Abstract
Cancer cells reprogram their metabolic pathways to facilitate fast proliferation. Previous studies have shown that overexpression of NF-kappa B2/p52 (p52) in prostate cancer cells promotes cell growth and leads to castration resistance through aberrant activation of androgen receptor. In addition, these cells become resistant to enzalutamide. In this study, we investigated the effects of p52 activation on glucose metabolism and on response to enzalutamide therapy. Data analysis of gene expression arrays showed that genes including Glut1, PKM2, G6PD, and ME1 involved in regulating glucose metabolism were altered in LNCaP cells overexpressing p52 compared to the parental LNCaP cells. We demonstrated an increased amount of glucose flux in the glycolysis pathway, as well as the pentose phosphate pathway (PPP) upon p52 activation. The p52 overexpressing cells increase glucose uptake and are capable of higher ATP and lactate production compared to the parental LNCaP cells. The growth of p52 overexpressing cells depends on glucose in the culture media and is sensitive to glucose deprivation compared to the parental LNCaP cells. Targeting glucose metabolism by glucose analog 2-Deocxy-D-Glucose (2-DG) synergistically inhibits cell growth when combined with enzalutamide, and re-sensitizes p52 overexpressing cells to enzalutamide treatment. These results suggest that p52 modulates glucose metabolism, enhances glucose flux to glycolysis and pentose phosphate pathway, thus facilitating fast proliferation of the cells. Co-targeting glucose metabolism together with androgen receptor axis synergistically inhibits cell growth, and restores enzalutamide-resistant cells to enzalutamide treatment.
Keywords: Prostate cancer, glucose metabolism, NF-κB2/p52, enzalutamide
Introduction
As the second leading cause of cancer-related death among men in the United States, prostate tumors respond to androgen deprivation therapy initially; however, after time, relapse occurs and castration-resistant (CRPC), a lethal form of prostate cancer develops (Chen, et al. 2004; Harris, et al. 2009). The NF-kappa B family has been identified as an important mediator in many cancer-causing processes. The family consists of five members: RelA/p65, NF-kappa B1/p50, c-Rel, RelB, and NF-kappa B2/p52, in which p65/p50 heterodimer and RelB/p52 heterodimer are involved in the canonical and non-canonical NF-kappa B pathways respectively (Betts and Nabel 1996; Fan and Maniatis 1991; Karin and Greten 2005; Lin, et al. 1998). The non-canonical pathway which involves the processing of NF-kappa B2 p100 to p52 is inducible and tightly regulated (Nadiminty, et al. 2006; Xiao, et al. 2001a; Xiao, et al. 2004; Xiao, et al. 2001b). Overproduction of p52 from p100 causes hyperplastic growth in the liver, lymphocytes, and mammary glands of mice (Connelly, et al. 2007; Ishikawa, et al. 1997). In several solid tumors, including breast and prostate, overproduction of p52 has been observed (Cogswell, et al. 2000; Lessard, et al. 2006). In previous studies, we have shown that NF-kappa B2/p52 induces castration-resistant growth in prostate cancer cells through aberrant activation of androgen receptor (AR) (Nadiminty, et al. 2008; Nadiminty, et al. 2010b). In addition, p52 overexpressing cells are resistant to enzalutamide, a newly approved anti-androgen drug for CRPC patients (Nadiminty, et al. 2013; Tran, et al. 2009). Yet, the effect of p52 on cellular metabolism has not previously been studied.
Different from highly differentiated normal cells, cancer cells reprogram their metabolism to facilitate fast proliferation. One typical feature is known as the “Warburg effect”, also called aerobic glycolysis, where cancer cells mainly depend on glycolysis for their energy production in the presence of oxygen (Warburg 1956). This process produces far less energy than oxidative phosphorylation exhibited in normal cells, however, it produces more intermediate products for anabolic building blocks and much less oxidative stress to keep the redox balance (Cairns, et al. 2011; DeBerardinis and Thompson 2012; Teicher, et al. 2012; Vander Heiden 2011). It has been reported that metabolic enzymes such as pyruvate kinase can be alternatively spliced to isoform M2, which supports anabolic growth and promotes tumorigenesis (Christofk, et al. 2008a; Sun, et al. 2011). Activation of many oncogenes, such as PI3K, Akt, mTORC1, and myc, can also impact mitochondria metabolism (Berwick, et al. 2002; Buzzai, et al. 2005; Cunningham, et al. 2007; Hatzivassiliou, et al. 2005).
In this study, we demonstrated that genes involved in glucose metabolic pathways in p52 overexpressing LNCaP cells were upregulated through gene expression array data analysis. Further analysis on glucose metabolism revealed enhancements in both the glycolysis and pentose phosphate pathways accompanied by higher glucose consumption and lactate production. Moreover, targeting glucose metabolism by glucose analog 2-Deocxy-D-Glucose (2-DG) re-sensitized p52 overexpressing prostate cancer cells response to enzalutamide.
Materials and methods
Cell culture and Reagents
LNCaP and C4-2B cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin and 0.1 mg/mL streptomycin and maintained at 37°C in a humidified incubator with 5% CO2. LNCaP-neo and LNCaP-p52 stale cells are generated as previously described (Nadiminty, et al. 2010a). C4-2B MDVR cells were generated by culturing C4-2B cells in media containing 20 μM Enzalutamide until the cells became resistant to Enzalutamide as described previously (Nadiminty, et al. 2013). Cells were seeded in 24-well plates and treated with 20 μM Enzalutamide and/or 1mM/2mM 2-Deocxy-D-Glucose (2-DG) after 24 hours. After 48-72 hours of treatment, cells were counted and harvested; medium was collected for glucose consumption, lactate production, and PSA concentration assays. 2-DG was purchased from Sigma-Aldrich (Saint Louis, MO). ATP assay kit was purchased from Perkin Elmer (Santa Clara, California). Glucose consumption, lactate production, pyruvate kinase activity, and NADPH/NADP assay kits were all purchased from Biovision (Milpitas, California). PSA ELISA kit was purchased from Abnova (Walnut, California).
Microarray pathway analysis
Metabolic pathways of NF-κ B2/p52 were analyzed using Ingenuity Pathway Analysis Software (Ingenuity). This method uses gene identities in conjunction with controlled, vocabulary-based data mining of literature associations, protein– protein interaction databases, and a metabolism pathway database.
Preparation of Whole Cell Extracts
Cells were washed twice with PBS and lysed for 20 min on ice with cell lysis buffer containing 10mM HEPES pH 7.9, 250mM NaCl, 1mM EDTA, 1% NP-40, supplemented with 1mM DTT, 1mM PMSF, 5mM NaF, 1mM Na3VO4 and protease inhibitors (Roche, Basel, Switzerland).
Western Blot Analysis
The cell extracts were obtained and separated by SDS-PAGE and the proteins were transferred onto nitrocellulose membranes. The membranes were blocked by 5% non-fat milk in PBS/0.05%Tween-20, followed by probing with appropriate primary and secondary antibodies.
Cell transfection
CWR22Rv1 cells were transient transfected with HA tag labeled p52 plasmid. Cells were plated at density of 1× 105/ml one day before transfection, which was done by Attractene (Qiagen) following the manufacturer's instruction.
Measurement of ATP production, glucose consumption, and lactate production
ATP levels in the cell lysate were determined by luminescence-based assay kit (ATPlite; Perkin Elmer) according to the manufactures’ instruction. Cells were seeded in the culture dishes and the medium was changed after 12 hours. The culture medium was collected for measurement of glucose and lactate concentration after 24-48 hours. Glucose levels were determined by comparing difference in the collected medium and fresh medium using a glucose assay kit (Biovision). Lactate levels were measured by lactate assay kit (Biovision). ATP production, glucose consumption, and lactate production levels were normalized to cell number.
NADPH/NADP assay
Cells were harvested after 48 hours of culture. NADP total (NADPH and NADP) levels and NADPH levels were determined by a commercially available assay kit (Biovision). NADP levels were determined by the difference of NADP total and NADPH levels. The ratio was calculated thereafter.
Glucose deprivation assay
The cells were seeded in 12-well plates one day before switching to RPMI-1640 glucose free medium containing 10% dialyzed FBS. Cells were counted after 3 days of changing medium.
PSA ELISA Assay
Culture medium was collected after two days of seeding cells or drug treatment. PSA levels in the medium were measured by a commercially available kit following the manufacturer's instruction. Data are expressed as means±SD from 3 independent experiments.
Statistical Analysis
All data present are shown as means ± SD. Student's t-test was used to compare difference between two groups. P<0.05 was considered significant.
Results
Alteration of glucose pathways by p52
Our previous studies have demonstrated p52 overexpressing LNCaP prostate cancer cells are more aggressive in terms of tumor growth, resistance to androgen deprivation and enzalutamide treatment (Nadiminty et al. 2008; Nadiminty et al. 2010a; Nadiminty et al. 2006; Nadiminty et al. 2010b; Nadiminty et al. 2013). Since it is well documented that cancer cells reprogram their metabolism to facilitate fast proliferation, we examined the metabolism pathways in p52 overexpressing LNCaP cells by analyzing the gene expression microarray data generated from p52 retrovirus infected cells (Nadiminty et al. 2010a).
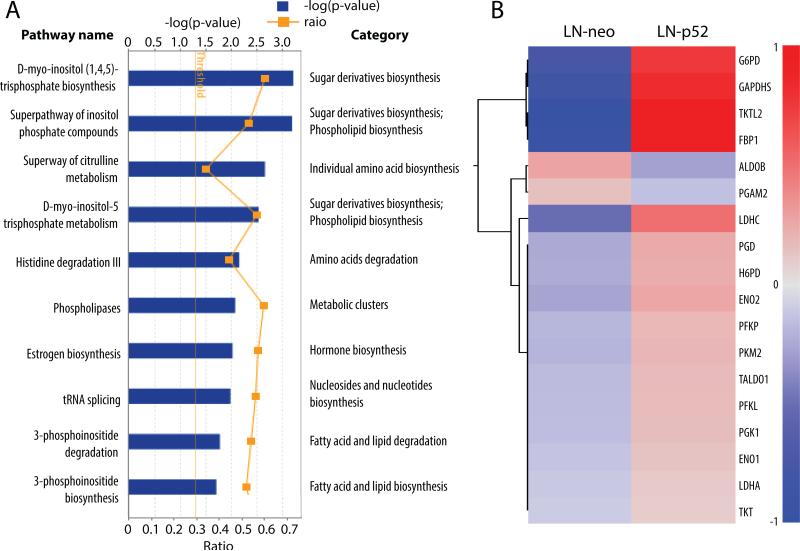
Within the top 30 altered pathways by overexpression of p52 in LNCaP cells compared to the parental LNCaP cells, the intermediate or final products of 11 pathways, including the top 2 pathways, are involved in sugar metabolism, fatty acid synthesis and/or lipid biosynthesis and degradation. Five pathways are correlated to amines, polyamines, amino acids biosynthesis and degradation. In addition, pathways involved in nucleoside and nucleotide biosynthesis and degradation as well as vitamin biosynthesis, NAD biosynthesis and polysaccharides biosynthesis are all significantly enhanced. The most altered metabolism pathways are listed in Fig 1A. These findings suggest that in p52 cells, anabolic metabolism pathways are enhanced, providing additional byproducts necessary for cell division, thus facilitating quicker cell proliferation.
Figure 1. Metabolic pathways and genes altered in LNCaP-p52 cells.
(A) Top 10 altered metabolic pathways are listed. Blue boxes show in –log (p-value). Yellow dots show in Log2 (Fold Change). Fold change threshold is set as 1.2. (B) Heat map of gene expression within glycolysis and pentose phosphate pathway. All genes of the glycolysis and pentose phosphate pathways with a > 1.2 fold change are included in the heat map. Color scale shows in the format of Log2 (Fold Change).
In correspondence with the enhanced macromolecular biosynthesis, glycolysis processes including pyruvate fermentation to lactate is at top of the list. Many key enzymes within glycolysis, such as PFKP, GAPDHS, PGK1, ENO1, ENO3, PKM2 and LDHA, have elevated expression levels in LNCaP-p52 cells compared to the parental LNCaP cells (Fig 1B). In addition to the upregulated genes in glycolysis, genes within the pentose phosphate pathway (PPP) such as G6PD, PGD, TALDO1, TKT are also up regulated (Fig 1B).
p52 enhances glucose metabolism
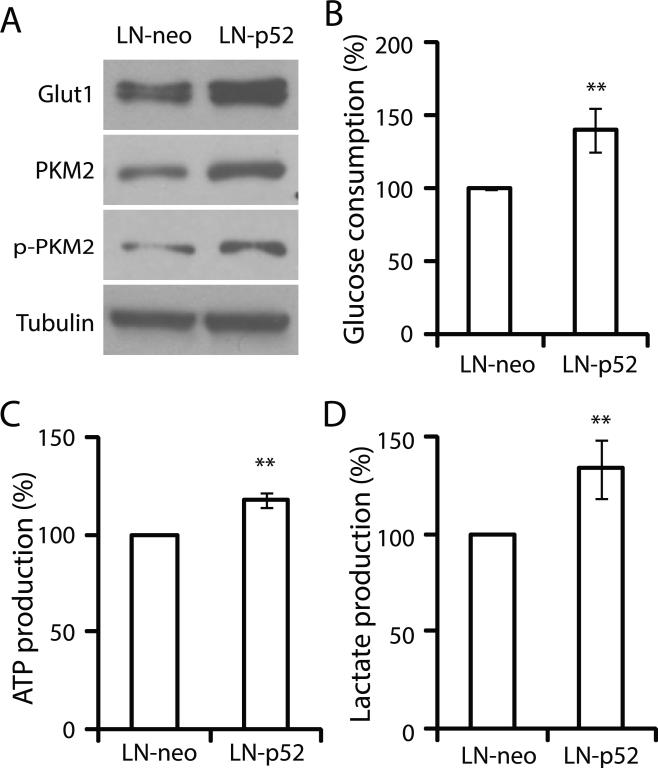
Gene expression array data suggests the alteration of glucose metabolic pathway by p52 in LNCaP cells. To further understand reprogrammed glucose metabolism by p52, we assayed several indicators within glucose metabolic pathways. Glucose uptake is the first step in glucose metabolism, which can be measured by the expression levels of glucose transporter, Glut1, and by glucose consumption assays. LNCaP-p52 cells expressed Glut1 at higher levels compared to the control (Fig 2A), consistent with higher glucose consumption in p52 overexpression cells compared to the control (Fig 2B). These data suggest an increased glucose uptake and consumption by the cells overexpression of p52. As a rate-limiting final step of glycolysis, catalysis of PEP to pyruvate by PKM2, a splice isoform of pyruvate kinase, plays important roles in cancer metabolism. Expression of PKM2 has a growth advantage for tumor cells in vivo (Christofk et al. 2008a; Christofk, et al. 2008b). Our gene expression array data indicated an increased expression of PKM2 mRNA by overexpression of p52. To confirm whether p52 enhances PKM2 protein expression, we analyzed the expression of PKM2 and phosphorylated PKM2. As shown in Fig 2A, the protein levels of both PKM2 and phosphorylated PKM2 were up-regulated in LNCaP-p52 cells compared to the control. Since cancer cells mainly generate energy from aerobic glycolysis of glucose, we measured ATP production as an indicator of aerobic glycolysis. The p52 overexpressing LNCaP cells are capable of generation of higher ATP production compared to the parental LNCaP cells (Fig 2C). In addition to ATP production, lactate production was also increased in LNCaP-p52 cells compared to the parental LNCaP cells (Fig 2D).
Figure 2. p52 increases LNCaP cells glucose uptake and aerobic glycolysis.
(A) Western blots for Glucose transporter 1 (Glut1), PKM2, phopho-PKM2. Tubulin was used as a loading control. Glucose consumption (B), ATP production (C), lactate production (D) of LNCaP-p52 cells compared to LNCaP-neo cells. Data are presented in mean±SD of at least three independent experiments. **; p<0.01.
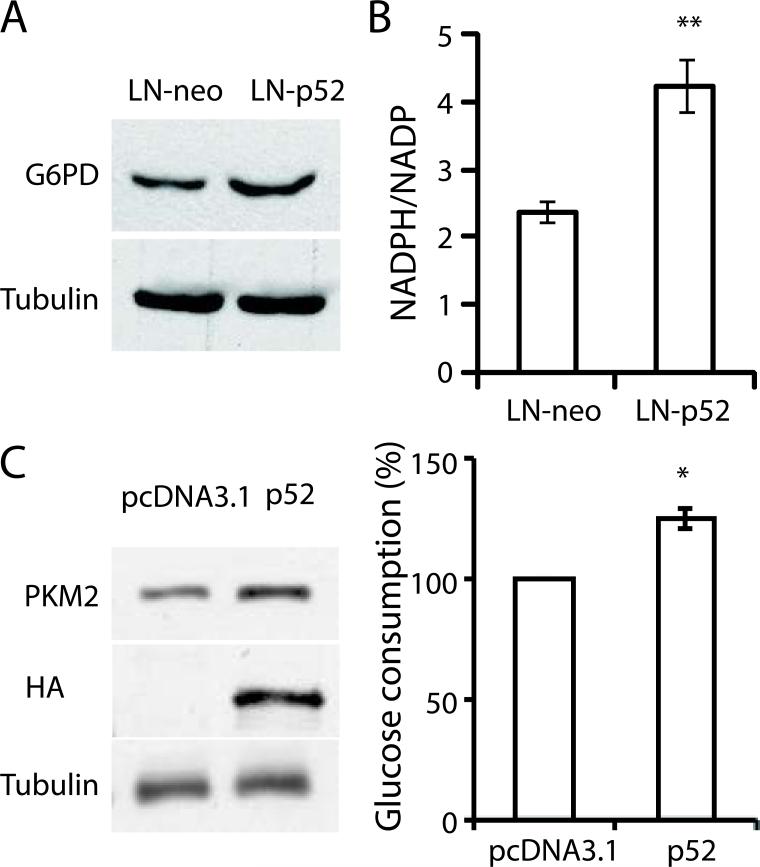
Pentose phosphate pathway is a branch shunt from glycolysis, which provides intermediate products for nucleoside synthesis, and more importantly provides reductants such as NADPH to maintain the redox balance of fast proliferating cells. The enzyme involved in the first step of PPP flux, G6PD, was up regulated in LNCaP-p52 cells (Fig 3A). In addition, the NADPH/NADP ratio was also much higher in LNCaP-p52 cells than control cells (Fig 3B), suggesting an overall enhanced PPP in LNCaP-p52 cells. To test if p52 mediated glucose metabolism is not LNCaP cell specific, CWR22Rv1 cells were transiently transfected with p52. As shown in Figure 3C, transient transfection of p52 increased PKM2 expression and glucose consumption in CWR22Rv1 cells (Fig 3C). Collectively, these data suggest that overexpression of p52 enhances glucose metabolism in LNCaP cells.
Figure 3.
(A) Western blots for G6PD of LNCaP-neo and LNCaP-p52 cells. Tubulin was used as a loading control. (B) NADPH/NADP ratio of LNCaP-p52 cells compared to LNCaP-neo cells. (C) Transient transfection of p52 enhances glucose metabolism in CWR22Rv1 cells. Immunoblots of HA tagged p52, PKM2 and tubulin in transient transfected LNCaP cells (left). Glucose consumption assay in transient vehicle and p52 transfected CWR22Rv1 cells (right). Data was presented as mean ±SD of three independent experiments. *, p < 0.05, **, p<0.01.
Overexpression of p52 increases cell sensitivity to glucose deprivation and 2-Deoxy-D-gluocose treatment
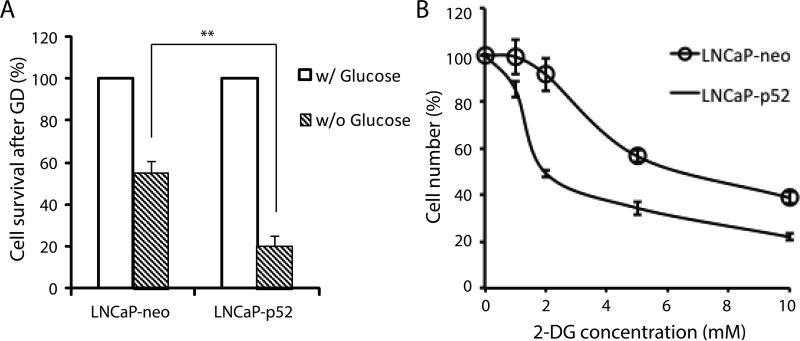
Since LNCaP-p52 cells have higher glucose uptake and rate of glucose metabolism, we hypothesized that p52 overexpressing LNCaP cells might be dependent on glucose for survival, and were more sensitive to glucose deprivation than parental LNCaP cells. To test that, we monitored cell growth in the absence of glucose. As shown in Fig 4A, more cells were dead in p52 overexpressing LNCaP cells compared to the parental LNCaP cells when they grew in media deprived from glucose. To further confirm this observation, we treated the cells with an analog of glucose, 2-deoxy-D-glucose (2-DG), an inhibitor of glucose metabolism. As shown in Fig 4B, p52 overexpressing LNCaP cells were more sensitive to 2-DG treatment than parental LNCaP cells. These results suggest that p52 overexpressing LNCaP cells are more sensitive to glucose deprivation than parental LNCaP cells.
Figure 4. LNCaP-p52 cells are more sensitive to glucose deprivation and 2-DG treatment.
(A) Cell growth comparison after three days of glucose deprivation. Cell numbers of LNCaP-neo and LNCaP-p52 cells in absence of glucose are normalized to the ones with glucose respectively. (B) Cell growth of LNCaP-p52 and LNCaP-neo at different concentrations of 2-DG. Data are presented in mean±SD in three independent experiments. **; p<0.01.
Targeting glucose metabolism by 2-DG re-sensitizes LNCaP-p52 cells to enzalutamide treatment
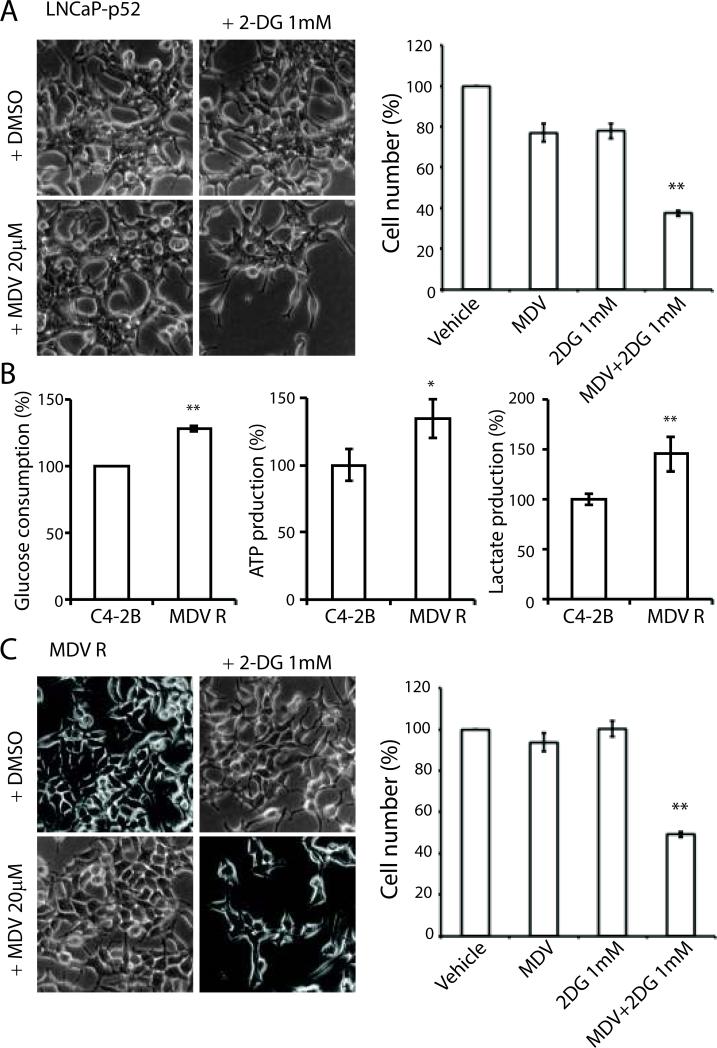
Our previous studies showed that LNCaP-p52 cells were resistant to enzalutamide treatment (Nadiminty et al. 2013). Since LNCaP-p52 cells exhibit enhanced glucose consumption and are more sensitive to glucose deprivation and 2-DG treatment, we combined 2-DG with enzalutamide to examine if the combination treatment could restore the cells sensitivity to enzalutamide. As shown in Fig 5A, a low dose of 2-DG (1mM) combined with 20 μM enzalutamide, dramatically reduced LNCaP-p52 cell number.
Figure 5. 2-DG re-sensitizes cells to enzalutamide treatment.
(A) Cell morphology of LNCaP-p52 cells was shown after two days treatment with 2-DG in combination with or without enzalutamide. Vehicle control and 2-DG treatment were shown in top panel. Enzalutamide and combination treatment with 1 mM 2-DG were shown in bottom panel. Right panel: Cell growth after two days of treatment. All numbers were normalized to vehicle group. Data were presented in mean±SD in at least three independent experiments. (B) Glucose consumption (left), ATP production (middle), and lactate production (right) in C4-2B MDVR enzalutamide-resistant cells compared to parental C4-2B enzalutamide-sensitive cells. (C) 2-DG re-sensitizes C4-2B MDVR enzalutamide-resistant cells' response to enzalutamide treatment. Cell numbers were normalized to vehicle group. Data were presented in mean±SD in three independent experiments; *, p < 0.05, **, p < 0.01.
We previously generated enzalutamide resistant C4-2B MDVR cells (Nadiminty et al. 2013). Similar to LNCaP-p52 cells, C4-2B MDVR cells have enhanced glucose consumption, ATP and lactate production (Fig 5B). Since 2-DG can greatly re-sensitize the response of LNCaP-p52 cells to enzalutamide, we tested whether 2-DG would have a similar effect to MDV-resistant cells. Combination treatment with 2-DG and enzalutamide significantly decreased cell number in C4-2B MDVR cells (Fig 5C,D). Collectively, these data suggest that targeting glucose metabolism can resensitize enzalutamide treatment in enzalutamide resistant prostate cancer cells.
Discussion
Our previous studies demonstrated that p52 overexpressing LNCaP cells grew significantly larger tumors in vivo, became castration-resistant through aberrant AR activation, and resistant to enzalutamide treatment. Cancer cells have reprogrammed metabolism to facilitate fast proliferation. In the present study, we analyzed glucose metabolism in p52 overexpressing prostate cancer cells, and found that glycolysis and pentose phosphate pathway are both up-regulated, indicating upregulation of glucose metabolism and ATP production by p52. Targeting glucose metabolism by 2-DG re-sensitizes the cell's response to enzalutamide of not only LNCaP-p52 cells, but also other enzalutamide-resistant cells, like C4-2B MDVR cells.
The Warburg effect is a widely observed feature in tumors with elevated glucose uptake, glucose consumption, and lactate production. Several enzymes including Glut1, PKM2, and LDHA are critically involved in glucose metabolism. We have shown that the levels of Glut1, PKM2, and LDHA gene expression were upregulated in p52-overexpressing LNCaP cells, which correlated with higher glucose metabolism. The upregulation of these rate-limiting glucose metabolic enzymes may play a critical role in the p52-induced Warburg effect, featuring elevated glucose consumption and higher lactate production. One typical feature of cancer cells is switching energy production from oxidative phosphorylation to glycolysis to generate additional precursors for macromolecular biosynthesis. Gene expression microarray data analysis showed an enhanced macromolecular biosynthesis in p52 overexpressing LNCaP cells compared to the parental LNCaP cells, suggesting an enhanced production of building blocks for these macromolecules derived from glucose metabolites. This is in consistence with the upregulated glucose metabolism pathways observed in p52 overexpressing LNCaP cells.
Due to an altered glucose metabolism that cancer cells usually have, targeting glucose metabolism to inhibit cancer progression is an attractive approach for cancer therapy. 2-DG, a glucose analog, is the most widely invested drug in experimental and clinical oncology (Dwarakanath and Jain 2009). 2-DG is competitively taken up by cells through the same transporter as glucose and transformed by hexokinase to 2-DG-6-phosphate which cannot be metabolized further, therefore decreasing the glucose flux to glycolysis and pentose phosphate pathway. However, as a single therapeutic agent, the clinical trial of 2-DG has been discontinued due to persistent side effects such as diaphoresis and disturbance of the CNS (Dwarakanath, et al. 2009; Gupta, et al. 2009). A potential promising approach is to combine 2-DG therapy with radiation or chemotherapy drugs (Gupta et al. 2009). Enzalutamide, a newly approved anti-androgen drug, can effectively inhibit CRPC cell growth in vivo (Tran et al. 2009). Despite its successes and continuing wide-spread use, development of resistance is inevitable. Our previous studies demonstrated that p52 overexpressing LNCaP cells were resistant to enzalutamide (Nadiminty et al. 2013). In the present study, we combined 2-DG and enzalutamide and found that a low dose of 2-DG resensitized the response of p52-overexpressing LNCaP cells to enzalutamide treatment. The combination of these two drugs has profound synergistic effects on inhibiting cell growth. We further validated the synergistic effects of combination treatment of 2-DG with enzalutamide in another enzalutamide-resistant C4-2B-MDVR cells.
In summary, these results suggest that p52 modulates glucose metabolism, enhances glucose flux to glycolysis and pentose phosphate pathway, thus facilitating fast proliferation of the cells. Targeting glucose metabolism by deprivation of glucose or using glucose analog such as 2-DG inhibits cell growth. We found that combination treatment of 2-DG with enzalutamide could resensitize enzalutamide-resistant prostate cancer cells to enzalutamide treatment, suggesting a potential therapeutic approach for CRPC patients by co-targeting glucose metabolism and androgen receptor pathways.
Acknowledgments
Grant Support: This work is supported in part by grants NIH/NCI CA140468, CA168601, CA179970, and US Department of Veterans Affairs, Office of Research and Development VA Merits I01 BX000526 (A.C. Gao), and by resources from the VA Northern California Health Care System, Sacramento, California.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed
Reference
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- Betts JC, Nabel GJ. Differential regulation of NF-kappaB2(p100) processing and control by amino-terminal sequences. Mol Cell Biol. 1996;16:6363–6371. doi: 10.1128/mcb.16.11.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzai M, Bauer DE, Jones RG, Deberardinis RJ, Hatzivassiliou G, Elstrom RL, Thompson CB. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005;24:4165–4173. doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008a;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008b;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- Connelly L, Robinson-Benion C, Chont M, Saint-Jean L, Li H, Polosukhin VV, Blackwell TS, Yull FE. A transgenic model reveals important roles for the NF-kappa B alternative pathway (p100/p52) in mammary development and links to tumorigenesis. J Biol Chem. 2007;282:10028–10035. doi: 10.1074/jbc.M611300200. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwarakanath B, Jain V. Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol. 2009;5:581–585. doi: 10.2217/fon.09.44. [DOI] [PubMed] [Google Scholar]
- Dwarakanath BS, Singh D, Banerji AK, Sarin R, Venkataramana NK, Jalali R, Vishwanath PN, Mohanti BK, Tripathi RP, Kalia VK, et al. Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther. 2009;5(Suppl 1):S21–26. doi: 10.4103/0973-1482.55136. [DOI] [PubMed] [Google Scholar]
- Fan CM, Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature. 1991;354:395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- Gupta S, Farooque A, Adhikari JS, Singh S, Dwarakanath BS. Enhancement of radiation and chemotherapeutic drug 305 responses by 2-deoxy-D-glucose in animal tumors. J Cancer Res Ther. 2009;5(Suppl 1):S16–20. doi: 10.4103/0973-1482.55135. [DOI] [PubMed] [Google Scholar]
- Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Carrasco D, Claudio E, Ryseck RP, Bravo R. Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-kappaB2. J Exp Med. 1997;186:999–1014. doi: 10.1084/jem.186.7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Lessard L, Karakiewicz PI, Bellon-Gagnon P, Alam-Fahmy M, Ismail HA, Mes-Masson AM, Saad F. Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res. 2006;12:5741–5745. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- Lin X, Mu Y, Cunningham ET, Jr., Marcu KB, Geleziunas R, Greene WC. Molecular determinants of NF-kappaB-inducing kinase action. Mol Cell Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadiminty N, Chun JY, Lou W, Lin X, Gao AC. NF-kappaB2/p52 enhances androgen-independent growth of human LNCaP cells via protection from apoptotic cell death and cell cycle arrest induced by androgen-deprivation. Prostate. 2008;68:1725–1733. doi: 10.1002/pros.20839. [DOI] [PubMed] [Google Scholar]
- Nadiminty N, Dutt S, Tepper C, Gao AC. Microarray analysis reveals potential target genes of NF-kappaB2/p52 in LNCaP prostate cancer cells. Prostate. 2010a;70:276–287. doi: 10.1002/pros.21062. [DOI] [PubMed] [Google Scholar]
- Nadiminty N, Lou W, Lee SO, Lin X, Trump DL, Gao AC. Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci U S A. 2006;103:7264–7269. doi: 10.1073/pnas.0509808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung HJ, Evans CP, Zhou Q, Gao AC. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 2010b;70:3309–3319. doi: 10.1158/0008-5472.CAN-09-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, Gao AC. NF-kappaB2/p52 Induces Resistance to Enzalutamide in Prostate Cancer: Role of Androgen Receptor and Its Variants. Mol Cancer Ther. 2013;12:1629–1637. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, Wang Y, Jing Y, Yang H, Chen R, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher BA, Linehan WM, Helman LJ. Targeting cancer metabolism. Clin Cancer Res. 2012;18:5537–5545. doi: 10.1158/1078-0432.CCR-12-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC. Retroviral oncoprotein Tax induces processing of NF-kappaB2/p100 in T cells: evidence for the involvement of IKKalpha. EMBO J. 2001a;20:6805–6815. doi: 10.1093/emboj/20.23.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem. 2004;279:30099–30105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001b;7:401–409. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]