin EB, Jacks LM, Yee DS, Jang TL, Laudone VP, et al. Comparative effectiveness of prostate cancer surgical treatments: A population based analysis of postoperative outcomes. J Urol. 2010;183:1366–72. doi: 10.1016/j.juro.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe J, Castellucci S, Cathelineau X, Harmon J, Rozet F, Barret E, et al. Robot-assisted laparoscopic prostatectomy: A single-institutions learning curve. Urology. 2009;73:127–33. doi: 10.1016/j.urology.2008.08.482. [DOI] [PubMed] [Google Scholar]
- 7.Samadi D, Levinson A, Hakimi A, Shabsigh R, Benson MC. From proficiency to expert, when does the learning curve for robotic-assisted prostatectomies plateau? The Columbia University experience. World J Urol. 2007;25:105–10. doi: 10.1007/s00345-006-0137-4. [DOI] [PubMed] [Google Scholar]
- 8.Vickers AJ, Bianco FJ, Gonen M, Cronin AM, Eastham JA, Schrag D, et al. Effects of pathologic stage on the learning curve for radical prostatectomy: Evidence that recurrence in organ-confined cancer is largely related to inadequate surgical technique. Eur Urol. 2008;53:960–6. doi: 10.1016/j.eururo.2008.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein EA, Bianco FJ, Serio AM, Eastham JA, Kattan MW, Pontes JE, et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. J Urol. 2008;179:2212–6. doi: 10.1016/j.juro.2008.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albani JM, Lee DI. Virtual reality-assisted robotic surgery simulation. J Endourol. 2007;21:285–7. doi: 10.1089/end.2007.9978. [DOI] [PubMed] [Google Scholar]
- 11.Halvorsen FH, Elle OJ, Dalinin VV, Mørk BE, Sørhus V, Røtnes JS, et al. Virtual reality simulator training equals mechanical robotic training in improving robot-assisted basic suturing skills. Surg Endosc. 2006;20:1565–9. doi: 10.1007/s00464-004-9270-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Mucksavage P, Kerbl DC, Huynh VB, Etafy M, McDougall EM. Validation study of a virtual reality robotic simulator - Role as an assessment tool? J Urol. 2012;187:998–1002. doi: 10.1016/j.juro.2011.10.160. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Mucksavage P, Kerbl DC, Osann KE, Winfield HN, Kahol K, et al. Laparoscopic warm-up exercises improve performance of senior-level trainees during laparoscopic renal surgery. J Endourol. 2012;26:545–50. doi: 10.1089/end.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mucksavage P, Lee J, Kerbl DC, Clayman RV, McDougall EM. Preoperative warming up exercises improve laparoscopic operative times in an experienced laparoscopic surgeon. J Endourol. 2012;26:765–8. doi: 10.1089/end.2011.0134. [DOI] [PubMed] [Google Scholar]
- 15.Wignall GR, Denstedt JD, Preminger GM, Cadeddu JA, Pearle MS, Sweet RM, et al. Surgical simulation: A urological perspective. J Urol. 2008;179:1690–9. doi: 10.1016/j.juro.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Kahol K, Satava RM, Ferrara J, Smith ML. Effect of short-term pretrial practice on surgical proficiency in simulated environments: A randomized trial of the “preoperative warm-up” effect. J Am Coll Surg. 2009;208:255–68. doi: 10.1016/j.jamcollsurg.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Kwon EO, Bautista TC, Blumberg JM, Jung H, Tamaddon K, Aboseif SR, et al. Rapid implementation of a robot-assisted prostatectomy program in a large health maintenance organization setting. J Endourol. 2010;24:461–5. doi: 10.1089/end.2009.0212. [DOI] [PubMed] [Google Scholar]
- 18.Ou YC, Yang CR, Wang J, Yang CK, Cheng CL, Patel VR, et al. The learning curve for reducing complications of robotic-assisted laparoscopic radical prostatectomy by a single surgeon. BJU Int. 2011;108:420–5. doi: 10.1111/j.1464-410X.2010.09847.x. [DOI] [PubMed] [Google Scholar]
- 19.Raman JD, Dong S, Levinson A, Samadi D, Scherr DS. Robotic radical prostatectomy: Operative technique, outcomes, and learning curve. JSLS. 2007;11:1–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Seymour NE, Gallagher AG, Roman SA, O’Brien MK, Bansal VK, Andersen DK, et al. Virtual reality training improves operating room performance: Results of a randomized, double-blinded study. Ann Surg. 2002;236:458–63. doi: 10.1097/00000658-200210000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg PL, Merguerian PA, Bihrle W, 3rd, Seigne JD. The cost of learning robotic-assisted prostatectomy. Urology. 2008;72:1068–72. doi: 10.1016/j.urology.2007.11.118. [DOI] [PubMed] [Google Scholar]
- 22.Lowrance WT, Eastham JA, Yee DS, Laudone VP, Denton B, Scardino PT, et al. Costs of medical care after open or minimally invasive prostate cancer surgery: A population-based analysis. Cancer. 2012;118:3079–86. doi: 10.1002/cncr.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Hu JC. Hospital volume, utilization, costs and outcomes of robot-assisted laparoscopic radical prostatectomy. J Urol. 2012;187:1632–7. doi: 10.1016/j.juro.2011.12.071. [DOI] [PubMed] [Google Scholar]
- 24.Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol. 2010;57:453–8. doi: 10.1016/j.eururo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Burgess SV, Atug F, Castle EP, Davis R, Thomas R. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol. 2006;20:827–30. doi: 10.1089/end.2006.20.827. [DOI] [PubMed] [Google Scholar]
- 26.Scales CD, Jr, Jones PJ, Eisenstein EL, Preminger GM, Albala DM. Local cost structures and the economics of robot assisted radical prostatectomy. J Urol. 2005;174:2323–9. doi: 10.1097/01.ju.0000181830.43340.e7. [DOI] [PubMed] [Google Scholar]


