Abstract
Generalized linear mixed models were used to examine longitudinal trajectories of everyday functional limitations by diagnostic stability/progression. Older adults (N=384) were followed an average 3.6 years; participants were grouped by diagnosis at study baseline and last follow-up (normal cognition, Mild Cognitive Impairment (MCI) or dementia at each time point). At study baseline there were clear group differences; most notably, among participants initially characterized as cognitively normal, those who developed MCI or dementia over follow-up already demonstrated greater functional impairment compared to those who remained cognitively normal. Change in functional impairment progressed slowly in the early disease groups, but showed an accelerated worsening in those converting to dementia.
Keywords: Everyday Function, Activities of daily living, Mild Cognitive Impairment, Dementia, Cognition, Aging
Previous studies have shown considerable heterogeneity in trajectories of cognitive change in late life, with variable rates of decline, as well as stable function in many cases (Wilson et al., 2012). A substantial part of this variability reflects the development of neurodegenerative diseases of aging, particularly Alzheimer’s disease (AD) and its prodrome, Mild Cognitive Impairment (MCI). Comparatively little research has been aimed at characterizing decline in everyday function. Cognition and everyday function are certainly related, but their trajectories may also differ in important ways. Further, it is impairments in everyday function that ultimately affect individual autonomy and independence, substantially reduce quality of life, increase caregiver burden, and contribute to disease-related financial costs (Andersen, Wittrup-Jensen, Lolk, Andersen, & Kragh-Sorensen, 2004; Gaugler et al., 2012; Razani et al., 2007). Dementia is clearly associated with loss in functional abilities. Because MCI can reflect a transition state between normal cognition and dementia, it is likely characterized by a continuum of functional loss falling between the subtle decrements associated with normal aging and the frank disability associated with dementia (Wadley, Crowe, Marsiske et al., 2007). A number of studies have now demonstrated that MCI is often associated with mild functional impairments (Farias et al., 2006; Jefferson et al., 2008), and this is further reflected in the new diagnostic criteria for MCI (Albert et al., 2011). Additionally, a greater degree of functional impairment in MCI is associated with faster subsequent cognitive decline and conversion to dementia (Farias, Mungas, Reed, Harvey, & Decarli, 2009; Farias, Mungas, Hinton, & Haan, 2011; Purser, Fillenbaum, Pieper, & Wallace, 2005). What is not currently known is more precisely when functional limitations emerge and how rate of change in everyday function may differ throughout the disease process (e.g. normal cognition to MCI to dementia), and around the transitions from each of these states.
The aim of the present study was to characterize trajectories of change in everyday function according to diagnostic in/stability over the study follow-up (i.e. whether diagnosis remained the same or progressed to the next ‘stage’). One previous impediment to research examining functional trajectories has been the dearth of functional instruments sensitive to a wide range of functional impairments, particularly to early and subtle functional impairment characteristic of MCI and possibly even normal aging. The present study utilizes the Everyday Cognition (ECog) scale which was specifically designed to be sensitive to mild functional changes that predate loss of independence (Farias et al., 2006; Farias et al., 2008). The basic hypotheses were that 1), more advanced disease stages would be associated with greater functional impairment at study baseline and 2) participants who had a change in diagnostic status (i.e. Normal to MCI or MCI to dementia) would exhibit a faster rate of increase in functional impairments compared to those who remained in the same diagnostic category over the follow-up period.
Methods
Participants
Participants were part of a longitudinal research cohort at the University of California, Davis Alzheimer’s Disease Center. All participants underwent a multidisciplinary diagnostic assessment that included a physical and neurological exam, clinical neuropsychological testing, imaging, and lab work. Dementia was diagnosed using DSM-III R (American Psychiatric Association, 1987) criteria for dementia, modified such that dementia could be diagnosed in the absence of memory impairment if there was significant impairment in two or more other cognitive domains. MCI was diagnosed according to standard clinical criteria and subtyped according to current Alzheimer’s Disease Centers Uniform Data Set guidelines (Morris et al., 2006). Individuals with MCI could either have amnestic MCI (single or multiple domain) or non-amnestic MCI (single or multiple domain). Individuals with MCI could not have impairments in basic ADLs or be dependent on others in any instrumental ADLs. For the purposes of the clinical diagnosis, functional change was assessed using a variety of standardized instruments and clinical interview. Clinical diagnoses were made without knowledge of the primary functional outcome measure in the present study (the ECog). All participants signed informed consent, and all human subject involvement was overseen by institutional review boards at University of California at Davis, the Veterans Administration Northern California Health Care System and San Joaquin General Hospital in Stockton, California.
Measurement of Everyday Function
The ECog is an informant-rated measure of cognitively-relevant everyday abilities comprised of 39 items, covering six domains: Everyday Memory, Everyday Language, Everyday Visuospatial Abilities, and Everyday Planning, Everyday Organization, and Everyday Divided Attention (for example items see – Farias, Mungas, Harvey, et al., 2011; Farias et al., 2008; Farias, 2013). For the present study, a total score including all completed items was used. For each item, informants compare the participant’s current level of everyday functioning with how he or she functioned 10 years earlier. In this way, individuals serve as their own control. Ratings are made on a four-point scale: 1 = better or no change compared to 10 years earlier, 2 = questionable/occasionally worse, 3 = consistently a little worse, 4 = consistently much worse. A total score is calculated by summing all of the ratings and dividing by the number of items completed, which allows for some missing/non-answered items (at least half of the items need to be completed to calculate a score). The ECog has been shown to have excellent psychometric properties including good test-retest reliability (r = .82, p<.001), as well as evidence of various aspects of validity including content, construction, convergent and divergent, and external validity (Farias et al., 2008). It is sensitive to many of the functional changes associated with MCI as well as dementia (Farias et al., 2006; Farias et al., 2008).
Data Analysis
Demographics and ECog scores were characterized descriptively overall and by diagnostic group. In further analysis, because the ECog scores were not normally distributed but instead showed a fairly flat to a U shaped distribution across the entire potential range, (depending somewhat on the participant subsample) we rescaled the ECog scores so that the outcome ranged from 0 (no impairment reported, or a mean score of 1 on the original scale) to 1 (worst impairment possible on all items, or a mean score of 4 on the original scale). We refer to this as the ECog impairment index, the proportion of impairment reported, with 0 corresponding to 0% impaired and 1 corresponding to 100% of potential impairment. Since the ECog scores had both floor and ceiling effects in this sample, we assumed a sigmoidal shape for the mean overall path of the predicted impairment index, denoted p, as it progressed from stable healthy everyday cognition in all items (impairment index of 0) to much worse function in all items (impairment index of 1). We modeled longitudinal trajectories of this sigmoidal shape by generalized linear mixed models with a logistic link. To account for variation from the predicted levels, we also assumed that individual trajectories had a person-specific random component and that the within-person variation from the person-specific curve was proportional to p(1−p), so that the greatest variation occurred in people with mid-range index scores and the least degree of variation in those with either completely normal functional capacity (p=0) or maximal functional impairment (p=1). This model is linear on the logit scale, meaning that ln(p/(1−p)) (log “odds”) has a linear association with the predictors. Therefore, positive coefficients for predictors indicate an increase in the log “odds” which implies an increase in the impairment proportion p. Results are presented both in terms of estimated coefficients as well as “Impairment Odds Ratios” to quantify changes in the impairment “odds” between groups. The model included main effects for predictors, including age and diagnosis. Age was centered at 75. We subdivided the normal and MCI groups into those who had stable diagnoses and those who progressed. The diagnostic predictors were defined as indicator variables to test specifically for differences between normals who converted to MCI compared to those who remained normal, whether normals who converted to dementia differed from those who converted to MCI, and whether MCIs who converted to dementia differed from those whose diagnosis stayed MCI throughout. We characterized change by including a term for the calculated time in years since baseline, and we examined effects of predictors on rate of change by including interaction terms. Model assumptions (logistic shape of curve, logit-linear effects of baseline age and rate of change, variance assumptions) were assessed by residual plots and diagnostics. All models were analyzed using R, version 2.15.1.
Results
Sample Characteristics
Table 1 shows the demographic and follow-up characteristics by baseline. Of the entire sample, 50% were Caucasian, 26% African American, 20% were Hispanic; the remainder were of another racial/ethnic group. According to baseline diagnosis alone, mean age was slightly higher for those with dementia as compared to normals or MCI, (ps = .001). Those normal at baseline were more likely females compared to the MCI group, p < .01, and the dementia group, p = .05. Education was significantly lower in those with dementia at baseline compared to normal, p = .03, or MCI, p < .01. Numbers of annual evaluations by diagnostic change are presented in Table 1; for the sample as a whole, the average number of evaluations was 3.6, (SD = 1.4, range = 2 to 8). Average length of follow-up did not differ significantly by diagnosis, p= .21 or by diagnostic change, p = .64. In terms of diagnostic change over the course of all follow-up visits, 164 participants remained normal over all follow-up visits, 18 normals converted to MCI, and 7 normals converted to dementia over follow-up. Of those with MCI at baseline, 52 remained MCI over the follow-up visits, and 65 converted to dementia. Ten individuals who back-converted from MCI to normal were excluded from the primary analysis; the impact of exclusion was assessed in secondary analysis.
Table 1.
Clinical and Demographic Characteristics and Length of Follow-up
| Variable | Diagnostic Groups
|
|||||
|---|---|---|---|---|---|---|
| Normal-Normal n = 164 | Normal-MCI n = 18 | Normal-Dementia n =7 | MCI-MCI n =52 | MCI-Dementia n =65 | Dementia- Dementia n = 78 | |
| Age (Years) | ||||||
| M (SD) | 73.37 (6.39) | 79.28 (7.39) | 79.71 (8.67) | 74.04 (7.73) | 75.66 (8.04) | 78.44 (7.83) |
| Education (years) | ||||||
| M (SD) | 13.27 (3.90) | 12.5 (3.26) | 13.57 (2.82) | 13.96 (3.94) | 14.77 (3.78) | 12.09 (3.89) |
| ECog at baseline | ||||||
| M (SD) | 1.37 (0.50) | 1.66 (0.68) | 1.92 (0.55) | 1.70 (0.66) | 2.09 (0.60) | 2.89 (0.73) |
| Gender | ||||||
| N (% female) | 70 | 61 | 86 | 52 | 45 | 58 |
| Number of annual evaluations | ||||||
| 2 | 38 | 6 | 1 | 24 | 13 | 27 |
| 3 | 42 | 4 | 1 | 12 | 15 | 17 |
| 4 | 31 | 3 | 2 | 6 | 11 | 15 |
| ≥5 | 53 | 5 | 3 | 10 | 26 | 19 |
Note. MCI = Mild Cognitive Impairment; ECog = the Everyday Cognition scale. The ECog score is presented as an average score (range = 1–4) with high scores indicating greater impairment.
Differences in Everyday Function by Diagnostic Stability/Progression
First, we examined whether degree of limitations in everyday function at study baseline differed by diagnostic group. In all cases except one, there were pronounced baseline differences in everyday function across the group comparisons (see Figure 1). Specifically, the stable normal group showed a trend toward better baseline everyday function than the normals who converted to MCI over the follow-up period, p = .12. All other comparisons were statistically significant. From Table 1 and the Figure it is also clear that the stable normal group had better baseline everyday function than those normals who eventually progressed to dementia. The stable normal group also showed better baseline everyday function than the stable MCI group; the normals who converted to MCI by follow-up showed better baseline everyday function than the normals who converted to dementia by follow-up; the stable MCI group showed better baseline everyday function than the MCI group who converted to dementia over follow-up; and the stable MCI group showed better baseline everyday function than the dementia group. Overall, individuals who were further along in the disease course (both in terms of baseline diagnosis and whether they progressed to the subsequent diagnostic category over follow-up) showed increasingly greater functional impairment at study baseline.
Figure 1.
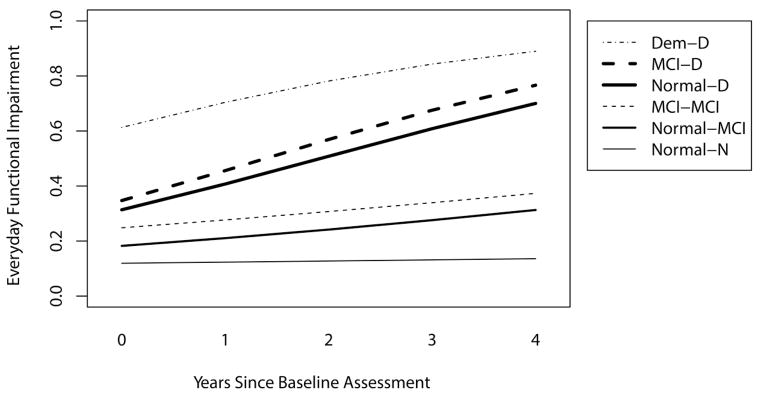
Plot of longitudinal ECog trajectories for each diagnostic group. Increasing scores indicate greater functional impairment. Group abbreviations are as follows: Dem-D = demented at first and at last evaluation, MCI-D = MCI at first evaluation, Demented at last, MCI-MCI = MCI at first evaluation and throughout follow-up, Normal-MCI = Normal at first visit, MCI at last follow-up visit, Normal-N = Normal at first and throughout follow-up.
Next, rate of change over time in everyday function by diagnostic group was examined. Rate of change in everyday function (worsening function) was significantly more rapid in those groups that converted to dementia over the follow-up period (either from normal or MCI to dementia) as compared to their counterparts who either remained normal or MCI, or who converted from normal to MCI (see Figure). The other diagnostic groups (stable normals, normal to MCI converters, and the stable MCI group) were all changing slightly (worsening function) but at a level that did not research statistical significance in any of the comparisons. None of these patterns were modified significantly by age at baseline.
Discussion
Functional abilities are key to personal autonomy and quality of life, yet the literature examining how everyday function changes in old age is very limited. The present study examined functional ability level in reference to cognitive status as reflected by whether diagnostic status changed over the course of study follow-up (Arnaiz et al., 2004). When comparing degree of functional impairment at study baseline across the diagnostic in/stability groups, we found statistically significant differences across all but one of the group comparisons such that, with successive stages of disease, greater levels of functional impairment were present. For example, participants who remained in the MCI category throughout follow-up demonstrated less functional impairment at study baseline than those with MCI who converted to dementia over follow-up, and not surprisingly, the dementia group had the greatest degree of functional impairment at study baseline of all the groups. Somewhat less expected, we found that among those initially considered to be cognitively normal at study baseline, those remaining cognitively normal had better everyday function than the normal elderly who converted to dementia at follow-up, and there was a trend for the stable normal group to have better baseline function than the normal elderly who developed MCI over follow-up. As far as we are aware, these findings are the first to suggest that very subtle changes in everyday function, as measured by the ECog, are detectable in individuals who are still considered cognitively normal but ultimately develop MCI or dementia within a few years.
Rate of change in everyday function showed a somewhat different pattern across diagnostic in/stability group comparisons. Here we observed a steeper rate of increasing functional impairment in individuals who experienced a transition to dementia over the course of follow-up as compared to all other groups. Specifically, those individuals who transitioned from either normal cognition to dementia or from MCI to dementia experienced an accelerated rate of increase in functional impairment compared to participants who remained normal or MCI, or transitioned from normal to MCI. Interestingly, we could not detect a significant difference in rate of change between the normal elderly who converted to MCI versus those who remained cognitively normal throughout the study. Those who transitioned from normal to MCI, as well as the stable MCI group, showed slightly worsening everyday function over time while the stable normal functional trajectory was quite flat. Overall, the pattern of findings suggests that functional deficits develop very gradually over time, starting when someone is still considered cognitively normal, and continuing at a similar rate throughout much of the early and intermediate MCI stage. Functional decline then begins to accelerate sometime before but likely in close temporal proximity to the time of conversion to dementia. Whether rate of change in everyday function continues at a similar rate once someone develops a full dementia syndrome is not entirely clear given the ceiling effects of the ECog in the dementia group. Examination of the trajectories drawn in the Figure indicates that the end point of the trajectory for those with MCI that convert to dementia is at about the same level of functional impairment as the starting point of the trajectory for dementia – suggesting that the latter is a continuation of the former trajectory.
The present results showing greater functional impairment at study baseline in MCI participants who eventually transition to dementia, relative to those remaining MCI, are consistent with other research demonstrating that greater functional impairment in MCI predicts faster subsequent disease progression and conversion to dementia (Farias, S. T. et al., 2009; Peres et al., 2006; Tabert et al., 2002). Also similar to the current results, one other study showed a steeper rate of functional decline in an MCI group who eventually converted to dementia compared to a stable MCI group (Okonkwo et al., 2010) although functional trajectories across the broader continuum of disease were not examined in that previous study. Contrasting the present results with studies of cognitive trajectories provides indication of some interesting similarities and differences. It has been demonstrated that cognitive decline in AD begins well before the development of dementia (Grober et al., 2008; Hall, Lipton, Sliwinski, & Stewart, 2000; Small & Backman, 2007; Wilson et al., 2012). Similarly, in the present study functional decline appears to begin when older adults are judged to be cognitively normal. Thus, both cognitive and functional changes commence well before a dementia diagnosis. Whether cognitive and functional impairment begin sequentially or in tandem is not really clear at this point, although some theoretical models would place the development of cognitive impairment earlier in the temporal course of AD than the development of functional impairment (Jack et al., 2010). When within the disease course cognitive versus functional impairment shows accelerated decline may certainly differ. Recent work suggests that cognitive decline may accelerate well before a dementia diagnosis (e.g. up to 5–7 years prior to dementia; Wilson et al., 2012), whereas the current study suggests that functional decline probably accelerates in closer proximity to dementia. While studies directly comparing rates of decline in cognition and everyday function across the disease spectrum will be needed to address this question, it seems plausible that cognitive and functional trajectories may take related but slightly different courses. We do anticipate that longitudinal declines in cognition and everyday function as measured by the ECog will be strongly correlated and this is an avenue of future study.
The acceleration of function impairment surrounding the time of the diagnostic change from MCI to dementia is not entirely surprising. A major threshold separating the syndrome of MCI and dementia is substantially worse everyday function in the latter (e.g. actual loss of independence in major spheres of life being associated with dementia, as compared to more subtle functional difficulties in MCI). However, it is important to bear in mind that ECog data was not used in the diagnostic process and further, in many cases level of functional impairment is not the sole criteria differentiating MCI from dementia (e.g. MCI can be diagnosed in the context of only one cognitive impairment, whereas multiple cognitive impairments are required for dementia).
Different rates of functional decline may ultimately reflect differences in underlying pathological processes. More rapid functional decline in those who worsen clinically and specifically convert from MCI to dementia, in many cases may be the consequence of accumulating brain pathology related to AD. Slower functional decline and/or more stable functional trajectories could reflect an earlier disease stage but could also be associated with a lack of true neurodegenerative disease, or a more benign pathological process such as less aggressive cerebrovascular disease.
There are a number of strengths to this study. It was carried out in a cognitively and ethnically diverse sample. There was uniform ascertainment of diagnosis and other characteristics, we used a well-validated functional measure that is sensitive to early and subtle functional changes, and the length of follow-up was substantial. There are also limitations. The sample, although diverse, was not population-based and as such there may be limits to generalizability. While the overall sample size was large, the number of individuals converting from normal cognition to MCI was rather small and, as a result, characterizations of related trajectories should be considered preliminary. Additionally, an important future avenue of inquiry will be to evaluate other factors, outside of clinical diagnosis, that influence rate of functional change. In the present study we did not find that age significantly contributed to rate of functional progression. However, there are many other possible factors that could substantially alter functional trajectories. For example, based on previous work we would expect that the degree and type of cognitive impairment present would likely influence rates of functional decline (Cahn-Weiner et al., 2007; Farias, S. T. et al., 2009; Rozzini et al., 2007). There has also been tremendous interest in how indicators of cognitive reserve impact risk of neurodegenerative disease and rates of disease progression (Stern, 2006, 2009; Stern, Albert, Tang, & Tsai, 1999). To date, very little work has examined how indicators of cognitive reserve may modify the expression of disease via everyday functional trajectories.
In summary, this is the first study to characterize, in detail, longitudinal trajectories of everyday function in older adults across a rather wide spectrum of cognitive function and disease severity. Findings indicate that early in the disease, functional changes emerge gradually, starting even when individuals are considered cognitively normal and continuing at a similar rate throughout early and mid-stage MCI. Rate of change in everyday function then accelerates as the disease progresses, particularly around the time of transition from MCI to dementia, and likely continues at this more rapid rate of decline throughout mild to moderate dementia. An implication of this study is that functional decline over time is an important indicator of underlying disease that might be useful for monitoring disease progression.
Table 2.
Comparison of Baseline and Change in Everyday Function by Diagnostic Status over Follow-up
| Variable | Estimate (SE) | Impairment Odds Ratio | p | |
|---|---|---|---|---|
| Stable Normal | Average baseline level | −2.00 (0.11) | 0.13 | < 0.001 |
| Average annual change | 0.04(0.03) | 1.04 | 0.13 | |
| Normal to MCI vs | Average baseline level | 0.50 (0.32) | 1.65 | 0.12 |
| Stable Normal | Average annual change | 0.10 (0.09) | 1.10 | 0.25 |
| Normal to dementia vs | Average baseline level | 0.72 (0.34) | 2.05 | 0.03 |
| Normal to MCI | Average annual change | 0.28 (0.10) | 1.32 | 0.008 |
| Stable MCI vs | Average baseline level | 0.89 (0.19) | 2.43 | <0.001 |
| Stable Normal | Average annual change | 0.09 (0.06) | 1.09 | 0.15 |
| MCI to dementia vs | Average baseline level | 0.48 (0.19) | 1.62 | 0.01 |
| Stable MCI | Average annual change | 0.32 (0.07) | 1.38 | 0.001 |
| Dementia vs | Average baseline level | 1.57 (0.21) | 4.81 | <0.001 |
| Stable MCI | Average annual change | 0.26 (0.10) | 1.30 | 0.001 |
Note. Estimated coefficients of Marginal Logistic Regression model adjusted for age. Impairment Odds Ratio for baseline level is the ratio of p/(p−1) in a target group divided by p/(p−1) in the reference group; for annual change it is the ratio of p/(p−1) divided by p/(p−1) one year earlier. It references the probability of a higher impairment index score associated with the target group or one year of time. MCI = Mild Cognitive Impairment.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington DC: American Psychiatric Association; 1987. Rev. [Google Scholar]
- Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sorensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Quality and Life Outcomes. 2004;2:52. doi: 10.1186/1477-7525-2-521477-7525-2-52. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaiz E, Almkvist O, Ivnik RJ, Tangalos EG, Wahlund LO, Winblad B, Petersen RC. Mild cognitive impairment: a cross-national comparison. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:1275–1280. doi: 10.1136/jnnp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Farias ST, Julian L, Harvey DJ, Kramer JH, Reed BR, Chui H. Cognitive and neuroimaging predictors of instrumental activities of daily living. Journal of the International Neuropsychological Society. 2007;13:747–757. doi: 10.1017/S1355617707070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias S, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Archives of Neurology. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Harvey D, Simmons A, Reed BR, Decarli C. The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales. Alzheimer’s & Dementia. 2011;2011:593–601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Hinton L, Haan M. Demographic, neuropsychological, and functional predictors of rate of longitudinal cognitive decline in Hispanic older adults. American Journal of Geriatric Psychiatry. 2011;19(5):440–450. doi: 10.1097/JGP.0b013e3181e9b9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, Decarli C. MCI is associated with deficits in everyday functioning. Alzheimer’s Disease & Associated Disorders. 2006;20:217–223. doi: 10.1097/01.wad.0000213849.51495.d900002093-200610000-00008. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Cahn-Weiner DA, Harvey DJ, Reed BR, Mungas D, Kramer JH, Chui H. Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. Clinical Neuropsychologist. 2009;23:446–461. doi: 10.1080/13854040802360558. 903059828 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Cahn-Weiner DA, Jagust W, Baynes K, DeCarli C. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Quitania Park L, Harvey D, Simon C, Reed BR, Carmichael O, Mungas D. Everyday cognition in older adults: Associations wiht neuropsychological performance and structural brain imaging. Journal of the International Neuropsychological Society. 2013 doi: 10.1017/S1355617712001609. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler JE, Hovater M, Roth DL, Johnston JA, Kane RL, Sarsour K. Analysis of Cognitive, Functional, Health Service Use, and Cost Trajectories Prior to and Following Memory Loss. Journals of Gerontology. Series B: Psychological Sciences and Social Sciences. 2012 doi: 10.1093/geronb/gbs078. [DOI] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Statistics in Medicine. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Byerly LK, Vanderhill S, Lambe S, Wong S, Ozonoff A, Karlawish JH. Characterization of activities of daily living in individuals with mild cognitive impairment. American Journal of Geriatric Psychiatry. 2008;16:375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Pa J, Boxer AL, Kramer JH, Freeman K, Yaffe K. Baseline predictors of clinical progression among patients with dysexecutive mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2010;30:344–351. doi: 10.1159/000318836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings JL, DeCari C, Ferris S, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from the Alzheimer Disease Centers. Alzheimer’s Disease & Associated Disorders. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Okonkwo OC, Alosco ML, Jerskey BA, Sweet LH, Ott BR, Tremont G Alzheimer’s Disease Neuroimaging Initiative. Cerebral atrophy, apolipoprotein E varepsilon4, and rate of decline in everyday function among patients with amnestic mild cognitive impairment. Alzheimers Dement. 2010;6(5):404–411. doi: 10.1016/j.jalz.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology. 2006;67:461–466. doi: 10.1212/01.wnl.0000228228.70065.f1. 67/3/461 [pii] [DOI] [PubMed] [Google Scholar]
- Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. Journal of the American Geriatrics Society. 2005;53:1966–1972. doi: 10.1111/j.1532-5415.2005.53566.x. JGS53566 [pii] [DOI] [PubMed] [Google Scholar]
- Razani J, Kakos B, Orieta-Barbalace C, Wong JT, Casas R, Lu P, Josephson K. Predicting caregiver burden from daily functional abilities of patients with mild dementia. Journal of the American Geriatrics Society. 2007;55:1415–1420. doi: 10.1111/j.1532-5415.2007.01307.x. JGS1307 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M, Padovani A. Conversion of amnestic Mild Cognitive Impairment to dementia of Alzheimer type is independent to memory deterioration. International Journal of Geriatric Psychiatry. 2007;22:1217–1222. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: a growth mixture modeling analysis. Cortex. 2007;43:826–834. doi: 10.1016/s0010-9452(08)70682-8. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer’s Disease & Associated Disorders. 2006;20:112–117. doi: 10.1097/01.wad.0000213815.20177.1900002093-200604000-00006. [pii] [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. S0028-3932(09)00123-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, Devanand DP. Functional deficits in patients with mild cognitive impairment. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Jack CR., Jr Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Archives of Neurology. 2007;64:1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The Natural History of Cognitive Decline in Alzheimer’s Disease. Psychology and Aging. 2012 doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]


