Abstract
Ascorbate, glutathione and α-tocopherol are the major low molecular weight antioxidants in the brain. The simultaneous changes in these compounds during normal development, and under a pro-oxidant condition are poorly understood. Ascorbate, glutathione and α-tocopherol concentrations in the olfactory bulb, cerebral cortex, hippocampus, striatum, hypothalamus, midbrain, cerebellum, pons and medulla oblongata were determined in postnatal day (P) 7, P14 and P60 male rats. A separate group of P14 and P60 rats were subjected to acute hypoglycemia, a pro-oxidant condition, prior to tissue collection. The concentrations of all three antioxidants were 100-600% higher in the brain regions at P7 and P14, relative to P60. The neuron-rich anterior brain regions (cerebral cortex and hippocampus) had higher concentrations of all three antioxidants than the myelin-rich posterior regions (pons and medulla oblongata) at P14 and P60. Hypoglycemia had a differential effect on the antioxidants. Glutathione was decreased at both P14 and P60. However, the decrease was localized at P14 and global at P60. Hypoglycemia had no effect on ascorbate and α-tocopherol at either age. Higher antioxidant concentrations in the developing brain may reflect the risk of oxidant stress during the early postnatal period and explain the relative resistance to oxidant-mediated injury at this age.
Keywords: α-tocopherol, ascorbate, brain, glutathione, hypoglycemia, rat
Introduction
Oxidants play a major role in acute brain injury and neurodegenerative disorders [1-3]. A system of antioxidant enzymes and low molecular compounds protects the brain against oxidant-mediated injury. Ascorbate, glutathione (GSH) and α-tocopherol are the major low molecular weight antioxidants in the brain. Ascorbate and GSH are water-soluble, while α-tocopherol is lipid-soluble [1]. Ascorbate and α-tocopherol are transported from plasma across the blood brain barrier via specialized transport systems, while GSH is locally synthesized from its constituents, glutamate, glycine and cysteine [4, 5]. The three compounds differ in their cellular localization. Ascorbate is primarily localized in neurons and GSH in glia [6]. A primary localization site for α-tocopherol has yet to be determined, although its concentration is higher in the gray matter than the white matter [7]. Supplementation studies confirm that all three compounds independently protect against oxidant-mediated injury [8-10]. Additionally, they interact synergistically and help resynthesize those depleted during oxidant exposure [1, 6, 11]. The three compounds also have non-antioxidant properties and influence neuronal and glial maturation and function [6, 12-14].
Ascorbate, GSH and α-tocopherol concentrations vary among the brain regions in adult rodents, with the concentrations being higher in the neuron-rich anterior regions (cerebral cortex and hippocampus) than the myelin-rich posterior regions (pons and medulla oblongata) [7, 15-18]. Whether similar interregional variations exist during development is not well understood. In humans and rats, peak brain development occurs postnatally with the posterior brain regions maturing earlier than the anterior regions [19]. Energy-demanding processes, such as synaptogenesis and myelination are primarily postnatal events in both species. Free radicals produced during this metabolically active period could impair brain development by altering gene expression, DNA replication and cell division, suggesting the need for an effective antioxidant system during development.
Previous studies demonstrate that ascorbate concentrations are indeed higher in the developing brain than the mature brain [15, 18, 20]. A higher brain GSH concentration during development has also been demonstrated in some, but not all studies [15, 21]. These studies have evaluated the changes in the whole brain or in a limited number of brain regions. To our knowledge, the developmental changes in α-tocopherol have never been evaluated. Similarly, no studies have compared the effects of a pro-oxidant condition on the three antioxidants in the developing and mature brains. The first aim of this study was to compare the ascorbate, GSH and α-tocopherol concentrations in nine brain regions in postnatal day (P) 7, P14 and P60 rats. The brain is still developing at P7 and P14, while at P60 it is mature [19]. The second aim was to compare the effect of acute hypoglycemia of equivalent severity and duration on the three antioxidants at P14 and P60. Free radicals initiate neuronal injury during hypoglycemia [2, 3]. Previous studies have demonstrated that the developing brain is more resistant to injury than the mature brain during hypoglycemia [22, 23], likely due to less severe oxidative stress [24]. Our purpose was to gain a better understanding of the role of antioxidants in the age-specific vulnerability, so that neuroprotective strategies can be developed using these compounds.
2. Materials and Methods
2.1 Animal Preparation
The Institutional Animal Care and Use Committee approved all procedures. Male P7 (body weight, 16±1 g), P14 (39±4 g) and P60 (311±20 g) Sprague-Dawley rats were used. Pregnant rats were purchased (Harlan Laboratories, Indianapolis, IN, USA) and allowed to deliver spontaneously. The litter size was culled to eight on P3. Only males were studied, in order to avoid the confounding effects of sex on the antioxidants [16, 25]. Animals in Aim 1 were killed after overnight fasting (n=6 at P14 and P60, and n=14 at P7). A separate set of P14 and P60 rats (n=6) was subjected to overnight fasting and hypoglycemia before tissue collection in Aim 2. P7 rats were not subjected to hypoglycemia, as they do not sustain brain injury in this model [22].
2.2 Induction of Hypoglycemia
Acute hypoglycemia was induced using previously published protocol [22, 24]. In brief, after overnight fasting, rats were injected subcutaneously with regular insulin in a dose of 10 IU/kg. Fasting was continued and the rats were maintained at an ambient temperature of 34±1°C. Blood glucose was monitored every 30 min using a glucometer (Accu-Check®, Roche Laboratories, Indianapolis, IN, USA) and maintained between 20 mg/dL and 40 mg/dL as in our previous studies [22, 24]. In this model, hypoglycemia (blood glucose <40 mg/dL) is achieved 30 min after the insulin administration and is maintained until 240 min [22, 24, 26]. The animals were killed 240 min after the insulin injection without correction of hypoglycemia and the brain was collected.
2.3 Tissue Preparation
Animals were deeply anesthetized using pentobarbital (120 mg/kg ip) and perfused with ice-cold saline. The brain was removed and the following nine regions were dissected as previously described [7, 16]: olfactory bulb, cerebral cortex, hippocampus, striatum, hypothalamus (anterior brain regions), cerebellum, pons, medulla oblongata (posterior brain regions) and midbrain. The tissue samples were manually homogenized and extractions for ascorbate and GSH analyses were performed immediately. The remainder of the homogenate was stored at −70°C for protein, cholesterol and α-tocopherol analyses. Samples from individual rats were used at P14 and P60, resulting in n=6. At P7, samples from two rats were combined to augment the tissue quantity, resulting in n=7.
2.4 Determination of Antioxidants
Tissue antioxidant concentrations were determined by HPLC using published methods described in Supplementary Material. Briefly, ascorbate was determined using the procedure of Margolis [27]. The modified method of Ubbink et al [28, 29] with minor revisions was used to determine free and total GSH. Cholesterol and α-tocopherol were determined in the hexane extracts of the tissue homogenate as previously described [30, 31]. Total protein was determined using modified Lowry technique [32].
2.5 Statistical Analysis
The effects of postnatal age and hypoglycemia on the regional antioxidant concentrations were determined using ANOVA. The intergroup differences were determined using Bonferroni-corrected unpaired t tests. Data are presented as mean ± SD. The ascorbate and GSH data are reported as nmol/mg protein. The α-tocopherol data are reported as nmol α-tocopherol/mmol cholesterol as is conventional [33]. A p value <0.05 was considered significant.
3. Results
3.1 Developmental Changes in the Antioxidant Concentrations in the Brain Regions
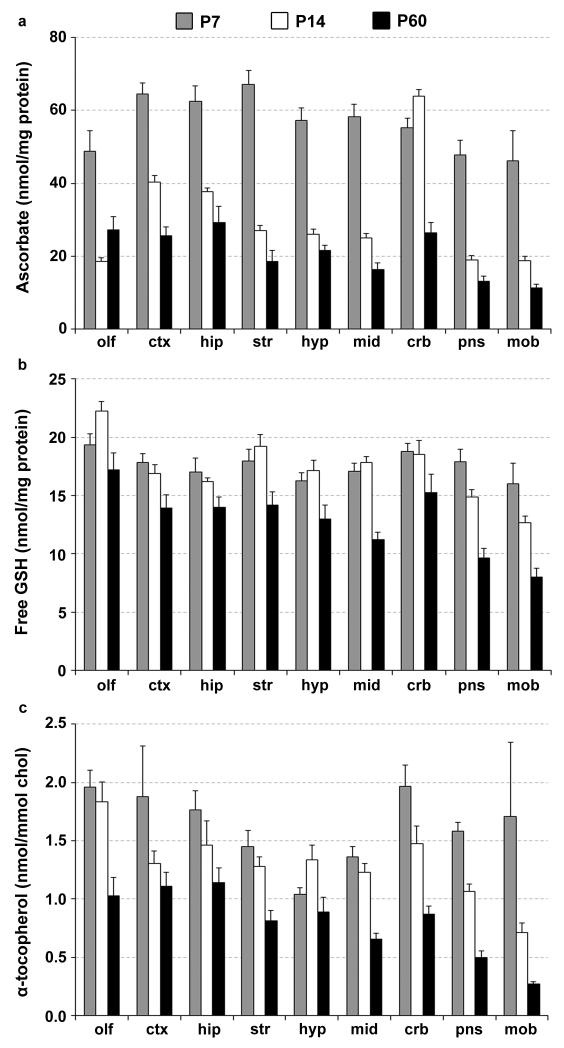
There was a main effect of postnatal age on all three antioxidants in all the nine brain regions (p<0.001; Fig 1). Ascorbate concentrations were higher at P7, compared with those at P14 and P60 (p<0.01; Fig. 1a). Between P7 and P14, ascorbate concentration decreased markedly in all the brain regions except the cerebellum (p<0.001). The concentration decreased further, but less markedly, between P14 and P60 in all the brain regions, except the olfactory bulb (p<0.001). There were inter-regional variations in the ascorbate concentrations at all the three ages. Overall, the concentrations were higher in the anterior regions than the posterior regions, particularly at P14 and P60 (p<0.001; Fig 1a).
Fig 1.
Developmental changes in ascorbate (a), free GSH (b), and α-tocopherol (c) concentrations in the brain regions of postnatal day (P) 7, P14 and P60 rats. Values are mean ± SD, n=6-7. There is a main effect of postnatal age on all three antioxidants in all the brain regions (p<0.001 for each, ANOVA). The concentration of each compound differs between P7, P14 and P60 in all the brain regions (p<0.01, Bonferroni-adjusted unpaired t tests) except, there are no difference in GSH concentrations between P7 and P14 in ctx, hip, str, crb, hyp and mid, and between P7 and P60 in olf, and no difference in α-tocopherol concentrations between P7 and P14 in olf and str, and between P7 and P60 in hyp. Abbreviations: olf, olfactory bulb; ctx, cerebral cortex; hip, hippocampus; str, striatum; hyp, hypothalamus; mid, midbrain; crb, cerebellum; pns, pons; mob, medulla oblongata; chol, cholesterol; and GSH, glutathione.
Free GSH concentrations were also higher during development in all the brain regions (p<0.001; Fig. 1b). Unlike ascorbate, GSH concentrations did not differ between P7 and P14; a P7>P14 difference was present only in the pons and medulla oblongata (p<0.005). Between P14 and P60, free GSH decreased in all the brain regions (p<0.001). An overall decrease along the anterior-to-posterior axis was also present at P14 and P60 (Fig 1b). The age and region-specific variations in total GSH mirrored those of free GSH (not shown).
Similar to ascorbate and GSH, the α-tocopherol concentrations were higher in all the brain regions at P7, compared with those at P14 and P60 (p<0.005; Fig. 1c). As with ascorbate, a P7>P14>P60 gradient was present, except in the olfactory bulb and striatum (P7=P14>P60), and the hypothalamus (P14>P7=P60). However, unlike ascorbate, the decrease in α-tocopherol concentration between P7 and P14 was less pronounced, except in the cerebral cortex and the posterior brain regions (Fig. 1c). There were minimal inter-regional variations in α-tocopherol concentrations at P7. Similar to ascorbate and GSH, an overall decrease along the anterior-to-posterior axis was present at P14 and P60 (Fig 1c).
3.2 Effect of Hypoglycemia on the Antioxidant Concentrations in the Brain Regions
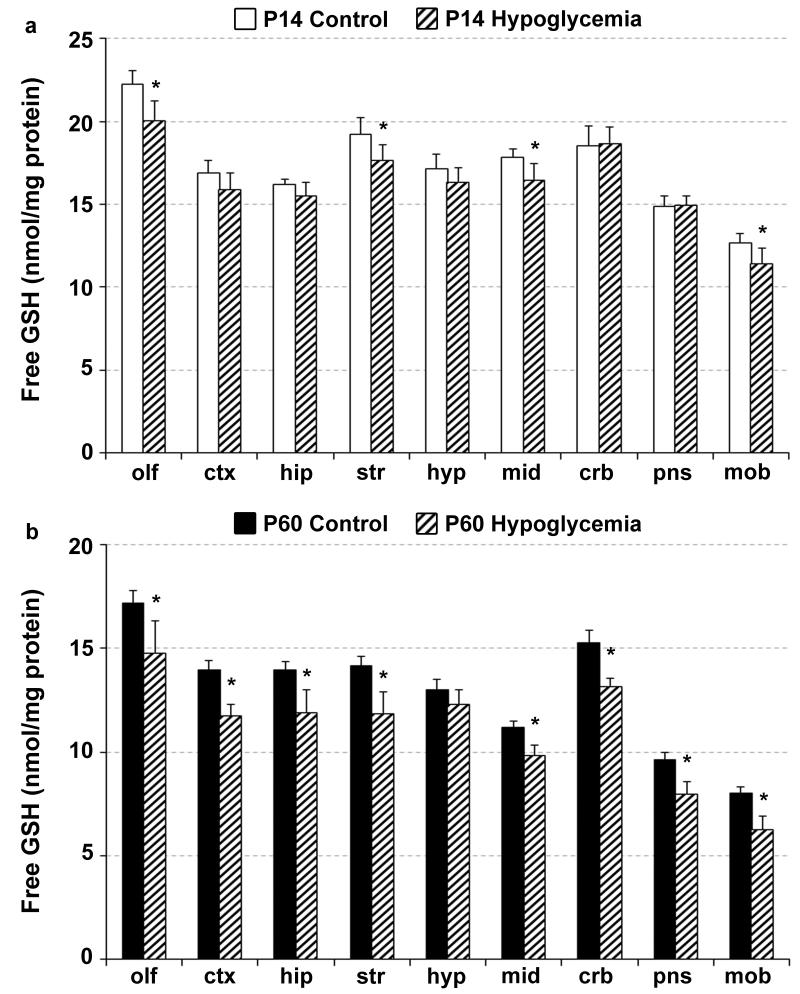
The severity of hypoglycemia at P14 and P60 was comparable (mean blood glucose concentration: P14, 28.5±3.9 mg/dL; P60, 28.2±1.5 mg/dL). Hypoglycemia had a differential effect on the three antioxidants. A main effect of hypoglycemia was present on free and total GSH concentrations at both P14 and P60 (p≤0.002 for each). Free GSH decreased in the olfactory bulb, striatum, midbrain and medulla oblongata at P14 (p<0.05; Fig 2a), and in all the brain regions, except the hypothalamus, at P60 (p<0.05; Fig. 2b). This was accompanied by decreased total GSH concentration in the olfactory bulb (-8%) and medulla oblongata (-8%) at P14, and in the olfactory bulb (-13%), hippocampus (-19%), striatum (-14%), cerebellum (-11%) and pons (-18%) at P60 (p<0.05 for all; not shown). There was no effect of hypoglycemia on ascorbate and α-tocopherol concentrations in any of the brain regions at either P14 or P60 (Supplementary Figure).
Fig 2.
Effect of hypoglycemia on free glutathione concentration in the brain regions of 14-day-old rats (a) and adult rats (b). Values are mean ± SD, n=6. There is a main effect of hypoglycemia on the glutathione concentrations at both postnatal ages (p<0.001 for each, ANOVA). *p<0.05, Control group vs. Hypoglycemia group (Bonferroni-adjusted unpaired t tests). Abbreviations: olf, olfactory bulb; ctx, cerebral cortex; hip, hippocampus; str, striatum; hyp, hypothalamus; mid, midbrain; crb, cerebellum; pns, pons; mob, medulla oblongata; and GSH, glutathione.
4. Discussion
In this comprehensive study, we found that the concentrations of all three major low molecular weight antioxidants varied between the developing and mature brain regions under basal conditions in rats. With few exceptions, the ascorbate, GSH and α-tocopherol concentrations were higher during development with some brain regions displaying six fold higher concentrations than the adult brain regions. The concentrations of all three antioxidants decreased postnatally. However, the rate of decline was not uniform, and varied among the antioxidants and the brain regions. Hypoglycemia also had a differential effect on the antioxidants in the developing and mature brains. Collectively, these data suggest that postnatal age influences antioxidant concentrations in the brain regions during normal development and under pro-oxidant conditions.
There were interregional variations in ascorbate, GSH and α-tocopherol at all the three ages. The variations at P7 were not in any particular order. In contrast, there was a progressive decrease along the anterior-to-posterior axis in all three antioxidants at P60. Interestingly, following just one week of development, the adult pattern was already established for ascorbate and α-tocopherol in the P14 brain with few exceptions. Overall, these results can be explained by the developmental stage of a brain region, such as the varying neuronal and glial densities and the stage of myelination at a given postnatal age [15, 16, 19]. However, this is unlikely to be the sole explanation, as discussed below.
Ascorbate contributed more than GSH to the water-soluble antioxidant pool, resulting in an ascorbate:GSH ratio as high as 4:1 at P7 and 2:1 at P60 in most regions. Higher ascorbate concentrations at P7 likely reflect the higher density of neurons, where ascorbate is localized, at this age [15, 20]. However, even in the relatively myelin-dense regions, such as the P60 medulla oblongata, the ascorbate:GSH ratio was >1.0. Previous studies attributed the postnatal decrease in the ascorbate:GSH ratio to the progressive increase in glia, where GSH is localized [6]. However, our data demonstrate that both ascorbate and GSH decrease postnatally, albeit at different rates, in both neuron-rich and glia-rich regions. In this respect, our data are similar to previous whole brain studies [18, 21].
The α-tocopherol concentrations also were higher during development. Since data are reported in relation to cholesterol, this could be a reflection of the relatively unmyelinated state of the developing brain. Between P7 and P60, α-tocopherol concentration decreased in all regions, except the hypothalamus. The percent decrease from P7 to P60 was similar to that of ascorbate, although the rates of decline of the two compounds were not identical. Whereas a marked decrease was already evident by P14 for ascorbate in all regions, except the cerebellum, α-tocopherol decreased minimally between P7 and P14 in most regions. The inter-regional variations at P60 are similar to previous data from our lab [7] with a progressive decrease along the neuron-rich anterior brain regions to myelin-rich posterior brain regions. Collectively, these data suggest that similar to ascorbate, α-tocopherol is primarily localized in neurons [15]. Additional studies are necessary to confirm this possibility.
Thus, the brain has a substantial pool of low molecular weight antioxidants during its peak development. This contrasts with the relatively low concentrations of the antioxidant enzymes, superoxide dismutase and glutathione peroxidase, at the corresponding postnatal period [34, 35]. Higher antioxidant concentrations are potentially necessary to prevent oxidant-mediated injury during the metabolically active phase of brain development. The robust expression of the nuclear enzyme poly(ADP-ribose)polymerase-1 that repairs oxidant-mediated DNA damage at the corresponding postnatal period [24] supports this interpretation. An additional explanation for the higher ascorbate, GSH and α-tocopherol concentrations during development is their role in neuronal and glial maturation and function [6, 12]. While all three compounds likely have major roles during brain development, the data suggest that ascorbate may be particularly important during the early postnatal period.
Hypoglycemia had a differential effect on the three antioxidants in the developing and mature brains. GSH was the only compound affected at both ages. The effect was localized at P14 involving only a few brain regions. Conversely, a more global involvement was seen at P60 and free GSH decreased in all brain regions, except the hypothalamus, a region known to be resistant to hypoglycemia-induced injury [22]. Further, in those regions affected at both ages (olfactory bulb, striatum, midbrain and medulla oblongata), the percent GSH decrease relative to the control was greater at P60 than at P14 (12-22% vs. 7-10%). These age-specific differences between P14 and P60 parallel the known vulnerability to injury during hypoglycemia and other pro-oxidant conditions at the corresponding ages [22-24, 36]. Decreased GSH during hypoglycemia has been previously demonstrated in adult rats [37]. GSH is an electron donor during the reduction of hydrogen peroxide by glutathione peroxidase, an enzyme that is upregulated in hypoglycemia [37]. However, total GSH was also decreased in several brain regions, suggesting inadequate GSH synthesis [11] also may be responsible for our results. Such a scenario is likely, since glutamate, a precursor of GSH is consumed for energy production during hypoglycemia [24].
In vitro studies demonstrate that ascorbate and α-tocopherol are consumed prior to GSH during oxidant exposure [38, 39]. This order of decrease was not present in our study. Our results are similar to previous in vivo studies in adult rats demonstrating decreased GSH without altered ascorbate during hypoxia-ischemia [11, 40], a condition characterized by cerebral energy failure, excitotoxicity and oxidative stress, similar to hypoglycemia. Thus, GSH likely plays a greater role than the other two antioxidants during a pathological stress. However, this possibility remains to be explained by studying other models of hypoglycemia, such as more severe, recurrent and prolonged hypoglycemia, or varying combinations of these, and by incorporating measurements of oxidant stress in the model.
5. Conclusions
The postnatal age influenced ascorbate, GSH and α-tocopherol concentrations in the brain regions during normal development and acute hypoglycemia in rats. The higher antioxidant concentrations in the early postnatal period may be reflective of the potential for oxidative stress during this metabolically active period or the necessity of these compounds for normal neurodevelopment. The antioxidant profile in the hypoglycemic mature brain implies the potential for oxidative stress and neuronal injury. Therapies directed at augmenting antioxidants, particularly GSH, may be beneficial in this setting.
Supplementary Material
Highlights.
Postnatal changes in antioxidant concentrations in the brain regions are unknown.
Ascorbate, glutathione and α-tocopherol were studied in 7, 14 and 60 day old rats.
The effect of acute hypoglycemia on the three antioxidants also was determined.
All three antioxidants were 100-600% higher during development than at adulthood.
Hypoglycemia led to less antioxidant decrease in developing brain than adult brain.
Acknowledgements
The authors gratefully acknowledge Maurice W. Dysken, MD, Director, GRECC Program, Minneapolis VA Health Care System for his support, and Kathleen Czerniak for assistance with the experiments. This project was supported with resources and the use of facilities at the Minneapolis VA Health Care System, and by grants from National Institutes of Child Health and Human Development (HD47276) and Viking Children’s Fund. The manuscript is dedicated to the memory of Govind T. Vatassery, PhD.
Abbreviations
- GSH
glutathione
- P
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare that they have no conflicts of interest.
Author Contributions: ARR conceived the study, designed and participated in all the experiments, analyzed data and wrote the manuscript. HQ and ES performed tissue analyses and assisted with manuscript preparation. GTV assisted with study design, data analysis and interpretation. RR was in overall charge of the project, data analysis and manuscript preparation.
References
- 1.Vatassery GT. Vitamin E and other endogenous antioxidants in the central nervous system. Geriatrics. 1998;53(Suppl 1):S25–7. [PubMed] [Google Scholar]
- 2.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–8. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGowan JE, Chen L, Gao D, Trush M, Wei C. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett. 2006;399:111–4. doi: 10.1016/j.neulet.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Spector R, Johanson CE. Vitamin transport and homeostasis in mammalian brain: focus on Vitamins B and E. J Neurochem. 2007;103:425–38. doi: 10.1111/j.1471-4159.2007.04773.x. [DOI] [PubMed] [Google Scholar]
- 5.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–71. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 6.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–16. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 7.Vatassery GT, Angerhofer CK, Knox CA, Deshmukh DS. Concentrations of vitamin E in various neuroanatomical regions and subcellular fractions, and the uptake of vitamin E by specific areas, of rat brain. Biochim Biophys Acta. 1984;792:118–22. doi: 10.1016/0005-2760(84)90211-x. [DOI] [PubMed] [Google Scholar]
- 8.Jayalakshmi K, Singh SB, Kalpana B, Sairam M, Muthuraju S, Ilavazhagan G. N-acetyl cysteine supplementation prevents impairment of spatial working memory functions in rats following exposure to hypobaric hypoxia. Physiol Behav. 2007;92:643–50. doi: 10.1016/j.physbeh.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Bano S, Parihar MS. Reduction of lipid peroxidation in different brain regions by a combination of alpha-tocopherol and ascorbic acid. J Neural Transm. 1997;104:1277–86. doi: 10.1007/BF01294728. [DOI] [PubMed] [Google Scholar]
- 10.Mejia-Toiber J, Montiel T, Massieu L. D-beta-hydroxybutyrate prevents glutamate-mediated lipoperoxidation and neuronal damage elicited during glycolysis inhibition in vivo. Neurochem Res. 2006;31:1399–408. doi: 10.1007/s11064-006-9189-5. [DOI] [PubMed] [Google Scholar]
- 11.Cooper AJ, Pulsinelli WA, Duffy TE. Glutathione and ascorbate during ischemia and postischemic reperfusion in rat brain. J Neurochem. 1980;35:1242–5. doi: 10.1111/j.1471-4159.1980.tb07882.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Chang MY, Park CH, Kim HY, Kim JH, Son H, Lee YS, Lee SH. Ascorbate-induced differentiation of embryonic cortical precursors into neurons and astrocytes. J Neurosci Res. 2003;73:156–65. doi: 10.1002/jnr.10647. [DOI] [PubMed] [Google Scholar]
- 13.Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–38. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 14.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 15.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–23. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 16.Gohil K, Oommen S, Quach HT, Vasu VT, Aung HH, Schock B, Cross CE, Vatassery GT. Mice lacking alpha-tocopherol transfer protein gene have severe alpha-tocopherol deficiency in multiple regions of the central nervous system. Brain Res. 2008;1201:167–76. doi: 10.1016/j.brainres.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajalakshmi R, Thrivikraman KV, Ramakrishnan CV. Protein deficiency & regional chemistry of the brain. I. Effects of protein deficiency on regional distribution of protein, glutathione & ascorbic acid in rat brain. Indian J Biochem. 1971;8:295–9. [PubMed] [Google Scholar]
- 18.Adlard BP, de Souza SW, Moon S. The effect of age, growth retardation and asphyxia on ascorbic acid concentrations in developing brain. J Neurochem. 1973;21:877–81. doi: 10.1111/j.1471-4159.1973.tb07532.x. [DOI] [PubMed] [Google Scholar]
- 19.Rice D, Barone S., Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terpstra M, Tkac I, Rao R, Gruetter R. Quantification of vitamin C in the rat brain in vivo using short echo-time 1H MRS. Magn Reson Med. 2006;55:979–83. doi: 10.1002/mrm.20854. [DOI] [PubMed] [Google Scholar]
- 21.Nanda D, Tolputt J, Collard KJ. Changes in brain glutathione levels during postnatal development in the rat. Brain Res Dev Brain Res. 1996;94:238–41. doi: 10.1016/0165-3806(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 22.Ennis K, Tran PV, Seaquist ER, Rao R. Postnatal age influences hypoglycemia-induced neuronal injury in the rat brain. Brain Res. 2008;1224:119–26. doi: 10.1016/j.brainres.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada KA, Rensing N, Izumi Y, De Erausquin GA, Gazit V, Dorsey DA, Herrera DG. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatr Res. 2004;55:372–9. doi: 10.1203/01.PDR.0000110523.07240.C1. [DOI] [PubMed] [Google Scholar]
- 24.Rao R, Sperr D, Ennis K, Tran P. Postnatal age influences hypoglycemia-induced poly(ADP-ribose) polymerase-1 activation in the brain regions of rats. Pediatr Res. 2009;66:642–7. doi: 10.1203/PDR.0b013e3181bbce69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci. 1975;258:103–18. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- 26.Rao R, Ennis K, Long JD, Ugurbil K, Gruetter R, Tkac I. Neurochemical changes in the developing rat hippocampus during prolonged hypoglycemia. J Neurochem. 2010;114:728–38. doi: 10.1111/j.1471-4159.2010.06797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margolis SA, Davis TP. Stabilization of ascorbic acid in human plasma, and its liquid-chromatographic measurement. Clin Chem. 1988;34:2217–23. [PubMed] [Google Scholar]
- 28.Ubbink JB, Hayward Vermaak WJ, Bissbort S. Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr. 1991;565:441–6. doi: 10.1016/0378-4347(91)80407-4. [DOI] [PubMed] [Google Scholar]
- 29.Castagna A, Le Grazie C, Accordini A, Giulidori P, Cavalli G, Bottiglieri T, Lazzarin A. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: effect of parenteral treatment with SAMe. Neurology. 1995;45:1678–83. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- 30.Vatassery GT, Smith WE, Quach HT. A liquid chromatographic method for the simultaneous determination of alpha-tocopherol and tocopherolquinone in human red blood cells and other biological samples where tocopherol is easily oxidized during sample treatment. Anal Biochem. 1993;214:426–30. doi: 10.1006/abio.1993.1518. [DOI] [PubMed] [Google Scholar]
- 31.Vatassery GT, Quach HT, Smith WE, Krick TP, Ungar F. Analysis of hydroxy and keto cholesterols in oxidized brain synaptosomes. Lipids. 1997;32:101–7. doi: 10.1007/s11745-997-0014-3. [DOI] [PubMed] [Google Scholar]
- 32.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–10. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 33.Lee ES, Smith WE, Quach HT, Jones BD, Santilli SM, Vatassery GT. Moderate hyperoxia (40%) increases antioxidant levels in mouse tissue. J Surg Res. 2005;127:80–4. doi: 10.1016/j.jss.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Aspberg A, Tottmar O. Development of antioxidant enzymes in rat brain and in reaggregation culture of fetal brain cells. Brain Res Dev Brain Res. 1992;66:55–8. doi: 10.1016/0165-3806(92)90139-n. [DOI] [PubMed] [Google Scholar]
- 35.Shivakumar BR, Anandatheerthavarada HK, Ravindranath V. Free radical scavenging systems in developing rat brain. Int J Dev Neurosci. 1991;9:181–5. doi: 10.1016/0736-5748(91)90010-j. [DOI] [PubMed] [Google Scholar]
- 36.Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res Dev Brain Res. 1997;100:149–60. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- 37.Singh P, Jain A, Kaur G. Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem. 2004;260:153–9. doi: 10.1023/b:mcbi.0000026067.08356.13. [DOI] [PubMed] [Google Scholar]
- 38.Vatassery GT. In vitro oxidation of vitamins C and E, cholesterol, and thiols in rat brain synaptosomes. Lipids. 1995;30:1007–13. doi: 10.1007/BF02536285. [DOI] [PubMed] [Google Scholar]
- 39.Vatassery GT, Smith WE, Quach HT, Lai JC. In vitro oxidation of vitamin E, vitamin C, thiols and cholesterol in rat brain mitochondria incubated with free radicals. Neurochem Int. 1995;26:527–35. doi: 10.1016/0197-0186(94)00147-m. [DOI] [PubMed] [Google Scholar]
- 40.Lyrer P, Landolt H, Kabiersch A, Langemann H, Kaeser H. Levels of low molecular weight scavengers in the rat brain during focal ischemia. Brain Res. 1991;567:317–20. doi: 10.1016/0006-8993(91)90811-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.