Abstract
Purpose
To compare the pharmacokinetics and retinal toxicity of various doses of intravitreal triamcinolone acetonide (TA) in rabbits.
Methods
The rabbits received intravitreal injections of 4 mg and 8 mg TA. The drug concentrations were determined with high-performance liquid chromatography after extraction from the vitreous at various time points. The main pharmacokinetics parameters were calculated with 3p97 pharmacokinetics software. The intraocular pressure, electroretinography, and pathological examinations were evaluated before and after intravitreal injection of different doses of TA.
Results
The half-life of intravitreal injection of 4 mg and 8 mg TA was 24 days and 34 days, respectively. No significant differences were found in intraocular pressure (p>0.05) and the electroretinography b-wave amplitudes (p>0.05) among the rabbits before and after intravitreal injection of 4 mg and 8 mg TA. Light and electron microscopy did not show any retinal damage in any group.
Conclusions
Intravitreal injection of 4 mg and 8 mg TA are safe for the rabbit retina. The injection of 8 mg TA produced a longer vitreous half-life and had a prolonged effect on the retina. This conclusion may be referenced in the clinical application of TA in retinal diseases.
Introduction
Intravitreal injection of triamcinolone acetonide (TA) is an efficient method for delivering the drug to the vitreous to avoid systemic side effects, and has been widely used for treating ophthalmic diseases such as diabetic retinopathy [1], retinal vein occlusion [2], and uveitis [3]. In addition, it has also been combined with photodynamic therapy recently [4].
Intravitreal injection of 4 mg/0.1 ml TA is the most popular choice for ophthalmologists [5,6]. Because diseases such as macular edema frequently recur, patients should receive repeated intravitreal injections of TA after a specific period. Jonas suggested that with a higher dose, considerably longer intraocular availability of TA might be achieved [7]. In this report, we speculated that increasing the dose of intravitreal TA could prolong the effects of the drug on the retina and relieve patients from suffering repeated injections. Although TA has already been applied on a large scale for years, little is known about the pharmacokinetics of the drug [8-17], especially since there is no consensus on whether TA has retinal toxicity [18-26]. Thus, the purpose of this study is to compare the pharmacokinetics and retinal toxicity of intravitreal commercial TA at various doses (4 mg and 8 mg) in rabbit eyes. These findings may provide important information for the clinical application of TA.
Methods
Drug preparations, animals, and animal experiments
The 4 mg/0.1 ml dose of TA was obtained directly from commercially available TA (40 mg/ml, Kunming Jida Pharmaceuticals, Kunming, China). The 8 mg/0.1 ml concentration of TA was obtained with the following method. Commercial TA of 0.2 ml was spun down in centrifuge for 5 min at 1698 ×g; thereafter, the half supernatant was removed, and the remnant was mixed. Methanol (high-performance liquid chromatography [HPLC] grade) was purchased from Merk (Hamburg, Germany).
Adult female and male New Zealand white rabbits (2.0–2.5 kg) were obtained from the Laboratory Animal Center of Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China), and all experimental procedures adhered to the guidelines of the Chinese Animal Administration and the Association for Research in Vision and Ophthalmology Statement for the use of animals in ophthalmic and vision research.
All rabbits were anesthetized with an intramuscular injection of ketamine hydrochloride (30 mg/kg) and chlorpromazine hydrochloride (15 mg/kg). Topical anesthesia was achieved with tetracaine hydrochloride. Tropicamide was applied to dilate the pupils. A baseline eye examination including slit-lamp biomicroscopy, funduscopy with direct ophthalmoscope, and intraocular pressure (IOP) measurement were performed. Before intravitreal injection, paracentesis with removal of 0.1 ml of aqueous humor was performed to avoid an increase in IOP. Intravitreal injection of 0.1 ml solution was performed after a sterile eyelid speculum was placed, using a 30-gauge needle in the superior temporal quadrant about 2.5 mm posterior to the limbus.
Pharmacokinetics
Forty-two rabbits were randomly divided into two groups of 21 animals each. Group 1 was intravitreally injected with 4 mg/0.1 ml TA, and six eyeballs were obtained by euthanizing three rabbits with an intravenous injection of 100 mg/kg sodium pentobarbital at 0, 7, 14, 30, 45, 70, and 130 days after TA injection. Group 2 received intravitreal injection of 8 mg/0.1 ml TA, and six eyeballs were obtained at 0, 10, 20, 35, 60, 90, and 150 days after TA injection.
Each eyeball was enucleated and immediately frozen in a −60 °C environment. The vitreous was isolated using the previously described technique by separating frozen vitreous from the sclera, retina, lens, cornea, and so on [13]. Each eyeball was enucleated and immediately frozen in a -60 °C environment, and the frozen vitreous was harvested carefully by separating sclera, retina, lens, cornea, and so on [13]. Vitreous samples were subjected to ultrasonic processor for 20 s at 0 °C (mixture with water and ice). The mixed vitreous of 0.1 ml was pretreated with the addition of 4.9 ml methanol. The mixture was blended and centrifuged at 1698 ×g for 30 min, and 20 μl of each supernatant was directly injected into reversed-phase HPLC (Shimadzu LC-10AD liquid chromatography equipped with SPD-10A UV detector, class-10A workstation system, Rheodyne injector, and 20-μl sample loop) to analyze the data. The Diamonsil C-18 chromatographic column (5 µm particle size, 250 mm × 4.6 mm, Dikma Technologies, Beijing, China) was used for separation, and detection was set at 254 nm. The mobile phase was composed of methanol:water (70:30) at a flow rate of 1.0 ml/min. The column oven was set at 40 °C. The TA retention time was 6.8 min, and the detection limit was 0.005 mg/ml. The standard curve was linear from 0.005 to 0.100 mg/ml (correlative >0.9998), using seven different amounts of TA standard. The concentrations of 0.01 mg/ml, 0.06 mg/ml, and 0.10 mg/ml TA in vitreous were measured for the recovery and intra- and inter-day reproducibility.
Retinal toxicity
Twenty rabbits were divided into two experimental groups: Ten right eyes in group A were injected with 4 mg/0.1 ml TA, and ten right eyes in group B were injected with 8 mg/0.1 ml TA. All left eyes were injected with 0.1 ml physiologic salt solution (PSS) as the control group.
IOP measurement was performed with a Schiötz ophthalmotonometer (Suzhou, China) before intravitreal injection and at 1, 4, 8, and 12 weeks following injection. All animals were kept in a quiet environment for about 30 min and then received topical anesthesia. After the eyelid was separated, the central part of the cornea was gently touched with the Schiötz ophthalmotonometer.
Electroretinography (ERG) was performed before intravitreal injection and at 1 and 3 months after injection using the method previously reported [27]. Briefly, the rabbits received dark adaption for at least 30 min before anesthesia and pupillary dilatation with the described methods. A silver electrode was placed on the cornea and served as a positive electrode. The reference electrode was placed subcutaneously between the base of the ear and the lateral canthus. The ground electrode was placed subcutaneously at the forehead area. The ERG included 30 Hz flicker, scotopic 0.5 Hz every 5 min during the 30 min of dark adaptation, with a flash intensity of 3.0 cd•s/m2.
The animals were euthanized, and both eyes were enucleated at 3 months after the last ERG. Enucleated eyes were fixed in 10% formalin for light microscopic examination, or 2.5% glutaraldehyde for transmission electron microscopy. Light microscopic sections were stained with hematoxylin-eosin, and transmission electron microscopic sections were stained with lead and uranyl acetate.
Statistical analysis
The elimination half-life (t1/2), initiate theoretical concentration (Co), and area under the concentration time curve (AUC) were calculated with 3p97 pharmacokinetics software (Practical Pharmacokinetics Software, Beijing, China). Using SPSS 13.0 (SPSS Inc., Chicago, IL), the IOP and ERG b-wave amplitude data were compared by using repeated measures ANOVA. p<0.05 was considered significant.
Results
Pharmacokinetics
Chromatograms of TA in samples are shown in Figure 1. TA was separated clearly and was not interfered by the other substances. The recovery and intra- and inter-day reproducibility results are shown in Table 1. The mean recovery from the vitreous was 98.36%. These data suggested that TA could be efficiently extracted from the vitreous via this method, and analyzing the TA concentration of intravitreal injection was reliable.
Figure 1.
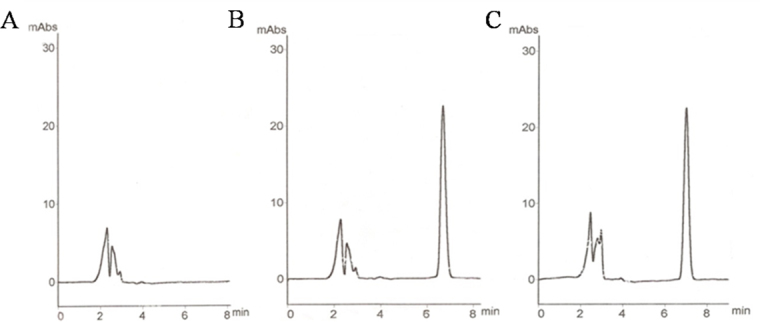
Triamcinolone acetonide (TA) concentration time profiles in the vitreous of rabbits following intravitreal injection of TA. The concentrations of intravitreal 4 mg (A) and 8 mg (B) TA decreased gradually with time. Values represent mean±SD (n=6). Typical high-performance liquid chromatography chromatograms of triamcinolone acetonide. A: Blank rabbit vitreous control. B: Blank rabbit vitreous+TA. C: The sample of rabbit vitreous after intravitreal injection of TA.
Table 1. Results of recovery and precision of TA.
| Concentration (mg/ml) | Recovery χ± RSD% | Intraday χ ± RSD% | Interday χ ± RSD% |
|---|---|---|---|
| 0.01 |
0.0097±0.0003 |
0.0090±0.0002 |
0.0094±0.0003 |
| 0.06 |
0.0591±0.0017 |
0.0605±0.0030 |
0.0582±0.0021 |
| 0.1 | 0.0997±0.0019 | 0.0997±0.0012 | 0.0962±0.0035 |
χ: mean values, n=5; RSD: relative standard deviation.
Figure 2 shows the time course of the TA concentration in the vitreous of the rabbits following intravitreal injection of 4 mg and 8 mg TA. The concentrations of both doses decreased gradually with time, and the peak concentration of intravitreal TA was the initial concentration. A concentration of 0.0524 mg/ml was maintained in the vitreous at 130 days after the injection of 4 mg TA, and 0.2606 mg/ml was detected at 150 days following the intravitreal injection of 8 mg TA. The calculated half-lives of intravitreal TA were 24 days for the 4 mg dose and 34 days for the 8 mg dose. The initiate theoretical concentration of intravitreal 4 mg and 8 mg TA were 2.745 mg/ml and 5.498 mg/ml, and the AUC were 95.560 (mg/ml)/d and 247.789 (mg/ml)/d, respectively. These data show that intravitreal injection of 8 mg TA achieved a longer half-life and higher concentration and bioavailability compared with the intravitreal injection of 4 mg TA.
Figure 2.
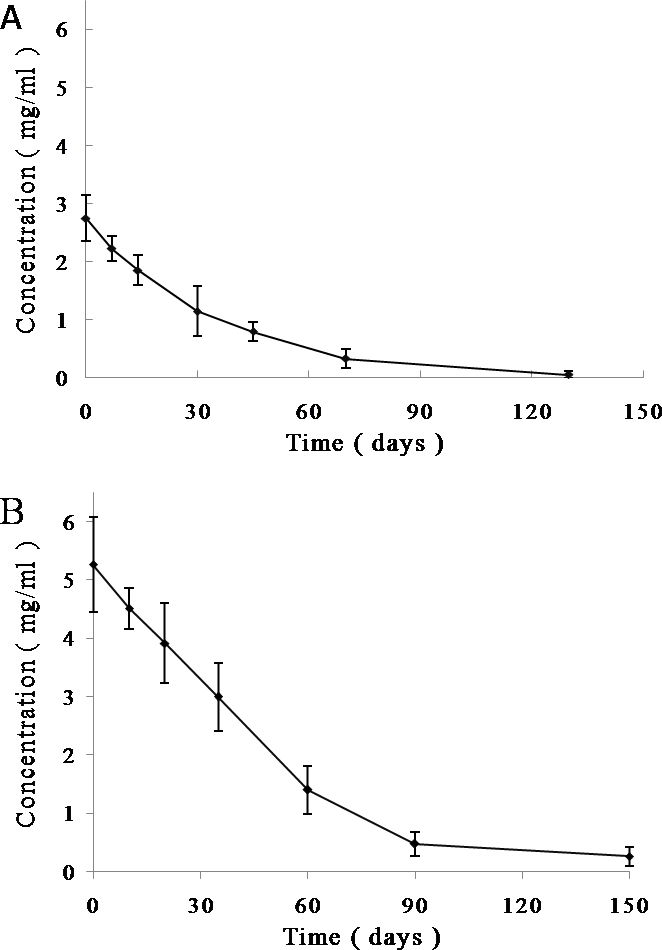
Triamcinolone acetonide concentration time profiles in the vitreous of rabbits following intravitreal injection of triamcinolone acetonide. The concentrations of intravitreal 4 mg (A) and 8 mg (B) triamcinolone acetonide (TA) decreased gradually with time. Values represent mean±SD (n = 6).
Retinal toxicity
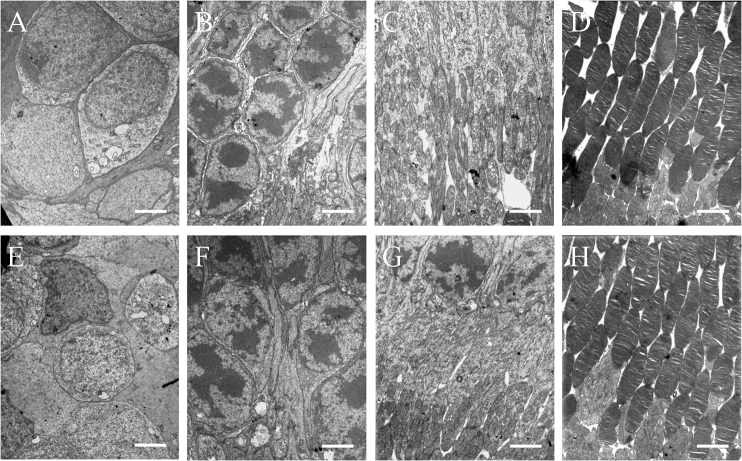
The vitreous was transparent with the intravitreal injection of PSS, and TA was visible as a white mass in the vitreous after the injection (Figure 3). The size of the mass became gradually smaller. No endophthalmitis, keratic damage, cataract, vitreous hemorrhage, or retinal detachment was discovered during the study period. The IOP and b-wave amplitudes for each group at different time points are shown in Figure 4 and Figure 5. There was no significant difference in IOP between any groups (F = 0.316, p = 0.732>0.05) or between any time points (F = 0.835, p =0.507>0.05), and there were no significant differences between any groups (F = 1.868, p = 0.167>0.05) or between any time points (F = 0.079, p = 0.925>0.05) in the ERG b-wave amplitudes. The histopathologic examination of both groups showed no structural abnormalities under light microscopy at 3 months, and normal layers of retina were revealed in both groups (Figure 6). Transmission electron microscopy confirmed these findings and demonstrated a complete normal retina in each group. The outer nuclear layer and the inner nuclear layer are normal in Figure 7A,B. The inner and outer segments of the photoreceptors are also normal at 3 months in Figure 7C,D. These results suggest that intravitreal injection of 4 mg and 8 mg TA is safe for the rabbit retina.
Figure 3.
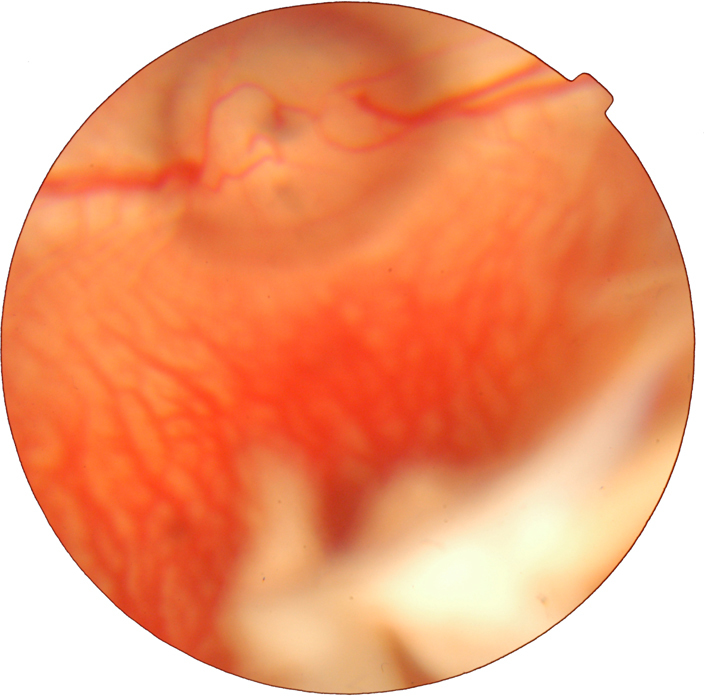
Digital color fundus photography of the rabbit after intravitreal injection of triamcinolone acetonide. Triamcinolone acetonide (TA) injected into the vitreous aggregated to form a white drug depot.
Figure 4.
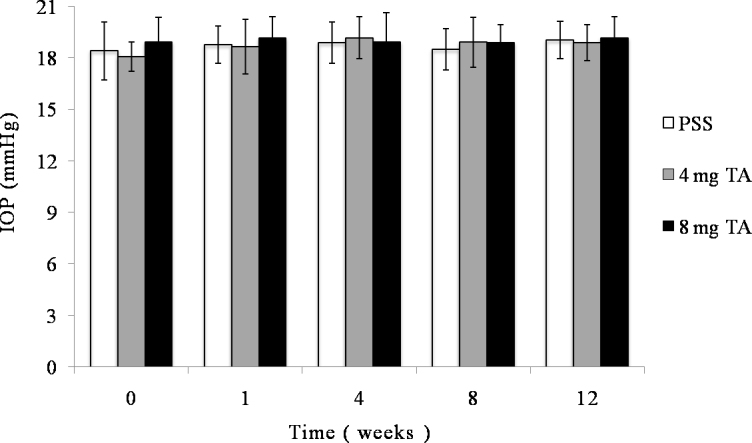
Intraocular pressure for each group at different time. Intraocular pressures was obtained with the Schiötz ophthalmotonometer before the intravitreal injection and at 1, 4, 8, and 12 weeks after injection. There was no significant difference in intraocular pressure between any groups (p>0.05) or between any time points (p>0.05).
Figure 5.
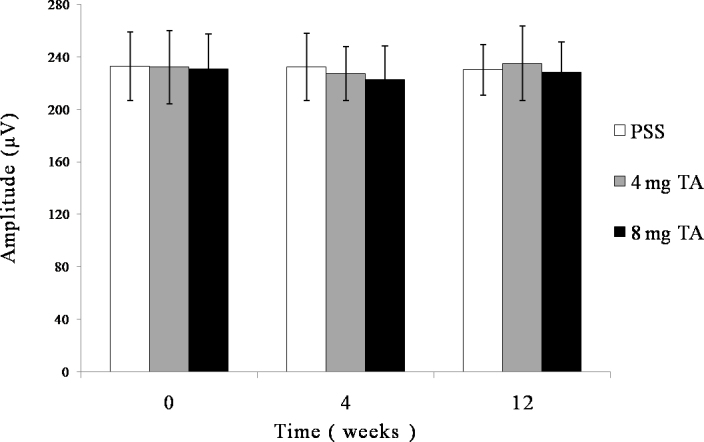
Electroretinography b-wave amplitudes for each group at different time points. Electroretinography was performed before intravitreal injection and at 4 and 12 weeks after injection. No significant difference was observed in b-wave amplitudes between any groups (p>0.05) or between any time points (p>0.05).
Figure 6.
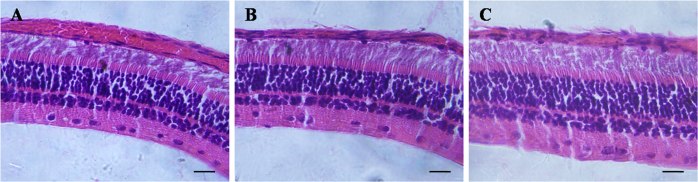
Light microscopic examination of the rabbit retina at 3 months (stain, hematoxylin-eosin; magnification, ×400). A: Intravitreal injection of physiologic salt solution. B: Intravitreal injection of 4 mg triamcinolone acetonide (TA). C: Intravitreal injection of 8 mg TA. The three figures show clear retina layers and normal structure. Bar = 20 μm.
Figure 7.
Transmission electron microscopic examination of rabbit retina at 3 months. Ultrastructure of rabbit retina at 3 months after intravitreal injection of PSS (A, B, C, D), 4 mg triamcinolone acetonide (TA; E, F) and 8 mg TA (G, H). No histological changes were verified in all retinas from the groups. The cells of outer nuclear layer (A, E), inner nuclear layer (B, F), inner segment of photoreceptor (C, G), and outer segment of photoreceptor (D, H) were all normal. Bar=2.5 μm in B, D and E, 2.0 μm in A, C, F, G, and H.
Discussion
Due to the inner and outer blood–retinal barriers, systemic drugs barely reach the vitreous. To increase local ophthalmic drugs and limit the risk of systemic adverse effects, intravitreal injection has been applied in clinical treatment [28]. In 1980, Tano et al. [29] reported intravitreal injection of TA for therapy of proliferative vitreoretinopathy for the first time, and thereafter the use of intravitreal TA has gradually increased in ophthalmic diseases.
However, there are few reports about the pharmacokinetics of intravitreal injection of TA. Chin et al. [13] reported that the half-lives of intravitreal 0.3 mg TA for rabbits were 1.57 days in the vitrectomized group and 2.89 days in the nonvitrectomized group. Kim et al. [14] demonstrated that the half-lives of intravitreal 4 mg Kenalog and TA preservative-free were 23 days and 24 days, respectively. From these studies, we can infer that drug clearance may be enhanced by vitrectomy, and different intravitreal doses of TA may cause different elimination half-lives. Ophthalmologists always intravitreally injected 4 mg TA for patients, and most did not undergo vitrectomy. Therefore, this study selected 4 mg as the small dose of TA for the nonvitrectomized rabbits. We analyzed the pharmacokinetics of intravitreal commercial TA of 4 mg and 8 mg because the report showed there was no difference in vitreous levels of TA compared to TA preservative-free [14]. Additionally, removing preservatives could increase the risk of contamination.
This study showed that the half-life of intravitreal injection of 8 mg TA in rabbits was longer than that of 4 mg TA, and the initiate concentration of intravitreal 8 mg TA was also significant higher than that of 4 mg TA. The longer vitreous half-life of higher dose of intravitreal drug might attribute to “saturation” of elimination mechanisms [30]. Thus, we could infer that the higher initial dose of TA could cause a longer half-life. Thus, the higher dose of TA in the vitreous could lead to longer drug exposure for treating chronic eye disorders. Clinically, a longer duration of a higher dose of TA may be of benefit for treating chronic eye diseases. In the study, the half-life of intravitreal 4 mg TA was 24 days, which was similar to the results of Kim et al. [14] that the half-life of intravitreal 4 mg Kenalog was 23 days. Taking account of the differences in anatomy and physiology between humans and rabbits, caution should be exercised when analyzing pharmacokinetic study using rabbit models.
Some researchers believed that the retinal toxicity associated with TA might be due to its preservative, benzyl alcohol [20,22,25,31]. Chang et al. [31] considered that the preserved commercial TA suspensions damaged human retinal pigment epithelial cells, but the preservative-free solutions did not. Macky et al. [32] believed benzyl alcohol produced severe ERG and structural damage to the retina when injected intravitreally. However, Dierks [19], Li [23], and Ruiz-Moreno [26] drew a complete opposite conclusion that the benzyl alcohol of 0.1 ml commercial TA did not show any toxicity to the retina. In this research, we used commercial TA, and the results demonstrated that IOP, ERG b-wave, light, and electron microscopy did not show significant differences in any groups, which were consistent with other studies [14,19,24,26,27]. In clinical analyses, intravitreal TA is associated with common side effects such as raised IOP and cataract, especially IOP elevation [33-35]. However, in most previous human reports regarding use of high-dose TA for intravitreal injections, no significant differences in IOP elevation were found between low-dose and high-dose intravitreal TA treatment [36-38]. Wei et al. [39] suggested that a higher dose of TA could be used if there was no IOP elevation with the initial lower dose of TA injection. Notwithstanding the histopathologic findings, ERG and IOP suggested no difference in the toxicity of 8mg TA, many other factors, especially the IOP elevation, should also be considered for the safety of intravitral 8mg TA in human eyes.
In conclusion, intravitreal injection of 4 mg and 8 mg TA was safe for the rabbits, and the higher TA dose had a longer effect. Consequently, these rabbit data provide a reasonable reference to the clinical application of intravitreal injection of TA.
Acknowledgments
This work was supported by the grant from National Natural Science Foundation of China (No. 81201026).
References
- 1.Yalcinbayir O Gelisken O, Kaderli B, Avci R. Intravitreal versus sub-tenon posterior triamcinolone injection in bilateral diffuse diabetic macular edema. Ophthalmologica. 2011;225:222–7. doi: 10.1159/000324714. [DOI] [PubMed] [Google Scholar]
- 2.Kwon SI Kim YW, Bang YW, Lee JY, Park IW. Comparison of natural course, intravitreal triamcinolone, and intravitreal bevacizumab for treatment of macular edema secondary to branch retinal vein occlusion. J Ocul Pharmacol Ther. 2013;29:5–9. doi: 10.1089/jop.2011.0249. [DOI] [PubMed] [Google Scholar]
- 3.Habot-Wilner Z, Sallam A, Pacheco PA, Do HH, McCluskey P, Lightman S. Intravitreal triamcinolone acetonide as adjunctive treatment with systemic therapy for uveitic macular edema. Eur J Ophthalmol. 2011;21(Suppl 6):S56–61. doi: 10.5301/EJO.2010.6062. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Moreno JM Montero JA, Amat P, Lugo F. Macular atrophy after combined intravitreal triamcinolone and photodynamic therapy to treat choroidal neovascularization. Int J Ophthalmol. 2010;3:161–3. doi: 10.3980/j.issn.2222-3959.2010.02.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JE, Pollack JS, Miller DG, Mittra RA, Spaide RF. ISIS-DME: a prospective, randomized, dose-escalation intravitreal steroid injection study for refractory diabetic macular edema. Retina. 2008;28:735–40. doi: 10.1097/IAE.0b013e318163194c. [DOI] [PubMed] [Google Scholar]
- 6.Lam DS, Chan CK, Tang EW, Li KK, Fan DS, Chan WM. Intravitreal triamcinolone for diabetic macular oedema in Chinese patients: six-month prospective longitudinal pilot study. Clin Experiment Ophthalmol. 2004;32:569–72. doi: 10.1111/j.1442-9071.2004.00903.x. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB. Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004;137:560–2. doi: 10.1016/j.ajo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Schindler RH, Chandler D, Thresher R, Machemer R. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982;93:415–7. doi: 10.1016/0002-9394(82)90130-1. [DOI] [PubMed] [Google Scholar]
- 9.Scholes GN, O'Brien WJ, Abrams GW, Kubicek MF. Clearance of triamcinolone from vitreous. Arch Ophthalmol. 1985;103:1567–9. doi: 10.1001/archopht.1985.01050100143037. [DOI] [PubMed] [Google Scholar]
- 10.Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–6. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 11.Mason JO, 3rd, Somaiya MD, Singh RJ. Intravitreal concentration and clearance of triamcinolone acetonide in nonvitrectomized human eyes. Retina. 2004;24:900–4. doi: 10.1097/00006982-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Takeda K, Morita K, Yamada M, Tanigawara Y, Oguchi Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004;138:1046–8. doi: 10.1016/j.ajo.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25:556–60. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Csaky KG, Gravlin L, Yuan P, Lutz RJ, Bungay PM, Tansey G. F DEM, Potti GK, Grimes G, Robinson MR. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina. 2006;26:523–30. doi: 10.1097/00006982-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kamppeter BA, Cej A, Jonas JB. Intraocular concentration of triamcinolone acetonide after intravitreal injection in the rabbit eye. Ophthalmology. 2008;115:1372–5. doi: 10.1016/j.ophtha.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SQ. Quantification of triamcinolone acetonide in ocular tissues after intravitreal injection to rabbit using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:548–52. doi: 10.1016/j.jchromb.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira RC, Messias A, Siqueira RC, Bonini-Filho MA, Haddad A, Damico FM, Maia-Filho A, Crispim PT, Saliba JB, Ribeiro JA, Scott IU, Cunha-Jr AS, Jorge R. Vitreous pharmacokinetics and retinal safety of intravitreal preserved versus non-preserved triamcinolone acetonide in rabbit eyes. Curr Eye Res. 2012;37:55–61. doi: 10.3109/02713683.2011.593722. [DOI] [PubMed] [Google Scholar]
- 18.McCuen BW, 2nd, Bessler M, Tano Y, Chandler D, Machemer R. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981;91:785–8. doi: 10.1016/0002-9394(81)90013-1. [DOI] [PubMed] [Google Scholar]
- 19.Dierks D, Lei B, Zhang K, Hainsworth DP. Electroretinographic effects of an intravitreal injection of triamcinolone in rabbit retina. Arch Ophthalmol. 2005;123:1563–9. doi: 10.1001/archopht.123.11.1563. [DOI] [PubMed] [Google Scholar]
- 20.Morrison VL, Koh HJ, Cheng L, Bessho K, Davidson MC, Freeman WR. Intravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rabbits. Retina. 2006;26:339–44. doi: 10.1097/00006982-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Kai W, Yanrong J, Xiaoxin L. Vehicle of triamcinolone acetonide is associated with retinal toxicity and transient increase of lens density. Graefe's archive for clinical and experimental ophthalmology. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2006;244:1152–9. doi: 10.1007/s00417-005-0251-9. [DOI] [PubMed] [Google Scholar]
- 22.Chang YS, Wu CL, Tseng SH, Kuo PY, Tseng SY. In vitro benzyl alcohol cytotoxicity: implications for intravitreal use of triamcinolone acetonide. Exp Eye Res. 2008;86:942–50. doi: 10.1016/j.exer.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Chen H, Hou J, Zhang Y, Li G, Gu X, Luo L, Shen H, Qu J, Cheng L. Further characterization of ocular safety profile of commercially available preserved and preservative-free triamcinolone acetonide. Retina. 2012;32:364–74. doi: 10.1097/IAE.0b013e31821e1f7c. [DOI] [PubMed] [Google Scholar]
- 24.Yi NY, Davis JL, Salmon JH, Gilger BC. Ocular distribution and toxicity of intravitreal injection of triamcinolone acetonide in normal equine eyes. Vet Ophthalmol. 2008;11(Suppl 1):15–9. doi: 10.1111/j.1463-5224.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhengyu S, Fang W, Ying F. Vehicle used for triamcinolone acetonide is toxic to ocular tissues of the pigmented rabbit. Curr Eye Res. 2009;34:769–76. doi: 10.1080/02713680903063082. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Moreno JM, Montero JA, Bayon A, Rueda J, Vidal M. Retinal toxicity of intravitreal triamcinolone acetonide at high doses in the rabbit. Exp Eye Res. 2007;84:342–8. doi: 10.1016/j.exer.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Albini TA, Abd-El-Barr MM, Carvounis PE, Iyer MN, Lakhanpal RR, Pennesi ME, Chevez-Barrios P, Wu SM, Holz ER. Long-term retinal toxicity of intravitreal commercially available preserved triamcinolone acetonide (Kenalog) in rabbit eyes. Invest Ophthalmol Vis Sci. 2007;48:390–5. doi: 10.1167/iovs.06-0145. [DOI] [PubMed] [Google Scholar]
- 28.Feigenbaum A, Kornbluth W. Intravitreal injection of penicillin in a case of incipient abscess of the vitreous following extracapsular cataract extraction; perfect cure. Ophthalmologica. 1945;110:300–5. doi: 10.1159/000300284. [DOI] [PubMed] [Google Scholar]
- 29.Tano Y, Chandler D, Machemer R. Treatment of intraocular proliferation with intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 1980;90:810–6. doi: 10.1016/s0002-9394(14)75196-7. [DOI] [PubMed] [Google Scholar]
- 30.Pearson PA, Jaffe GJ, Martin DF, Cordahi GJ, Grossniklaus H, Schmeisser ET, Ashton P. Evaluation of a delivery system providing long-term release of cyclosporine. Arch Ophthalmol. 1996;114:311–7. doi: 10.1001/archopht.1996.01100130307014. [DOI] [PubMed] [Google Scholar]
- 31.Chang YS, Wu CL, Tseng SH, Kuo PY, Tseng SY. Cytotoxicity of triamcinolone acetonide on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2792–8. doi: 10.1167/iovs.06-1146. [DOI] [PubMed] [Google Scholar]
- 32.Macky TA, Helmy D, El Shazly N. Retinal toxicity of triamcinolone's vehicle (benzyl alcohol): an electrophysiologic and electron microscopic study. Graefe's archive for clinical and experimental ophthalmology. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2007;245:817–24. doi: 10.1007/s00417-006-0459-3. [DOI] [PubMed] [Google Scholar]
- 33.Arikan G Osman Saatci A, Hakan Oner F. Immediate intraocular pressure rise after intravitreal injection of ranibizumab and two doses of triamcinolone acetonide. Int J Ophthalmol. 2011;4:402–5. doi: 10.3980/j.issn.2222-3959.2011.04.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan CK, Fan DS, Chan WM, Lai WW, Lee VY, Lam DS. Ocular-hypertensive response and corneal endothelial changes after intravitreal triamcinolone injections in Chinese subjects: a 6-month follow-up study. Eye (Lond) 2005;19:625–30. doi: 10.1038/sj.eye.6701585. [DOI] [PubMed] [Google Scholar]
- 35.Veritti D, Di Giulio A, Sarao V, Lanzetta P. Drug safety evaluation of intravitreal triamcinolone acetonide. Expert Opin Drug Saf. 2012;11:331–40. doi: 10.1517/14740338.2012.635141. [DOI] [PubMed] [Google Scholar]
- 36.Jonas JB, Degenring R, Kreissig I, Akkoyun I. Safety of intravitreal high-dose reinjections of triamcinolone acetonide. Am J Ophthalmol. 2004;138:1054–5. doi: 10.1016/j.ajo.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 37.Spandau UH, Derse M, Schmitz-Valckenberg P, Papoulis C, Jonas JB. Dosage dependency of intravitreal triamcinolone acetonide as treatment for diabetic macular oedema. Br J Ophthalmol. 2005;89:999–1003. doi: 10.1136/bjo.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tammewar AM, Cheng L, Kayikcioglu OR, Falkenstein IA, Kozak I, Goldbaum MH, Freeman WR. Comparison of 4 mg versus 20 mg intravitreal triamcinolone acetonide injections. Br J Ophthalmol. 2008;92:810–3. doi: 10.1136/bjo.2007.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Y Wang H, Chen F, Zu Z, Bi C, Yang X. Different dosages of intravitreal triamcinolone acetonide injections for macular edema secondary to central retinal vein occlusion. Eye Sci. 2012;27:152–7. doi: 10.3969/j.issn.1000-4432.2012.03.009. [DOI] [PubMed] [Google Scholar]