Abstract
Mycobacterium farcinogenes and M. senegalense are the causal agents of bovine farcy, a chronic, progressive disease of the skin and lymphatics of zebu cattle. The disease, which is prevalent mainly in sub-Saharan Africa, was in earlier times thought to be caused by Nocardia farcinica and can be described as one of the neglected diseases in cattle. Some aspects of the disease have been investigated during the last five decades but the major development had been in the bacteriological, chemotaxonomic, and phylogenetic aspects. Molecular analyses confirmed that M. farcinogenes and M. senegalense fall in a subclade together with M. houstonense and M. fortuitum. This subclade is closely related to the one accommodating M. peregrinum, M. porcinum, M. septicum, M. neworleansense, and M. alvei. DNA probes were designed from 16S-23S rRNA internal transcribed spacer and could be used for the rapid diagnosis of bovine farcy. An ELISA assay has been evaluated for the serodiagnosis of the disease. The zoonotic potentials of M. farcinogenes and M. senegalense are unknown; few studies reported the isolation of M. senegalense and M. farcinogenes from human clinical sources but not from environmental sources or from other domestic or wild animals.
1. Introduction
Bovine farcy (which is caused by Mycobacterium farcinogenes and M. senegalense) is a chronic granulomatous inflammation of the skin lymphatics and draining lymph nodes of zebu cattle. It has been reported in 19 countries in Africa, Asia, Latin America, and the Caribbean with tropical and subtropical climates. Historically, it existed in a belt that extends east to include south India, Sri Lanka, and Sumatra and west to include north parts of Latin America and the West Indies but mainly dominant in the sub-Saharan African countries [1]. It was in 1888 that Edmond Nocard first isolated and described the causal agent of “bovine farcy” [2]. In his original description, Nocard [2] noted a granulomatous disease of cattle with multiple abscesses, draining sinuses, pulmonary involvement, emaciation, and eventually death. Since then, the classification of the Nocardia organisms has undergone several changes.
Literature on the prevalence, transmission patterns, and risk factors of bovine farcy is deficient. The disease is not included within the categories of cattle diseases in List A or List B of the OIE categorization [3] due to its characteristic that it has neither international spread nor significant mortality and morbidity at the level of a country or a zone nor an apparent zoonotic property with severe consequences. Nevertheless, cattlemen and governments in Africa believe that bovine farcy is responsible for certain economic losses as a result of damaged hides. Besides, it is public-health burden since the lymphadenitis due to farcy resembles the lesions of bovine tuberculosis in carcasses and the meat is considered inappropriate for human consumption [1].
Laboratory diagnoses are hardly ever used to make routine diagnosis and to initiate treatment. This is because of logistic and practical difficulties encountered amongst rural communities in Africa. However, laboratory diagnoses can confirm the clinical diagnosis retrospectively on tissues and purulent materials taken during treatment or during meat inspection. Apart from the reasonable use of standard smear-and-culture methods, few diagnostic tests have been developed; the molecular and serological tests have not been evaluated for reproducibility and accuracy.
2. Taxonomy
Bovine farcy causing actinomycetes isolated from zebu cattle in eastern Africa were found to belong to the genus Mycobacterium and not to Nocardia [4–10]. The causal agents of bovine farcy contained mycolic acids, the esters of which yielded, on pyrolysis gas chromatography, a single peak that corresponded to the C24 ester characteristic of some mycobacteria. Chamoiseau [7] suggested that these bacteria be allocated in the genus Mycobacterium as M. farcinogenes; he distinguished two subspecies, M. farcinogenes subspecies tchadense and M. farcinogenes subspecies senegalense. On the ground of their phenotypic dissimilarity, Chamoiseau [11] raised the two subspecies to species levels as M. farcinogenes and M. senegalense which have first appeared in the 1st edition of Bergey's Manual of Systematic Bacteriology [12].
It is now known that M. farcinogenes and M. senegalense can also be distinguished from one another on the basis of histopathological behavior [11], DNA relatedness [13], mycobactin contents [14], chemotaxonomic and biochemical properties [15–17], pyrolysis mass spectrometry [18], and 16S rRNA sequence data [19–23].
Members of the two species have many properties in common both with one another and with the nonphotochromogenic rapidly growing mycobacteria, namely, M. fortuitum and M. peregrinum, M. septicum (sorbitol negative 3rd M. fortuitum biovar), M. porcinum [24], and, with the recently described species in the M. fortuitum complex, M. boenickei, M. houstonense (sorbitol negative 3rd M. fortuitum biovar), and M. neworleansense (Figure 1; [25, 26]).
Figure 1.
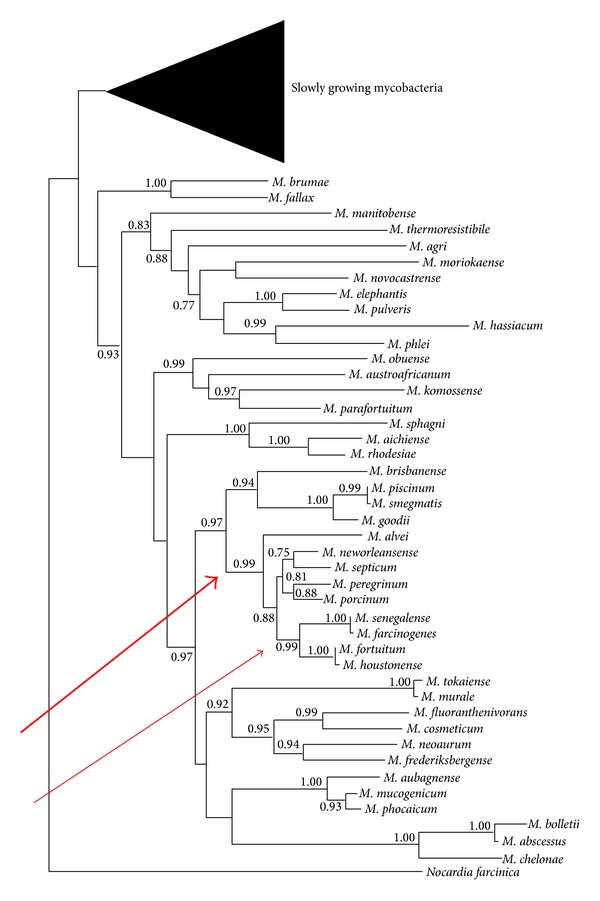
Estimate of mycobacterial phylogeny based on a multilocus seven-gene concatenate (hsp65, rpoB, 16S rRNA, smpB, sodA, tmRNA, and tuf) showing the position of Mycobacterium farcinogenes and M. senegalense within a branch that accommodates the rapidly growing nonphotochromogenic mycobacteria (thick arrow) and their close relationship to M. houstonense and M. fortuitum (thin arrow). The percentages of bootstrap values are shown next to the nodes. The tree was modified from Mignard and Flandrois [26].
M. porcinum is a known veterinary pathogen [27]. Phylogenetic tree of the combined rpoB + recA + soda + hsp65 + 16S rRNA gene sequences of 19 rapidly growing mycobacteria using the neighbor-joining method supported the designation of the M. fortuitum grouping into subclades which include M. peregrinum and M. septicum; this is joint by another subclade consisting of M. boenickei, M. neworleansense, and M. porcinum along with subclade in M. fortuitum and M. houstonense, M. farcinogenes and M. senegalense. Both subclades are related to another subclade of M. farcinogenes and M. senegalense. The three are related to the subclade which includes M. fortuitum and M. houstonense [28]. The 99% rpoB gene sequence similarity between M. houstonense and M. fortuitum suggested that these strains may be closely related subspecies, although M. houstonense showed resistance to pipemidic acid, biochemical differences such as mannitol, inositol, sorbitol, and trehalose utilization [22], and three base differences in the 16S rRNA gene sequence [25]. The isolation of M. fortuitum 3rd variant from cattle specimens [29] may be of epidemiologic and taxonomic implications. The M. fortuitum 3rd (sorbitol positive) variant is now reclassified as M. houstonense sp. nov. [25]. Recently, Guérin-Faublée et al. [30] described Mycobacterium bourgelatii, a new rapidly growing nonphotochromogenic species which they had isolated from cattle with lymphadenitis. These new isolates need to be compared with other cattle pathogens giving the similarity in both chromogenicity and the rapidly growing property.
N. farcinica is still though not often reported as a causal agent of bovine farcy when diagnosis based on morphological traits, which fail to discriminate Nocardia from other mycolic acid-containing actinomycetes, is used [31–40].
The conclusions from the many taxonomic studies could be summarized as follows:
M. farcinogenes and M. senegalense are morphologically similar to each other and to Nocardia farcinica (Figures 2 and 3). But M. farcinogenes is relatively a slow growing Mycobacterium compared to the rapidly growing M. senegalense.
On molecular basis, M. farcinogenes and M. senegalense are closely related to M. houstonense. The three species fall ina subclade including M. fortuitum. This subclade is closely linked to the one incorporating M. peregrinum, M. porcinum, M. septicum, M. neworleansense, and M. alvei (Figure 1).
Figure 2.
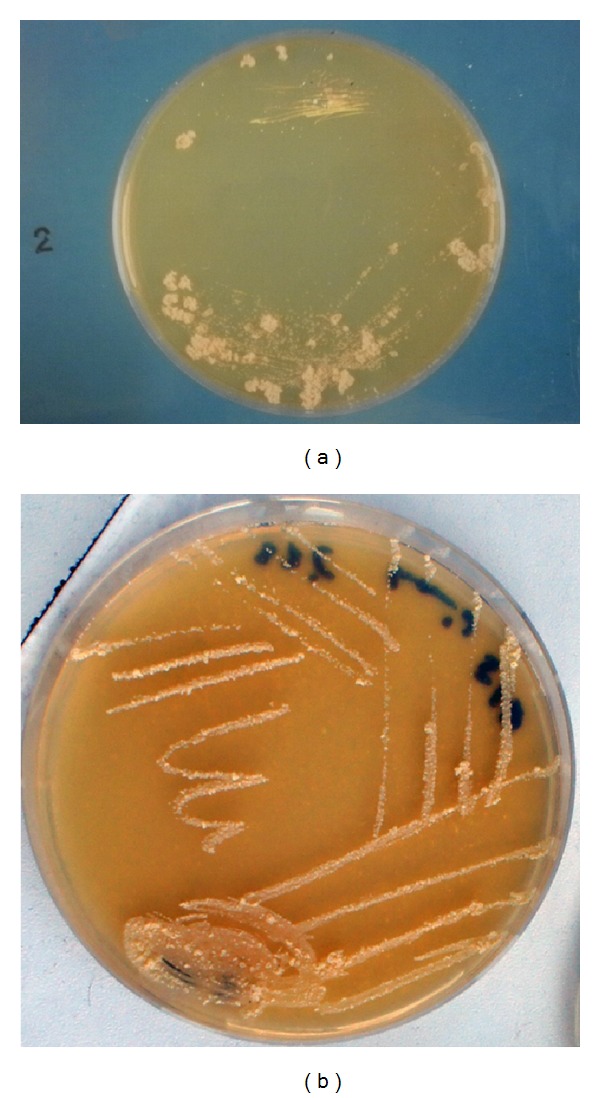
Growth of Mycobacterium farcinogenes on glucose yeast extract agar (a) and M. senegalense on glucose yeast extract malt extract agar (b) at 37°C for 7 days, showing nonchromogenic, wheat-colored rough convoluted irregular colonies.
Figure 3.
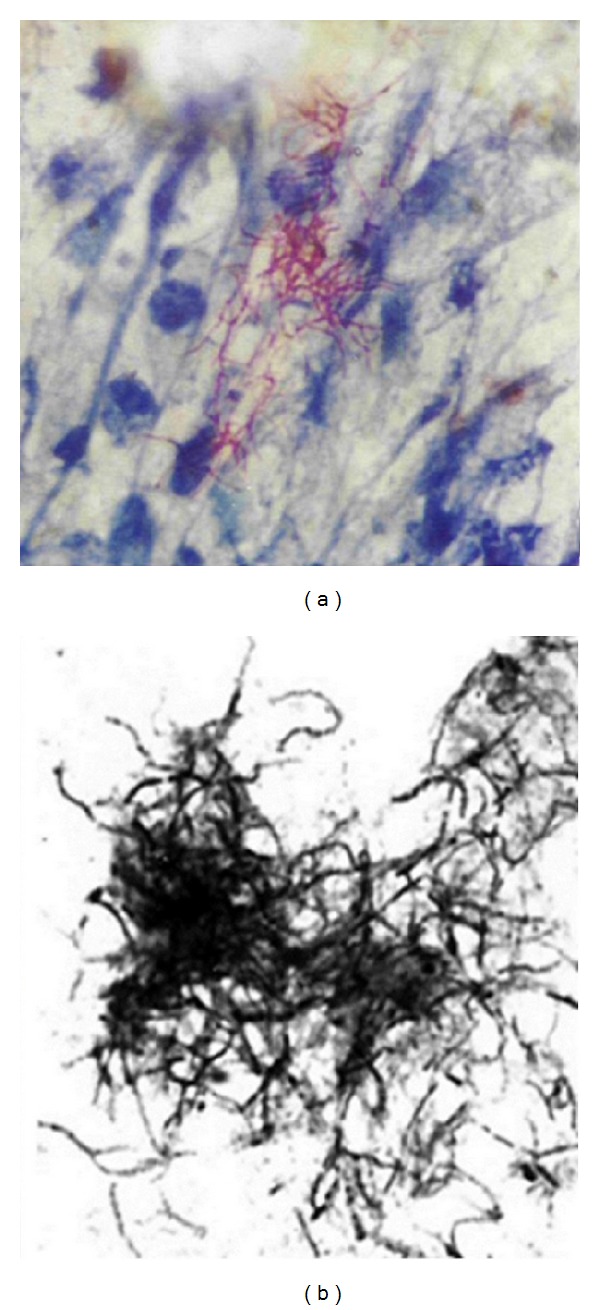
Smears made from a purulent material of M. farcinogenes-infected cow (a) showing acid fast branching filaments and smear made from a culture of M. senegalense (b). Note short or long filaments, bent and branched, in clumps or tangled lacy network which do not fragment into bacillary forms.
3. Habitats
There are no reports on isolating or detecting M. farcinogenes or M. senegalense from environmental samples. Epidemiological data have not reported bovine farcy in wild and other domestic animals. The zoonotic potentials of M. farcinogenes and M. senegalense are unknown; only few reports provided evidence that M. senegalense [41] and M. farcinogenes [42] cause infections in human.
The role of ticks in the transmission of farcy is not understood. Bovine farcy lesions start at the lymph nodes (usually peripheral, femoral, or parotid) and then spread slowly via lymphatic vessel to subcutaneous tissue on the dorsal parts. In contrast, ticks feed mostly on the ventral aspects. Furthermore, tick larvae and nymphs moult before feeding on another animal; therefore, it is not feasible that the bacterium is transferred from one host to another on the outside of ticks. Although transmission of the infection by ticks under field condition has not been established, it has long been believed by locals in the Sudan that the ticks are involved. Some authors associate bovine farcy with tick infestation (notably, the ixodid tick Amblyomma variegatum) [40, 43–45]. Additionally, Al Janabi et al. [46] successfully transmitted N. farcinica (which was believed at that time to be the agent of bovine farcy) from an experimentally infected rabbit to a control one via Amblyomma variegatum.
4. Isolation and Cultivation
Lowenstein-Jensen is the medium commonly used for selective isolation of M. farcinogenes as for many other mycobacteria from infected materials [47]. Glucose yeast extract agar ([48]; GYEA) is used for maintenance and bench work. Modified Sauton's broth [49] is routinely used to cultivate biomass of M. farcinogenes, M. senegalense, and some other mycobacteria for chemotaxonomic studies [16, 50–52]. Mycobacterium medium number 219 is recommended by DSMZ for routine culture [53].
M. farcinogenes and M. senegalense grow on a wide range of common synthetic media. Shigidi et al. [38] used diagnostic sensitivity test (DST) agar for culturing farcy organisms. Out of 13 diverse agar-based media, M. farcinogenes was found to grow particularly well on Mueller Hinton's medium followed by modified Bennett, Tryptic soya, glucose yeast extract, and DST agars [54]. A broth medium containing (g/L; w/v) yeast extract (4), glucose (15), magnesium sulphate (0.5), trisodium citrate (1.5), potassium sulphate (0.5), and ammonium ferric citrate (trace) and buffered with potassium dihydrogen phosphate (5) was formulated [55] and found to support a luxuriant growth of M. farcinogenes strains than media used before [49].
5. Morphological and Cultural Characteristics
Colonies appear after 2 to 5 days (M. senegalense) and 5 to 10 days (M. farcinogenes) at 25°C to 37°C on Lowenstein-Jensen medium. Colonies appear rough and convoluted that are firmly attached to the media. The grown colonies are usually nonchromogenic, wheat-colored. Growth on most agar-based media such as GYEA, DST agar, Tryptic Soya agar, and Mueller Hinton agar is seen as nonchromogenic irregular rough colonies (Figure 2) which are not firmly attached to the median (in contrast to their growth Lowenstein-Jensen slants) and are difficult to emulsify [11, 15, 45, 56]. These mycobacteria can be preserved for up to 10 years when cells are suspended in 20% glycerol and kept frozen at −20°C [57].
M. farcinogenes and M. senegalense have short or long filaments, bent and branched, in clumps or tangled lacy network whether seen in smears from culture or from lesions (Figure 3). These filaments do not fragment into bacillary forms and were strongly acid-alcohol fast. Scanning electron microscopy observations have confirmed the true-nonfragmenting filamentous nature of the M. farcinogenes and M. senegalense (Figure 4). It is obvious that these species could be distinguished from other mycobacteria because they form branched substrate mycelia. Moreover, M. senegalense exhibitsa characteristic fungal structure called “synnema” (plural synnemata) which is strand resembling stalks thread together (Figure 4).
Figure 4.
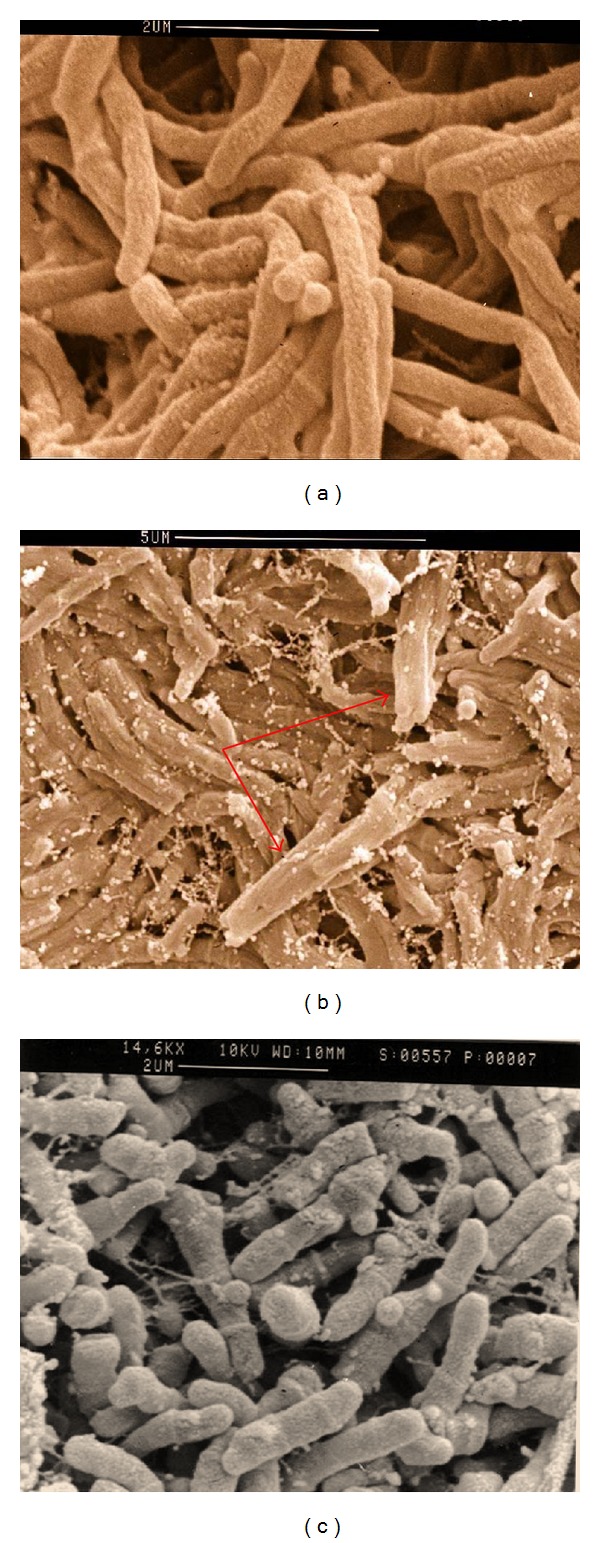
Scanning electron microscopy of M. farcinogenes (a), M. senegalense (b), and Nocardia farcinica (c). Note the true-nonfragmenting branched filaments in both species and the presence of “synnemata” in M. senegalense (arrow).
6. Biochemical Features
Members of the M. farcinogenes and M. senegalense produce a positive malonamidase test, an attribute that is rarely shown by other mycobacteria. Routinely, M. senegalense is more active biochemically than M. farcinogenes. Some of the biochemical properties, enzyme profile, tolerance to chemical inhibitors, and resistance pattern to antibiotics as well as morphological and cultural characteristics of the two species are shown in Table 1.
Table 1.
Phenotypic characteristics of Mycobacterium farcinogenes and Mycobacterium senegalense.
| Test | M. farcinogenes | M. senegalense | References* |
|---|---|---|---|
| Morphology and cultural characteristics | |||
| Growth at 30°C | + | + | [56] |
| Growth at 35–37°C | + | + | [56] |
| Growth after 3–5 days | − | + | [11, 15, 56] |
| Growth after 5–10 days | + | − | [11, 15, 56] |
| Colony wheat-colored | + | + | [11, 15, 56] |
| Colony convoluted | + | + | [11, 15, 56] |
| Colony rough | + | + | [11, 15, 56] |
| Colony very rough and grainy | + | − | [11, 56] |
| Colony easily detached from agar | + | − | [15, 56] |
| Colony relatively emulsifiable | − | + | [11, 56] |
| Colony nonemulsifiable | + | − | [56] |
| Aerial hyphae sparse | + | + | [15, 56] |
| Aerial hyphae abundant | − | − | [15, 56] |
| Biochemical (enzyme) tests | |||
| Acetamidase | + | + | [11, 15, 82] |
| Allantoinase | +/− | + | [11] |
| Arylsulphatase | − | + | [15, 76, 82] |
| Benzamidase | +/− | + | [11] |
| Catalase** | + | + | [11, 15, 56, 76, 82] |
| Iron citrate test | − | − | [11] |
| Isonicotinamidase | +/− | + | [11] |
| Beta-lactamase after 60 min | + | + | [15] |
| Neutral red test | − | − | [11] |
| Niacin production | − | − | [11] |
| Nicotinamidase | +/− | + | [11] |
| Nitrate reductase | + | + | [11, 15, 76] |
| Nitrophenol oxidase | − | − | [15] |
| Salicylamidase | +/− | + | [11] |
| Urease | −/+ | + | [11, 15, 82] |
| Urease | − | ND | [76] |
| Fluorogenic enzyme tests (cleavage of 4-methylumbelliferone glycosides substrates (4MU)) |
|||
| 4MU-α-L-arabinofuranoside | + | +/− | [56] |
| 4MU-α-L-arabinopyranoside | + | +/− | [56] |
| 4MU-β-D-fucoside | + | +/− | [56] |
| 4MU-β-D-galactoside | + | − | [56] |
| 4MU-β-D-glucoside | − | + | [56] |
| 4MU-β-D-glucoside | + | + | [56] |
| 4MU-p-guanidinobenzoate (HCl) | + | + | [56] |
| 4MU-α-D-mannopyranoside | − | + | [56] |
| 4MU-β-D-ribofuranoside | + | + | [56] |
| Degradation tests | |||
| Aesculin | + | + | [56] |
| Arbutin | + | + | [56] |
| Casein | − | − | [56] |
| Elastin | − | − | [56] |
| Guanine | − | − | [56] |
| Hypoxanthine | − | − | [56] |
| Keratin | − | − | [56] |
| Testosterone | + | + | [15] |
| Tyrosine | − | − | [15, 56] |
| Tweens | + | + | [56] |
| Xanthine | − | − | [56] |
| Growth in the presence of (%) | |||
| Cobalt chloride (0.005) | + | + | [56] |
| Copper sulphate (0.01) | + | + | [56] |
| Crystal violet (0.001) | − | − | [15] |
| Ferrous sulphate (0.01) | + | + | [56] |
| Hydroxylamine HCl (0.05) | − | − | [56] |
| Lead acetate (0.01) | + | + | [56] |
| o-Nitrobenzoic acid (0.05) | − | + | [56] |
| Oleic acid (0.25, v/v) | + | + | [56] |
| Phenol (0.01) | + | + | [56] |
| Phenol (0.1) | − | − | [15, 56, 82] |
| Phenyl ethanol (0.02%) | − | + | [15, 82] |
| Potassium tellurite (0.04%) | − | + | [15, 82] |
| Potassium tellurite (0.5) | − | − | [56] |
| Pyronin G (0.1) | − | − | [56] |
| Sodium azide (0.005) | − | + | [56] |
| Sodium azide (0.01) | − | − | [15, 82] |
| Sodium chloride (5) | − | − | [56] |
| Sodium chloride (5%) | − | + | [15, 82] |
| Sodium deoxycholate (0.01) | − | + | [56] |
| Sodium nitrate (1) | − | − | [56] |
| Sodium salicylate (0.1) | − | + | [56] |
| Sodium selenite (0.001) | − | − | [56] |
| Thallous acetate (0.05) | − | − | [56] |
| Tetrazolium chloride (0.01) | − | − | [56] |
| Toluidine blue (0.03) | − | + | [56] |
| Teepol HB6 (0.05, v/v) | − | − | [56] |
| Zinc chloride (0.005) | − | − | [56] |
| Zinc chloride (0.01) | − | − | [56] |
| Growth at | |||
| 45°C | − | − | [15, 76] |
| pH4 | − | − | [15] |
| pH5 | − | + | [15, 82] |
| pH10 | − | − | [15] |
| Survival at 60°C for 4 hours | − | − | [15] |
| Resistance to antibiotics and antibacterial agents (μgmL-1) | |||
| Amikacin (2) | − | − | [55] |
| p-Aminosalicylic acid, Na salt (64) | −/+ | + | [11, 55] |
| Amoxicillin (64) | + | + | [55] |
| Ampicillin (64) | +/− | + | [55] |
| Capreomycin sulphate (10) | − | + | [15] |
| Capreomycin sulphate (10) | −/+ | − | [55] |
| Cephaloridine (64) | + | + | [55] |
| Cephapirin Na salt (64) | + | + | [55] |
| Chlortetracycline HCl (64) | −/+ | −/+ | [55] |
| D-Cycloserine (2) | + | + | [55] |
| Cycloserine | − | − | [11] |
| Dapsone (16) | + | + | [55] |
| Dapsone (100) | − | + | [15, 82] |
| Doxycycline HCl (8) | − | +/− | [55] |
| Doxycycline HCl (64) | − | − | [55] |
| Erythromycin (64) | − | + | [55] |
| Ethambutol HCl (4) | + | + | [15, 55] |
| Ethambutol HCl (64) | −/+ | + | [15, 55, 82] |
| Ethionamide (5) | + | + | [11, 55] |
| Gentamycin sulphate (128) | − | − | [55] |
| Isoniazid (2) | + | + | [15, 55] |
| Kanamycin sulphate (2) | + | + | [55] |
| Kanamycin sulphate (16) | − | − | [11] |
| Lividomycin sulphate (16) | − | − | [55] |
| Lysozyme (50) | + | + | [15] |
| Lincomycin HCl (64) | + | + | [55] |
| Nalidixic acid Na salt (64) | − | + | [55] |
| Novobiocin (64) | +/− | + | [55] |
| Neomycin sulphate (128) | − | − | [55] |
| Oleandomycin phosphate (64) | − | + | [55] |
| Oxytetracycline HCl (64) | − | − | [55] |
| Paromomycin sulphate (64) | − | − | [55] |
| Penicillin (66 IU/mL) | − | +/− | [15] |
| Polymyxin B sulphate (64) | − | + | [55] |
| Prothionamide (10) | + | + | [15] |
| Rifampicin (16) | + | + | [55] |
| Rifampicin (20) | + | − | [15] |
| Streptomycin sulphate (1.6) | + | + | [11, 55] |
| Streptomycin sulphate (64) | − | + | [15] |
| Sulphamethazine (1.6) | + | + | [55] |
| Thiacetazone (10) | + | −/+ | [55] |
| Trimethoprim + sulphamethoxazole (8) | + | + | [55] |
| Vancomycin HCl (64) | − | − | [55] |
| Viomycin sulphate (64) | − | − | [11, 55] |
| Growth on sole carbon source (1%) | |||
| Acetamide | + | + | [56] |
| Acetic acid (Na salt) | + | + | [56] |
| Butane 1,3 diol | + | − | [15, 82] |
| Butane 2,3 diol | − | + | [15, 56, 82] |
| Ethanol | − | − | [15, 82] |
| Fructose | − | + | [56] |
| D(+)Galactose | − | − | [56] |
| D-Gluconic acid | + | + | [56] |
| D(+)Glucosamine HCl | − | + | [56] |
| Hippuric acid (Na salt) | − | − | [56] |
| Lactic acid (Na salt) | − | + | [56] |
| Malonic acid (Na salt) | − | − | [56] |
| Maltose | − | − | [56] |
| Mannitol | − | + | [56] |
| Oxalic acid (Na salt) | − | − | [56] |
| 1,2-Propanediol | − | + | [56] |
| Pyruvic acid (Na salt) | −/+ | + | [56] |
| D(+)Raffinose | − | − | [56] |
| Rhamnose | − | + | [56] |
| Rhamnose | − | − | [15, 82] |
| Salicin | − | + | [56] |
| Sucrose | + | − | [56] |
| Tartaric acid (Na salt) | − | − | [56] |
| Trehalose | − | + | [56] |
| D(+)Turanose | − | − | [56] |
7. Chemotaxonomy
Glycolipids and Phospholipids. M. farcinogenes and M. senegalense have been found to contain trehalose dimycolate (cord factor), phosphatidylethanolamine, and phosphatidylinositol [4, 58]. Glycopeptidolipids (GPL), the so-called C-mycosides, have been found in some M. senegalense strains [16, 58]. Four groups of antigenic glycolipids have been detected in some M. senegalense strains [16]. The M. senegalense strains were considered to belong to two major groups. The first group which includes the majority of the strains as well as the type strain (NCTC 10956) has an alkali-stable glycopeptidolipids class of antigens [16, 59, 60]. The second group belongs to the alkali-labile acyl trehalose lipooligosaccharide class of antigens [16, 60]. The first group was found to share its properties with those described in M. peregrinum [16, 61] and M. porcinum [59], the same unusual distribution of the alaninol end of the molecules. These data reinforce the close taxonomic relationships between the three mycobacterial species and demonstrate the antigenicity of the new variants of mycobacterial glycopeptidolipids, whereas the second group has structures similar to those produced by the antigenic lipooligosaccharides of M. fortuitum [61, 62].
Mycolic acids. M. farcinogenes and M. senegalense strains contain mycolic acids that can be separated into α, α′, and epoxymycolates. Similar mycolic acid patterns are characteristic of M. chitae, M. fortuitum, M. peregrinum, M. smegmatis, and M. porcinum [63, 64]. Mycolic acids are B-hydroxy fatty acids substituted at the a-position with a moderately long aliphatic chain. The distribution of these molecules is restricted to strains in the suborder Corynebacterineae which include the genera Hoyosella, Amycolicicoccus, Corynebacterium, Dietzia, Gordonia, Hoyosella, Millisia, Mycobacterium, Nocardia, Rhodococcus, Segniliparus, Skermania, Smaragdicoccus, Tsukamurella, and Williamsia [65, 66]. These actinomycetes have an arabinogalactan-based cell wall type IV. The causal agents of bovine farcy can easily be discriminated from N. farcinica type strains on the basis of mycolic acid analysis. Nocardia species show a single mycolic acid spot on thin layer chromatography [50, 52, 67].
Members of M. farcinogenes and M. senegalense, like many other mycobacteria, undergo a characteristic cleavage reaction on pyrolysis gas chromatography. In addition to meroaldehyde, they release tetracosanoic acid (C24) as major ester fragment [52, 68]; some other mycobacteria, for example, M. tuberculosis, release hexacosanoic acids (C26).
El Sanousi and Tag El Din [51], using modified precipitation technique of Kanetsuna and Bartoli [69], were able to assign bovine farcy strains to the genus Mycobacterium. Later, Hamid et al. [16] developed a new effective mycolic acid precipitation method for the distinction between mycobacteria and other mycolic acid-containing taxa. The method was based on the precipitation of mycolic acids methyl esters in a mixture of acetonitrile and toluene (3 : 2, v/v). The method was proven to be useful, particularly to accommodate many isolates of bovine farcy to the genus Mycobacterium by giving copious white precipitate when acetonitrile and toluene were used to precipitate mycolic acid methyl esters.
8. Antigenicity and Immunogenicity
Awad and Karib [32] found that bovine farcy animals induced sensitivity to avian and mammalian tuberculins. This finding was later supported by Mostafa [70] who in addition used immunogens prepared from the causal agents of bovine farcy and it was found to give a profound reaction with high specificity and sensitivity than did the preparations from avian and mammalian strains. Magnusson and Mariat [71] have developed an immunological method based on the specificity of delayed-type skin reactions on guinea pigs for comparing Nocardia strains including isolates from cases of bovine farcy. The method noticeably differentiated bovine farcy strains, which formed a homogeneous group that was readily separated from reference (type) strains of N. asteroides, N. brasiliensis, and N. farcinica. Comparative immunodiffusion studies by Ridell and Norlin [72], Ridell [73–75], and Ridell et al. [50] indicated that the bovine farcy organisms had stronger affinity to mycobacteria than to Nocardia. From these studies, two serological groups were mainly identified, the first group includes the N. farcinica ATCC 3318, which seemed to belong to the genus Nocardia, whereas members of the other group including farcy strains were more closely related to Mycobacterium strains. The distribution of precipitinogens showed that M. farcinogenes and M. senegalense were closely related and were found to share a large number of precipitinogens. Both species shared visible precipitinogens with some other mycobacterial strains, particularly M. fortuitum, M. peregrinum, and M. smegmatis [75].
Using gel diffusion precipitin test and immunoelectrophoresis, Shigidi et al. [38] found that most of the strains isolated from cases of bovine farcy in Sudan reacted with antiserum from N. farcinica but not with antiserum from M. tuberculosis and M. bovis. In other studies, these strains were proved to be mycobacteria and were classified as M. farcinogenes ([16, 51]). In separate studies, when antigen prepared from whole cells of M. farcinogenes was tested against sera collected from animals infected with bovine farcy, only traces of agglutinin were detected [76] but limited, though sharp, precipitin lines were detected in most of the sera [52]. These two findings imply that circulating antibodies were also involved in the immunity and protection mechanism of infection with M. farcinogenes. Enzyme linked immunosorbent assay (ELISA) was evaluated for the serodiagnosis of bovine farcy among clinically proved cattle. Whole cell homologous suspension of M. farcinogenes was used as antigen and the test revealed a sensitivity of 92.7% and a specificity of 97% [77].
On the basis of antigenic cell surface glycolipids, M. senegalense strains were found to fall into four serological groups [16], whereas the majority of M. farcinogenes did not contain such antigens. The structure of the main group of M. senegalense, which included the type strain, was determined as glycopeptidolipids [59, 60]. These glycolipids were highly reactive to homologous sera prepared from whole cell M. senegalense and to lesser extent with heterologous sera from M. peregrinum [16, 78].
A wild strain of M. farcinogenes (A24) was subjected first to serial passage (20) in modified Sauton's broth then in guinea pigs. The result of the vaccination with the attenuated strain in calves revealed that 75% of calves in the vaccinated group were protected and endured the challenge infection with a virulent freshly isolated M. farcinogenes [79].
9. Molecular Analysis
DNA-DNA homology studies have indicated that M. farcinogenes and M. senegalense were separate species. According to Baess [13], M. senegalense is moderately related to M. farcinogenes, M. fortuitum, and to M. peregrinum. This fact has been further authenticated by Rogall et al. [19]. Using genus specific oligonucleotide, M. farcinogenes and M. senegalense reacted positively in the mycobacterial system [80].
Earlier sequencing of the 16S rRNA showed a close relationship between M. chelonae, M. farcinogenes, M. fortuitum, and M. senegalense [19, 21]. M. farcinogenes, M. senegalense, M. chelonae, M. fortuitum, and M. peregrinum form a distinct evolutionary branch within the adaptive radiation accommodated by the genus Mycobacterium [19–21]. These species form the rapidly growing nonchromogenic mycobacteria.
Consequent studies indicated the close phylogenetic relationship of farcy agents to members of the M. fortuitum complex [81]. With the appearance of new rapidly growing species, Adékambi and Drancourt [28] accommodated these into two subclusters: (i) M. peregrinum, M. septicum, M. neworleansense, and M. porcinum and (ii) M. farcinogenes, M. senegalense, M. houstonense, and M. fortuitum. These clustering and the subclusters agree with that of Schinsky et al. [25].
16S rRNA sequences of M. farcinogenes and M. senegalense are very similar and when using the Kirschner diagnostic helix 10 and helix 18 it is not possible to differentiate between the two species (Table 2). However, when using 16S-23S rDNA spacer, Hamid et al. [23] were able to distinguish between the two species with ample number of base substitution. Two probes designed on the basis of all of the available spacer sequences were evaluated for specificity, namely, biotin–3-TCAGCCAGCATCTGTAG and biotin–3-AGGAGTCTGTGCGCTGT, as probes for the rapid diagnosis of the disease from clinical specimens or for identification of unknown strains of M. farcinogenes or M. senegalense, respectively [23].
Table 2.
Comparison of 16S rDNA signature sequences. The alignment comprises the two variable regions found in the 16S rRNA genes of selected members of species closely related to M. farcinogenes and M. senegalense; “-” indicates identity.
| 16S rRNA position number 177 (E. coli position) | 16S rRNA position number 254 (E. coli position) | |
|---|---|---|
| M. fortuitum | C GAAT ATGACCAC GCGCTTCAT GGTGT | TTGGTGGGG TAATGGCCT AC |
| M. houstonense | –G– -G––– –––— –— | –––— –––— – |
| M. senegalense | –G– -G––– –––— –— | –––— –––— – |
| M. farcinogenes | –G– -G––– –––— –— | –––— –––— – |
| M. boenickei | –— -G––G- –T––– –G– | –––— –––— – |
| M. neworleansense | –— -G––G- –T––– –G– | –––— –––— – |
| M. porcinum | –— -G––G- –T––– –G– | –––— –––— – |
| M. septicum | –— –––G- –A––C- –— | –––— –––— – |
| M. peregrinum | –— –––G- –A––C- –— | –––— –––— – |
| M. alvei | –— ––—T –A––C- –— | –––— –––— – |
10. Antimicrobial Susceptibility
Most of the M. farcinogenes and M. senegalense strains tested in vitro were found susceptible to cycloserine [11], dapsone [15], or amikacin, doxycycline HCl (64 μg/mL), oxytetracycline HCl (64 μg/mL), and paromomycin sulphate (64 μg/mL) [55]. Susceptibilities to other antimicrobial agents and to various chemical agents are shown in Table 1.
11. Conclusions
Basic information about M. farcinogenes and M. senegalense is available in the literature. These actinomycetes are unique in their morphologies and exhibit some distinctive characteristics. These characteristics, notably, cell wall chemical markers and DNA sequence data, separate them from Nocardia farcinica and from closely related nonphotochromogenic rapidly growing mycobacteria. There are hardly any new reports of isolating these bacteria from cattle in recent times. On the contrary, limited numbers of reports have incriminated M. farcinogenes and M. senegalense as causal agents of human diseases.
Acknowledgments
The author is grateful to Professor M. Goodfellow (University of Newcastle), Professor S.M. El Sanousi, Professor M.T. Abu Samra (University of Khartoum), Professor Galal E. Al Azhari (Sudan University for Science and Technology), Professor D.E. Minnikin (University of Birmingham), Dr. A. Roth (Berlin Frei Universität), and Dr. Moh. Ahmed Hamád (Ministry of Animal Resources, Sudan) for their help and encouragement during previous bovine farcy research projects. These projects received supports from Alexander Humboldt Foundation, The Wellcome Trust (Grant no. 043935/Z/95/A), the International Foundation for Science (IFS; Grant no. B/2903), and the British Council (KHT/991/21/Vet).
Conflict of Interests
The author declares that he has no financial or personal relationships which may have inappropriately influenced him in writing this paper.
References
- 1.Hamid ME. Epidemiology, pathology, immunology and diagnosis of bovine farcy: a review. Preventive Veterinary Medicine. 2012;105(1-2):1–9. doi: 10.1016/j.prevetmed.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Nocard E. Note sur la maladie de bceufs de la Guadeloupe connue sous le nom de farcin. Annales de l’Institut Pasteur. 1888;2:293–302. [Google Scholar]
- 3.World Organization for Animal Health (OIE) Terrestrial Animal Health Code. Chapter 1.2. Criteria for listing diseases, Articles 1.2.1., 1.2.2. and 1.2.3., 2010, http://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/2010/en_chapitre_1.1.2.htm#chapitre_1.1.2.
- 4.Asselineau J, Laneelle MA, Chamoiseau G. De l’aetiologie du farcin de zebus tchadiens. Nocardiose ou mycobacteriose? II Composition lipidique. Revue d’Elevage et de Medicine Veterinaire des Pays Tropicaux. 1969;22:205–209. [PubMed] [Google Scholar]
- 5.Chamoiseau G. De l’etiologie du farcin de zebus tchadiens Nocardiose ou mycobacteriose? I Etude bacteriologique et biochemique. Revue d’Elevage et de Medicine Veterinaire des Pays Tropicaux. 1969;22:195–204. [PubMed] [Google Scholar]
- 6.Chamoiseau G. De I’etiologie du farcin de zebus tchadiens Nocardiens ou mycobacteriose? I Activite amidasique. Revue d’Elevage et de Medicine Veterinaire des Pays Tropicaux. 1972;25:191–194. [PubMed] [Google Scholar]
- 7.Chamoiseau G. Mycobacterium farcinogenes agent causal du farcin du boeuf en Afrique. Annales de Microbiologie (Institut Pasteur) 1973;124(2):215–222. [PubMed] [Google Scholar]
- 8.Chamoiseau G. Mycobacterium farcinogenes agent causal du farcin du boeuf en Afrique. Revue d’Elevage et de Medicine Veterinaire des Pays Tropicaux. 1974;32:135–141. [PubMed] [Google Scholar]
- 9.Chamoiseau G, Asselineau J. Examen des lipides d’une souche de N farcinica: presence d’acides mycoloques. Compte Rendu Hebdomadaire des Seances de l’Academie des Sciences D. 1970;270:2603–2604. [PubMed] [Google Scholar]
- 10.El Sanousi SM, Tag El Din MH, Abdel Wahab SM. Classification of the bovine farcy organism. Tropical Animal Health and Production. 1977;9(2):p. 124. doi: 10.1007/BF02236391. [DOI] [PubMed] [Google Scholar]
- 11.Chamoiseau G. Etiology of farcy in African bovines: nomenclature of the causal organisms Mycobacterium farcinogenes (Chamoiseau) and Mycobacterium senegalense (Chamoiseau) comb nov. International Journal of Systematic Bacteriology. 1979;29(4):407–410. [Google Scholar]
- 12.Wayne LG, Kubica GP. Genus Mycobacterium Lehmann and Neumann 1896 363 ALM. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey’s Manual of Systematic Bacteriology. Vol. 2. Baltimore, Md, USA: Williams & Wilkins; 1986. pp. 1436–1457. [Google Scholar]
- 13.Baess I. Deoxyribonucleic acid relatedness among species of rapidly growing mycobacteria. Acta Pathologica et Microbiologica Scandinavica B: Microbiology and Immunology. 1982;90(5):371–375. doi: 10.1111/j.1699-0463.1982.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall RM, Ratledge C. Equivalence of mycobactins from Mycobacterium senegalense, Mycobacterium farcinogenes and Mycobacterium fortuitum . Journal of General Microbiology. 1985;131(7):1691–1696. doi: 10.1099/00221287-131-7-1691. [DOI] [PubMed] [Google Scholar]
- 15.Ridell M, Goodfellow M. Numerical classification of Mycobacterium farcinogenes, Mycobacterium senegalense and related taxa. Journal of General Microbiology. 1983;129(3):599–611. doi: 10.1099/00221287-129-3-599. [DOI] [PubMed] [Google Scholar]
- 16.Hamid ME, Minnikin DE, Goodfellow M. A simple chemical test to distinguish mycobacteria from other mycolic-acid-containing actinomycetes. Journal of General Microbiology. 1993;139(9):2203–2213. doi: 10.1099/00221287-139-9-2203. [DOI] [PubMed] [Google Scholar]
- 17.Hamid ME, Chun J, Magee JG, Minnikin DE, Goodfellow M. Rapid characterisation and identification of mycobacteria using fluorogenic enzyme tests. Zentralblatt für Bakteriologie. 1994;280(4):476–487. doi: 10.1016/s0934-8840(11)80507-4. [DOI] [PubMed] [Google Scholar]
- 18.Magee JG, Goodfellow M, Sisson PR, Freeman R, Lightfoot NF. Differentiation of Mycobacterium senegalense from related non-chromogenic mycobacteria using pyrolysis mass spectrometry. Zentralblatt für Bakteriologie. 1997;285(2):278–284. doi: 10.1016/s0934-8840(97)80035-7. [DOI] [PubMed] [Google Scholar]
- 19.Rogall T, Wolters J, Flohr T, Bottger EC. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium . International Journal of Systematic Bacteriology. 1990;40(4):323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 20.Stahl DA, Urbance JW. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. Journal of Bacteriology. 1990;172(1):116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitulle C, Dorsch M, Kazda J, Wolters J, Stackebrandt E. Phylogeny of rapidly growing members of the genus Mycobacterium . International Journal of Systematic Bacteriology. 1992;42(3):337–343. doi: 10.1099/00207713-42-3-337. [DOI] [PubMed] [Google Scholar]
- 22.Kirschner P, Kiekenbeck M, Meissner D, Wolters J, Bottger EC. Genetic heterogeneity within Mycobacterium fortuitum complex species: genotypic criteria for identification. Journal of Clinical Microbiology. 1992;30(11):2772–2775. doi: 10.1128/jcm.30.11.2772-2775.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamid ME, Roth A, Landt O, Kroppenstedt RM, Goodfellow M, Mauch H. Differentiation between Mycobacterium farcinogenes and Mycobacterium senegalense strains based on 16S-23S ribosomal DNA internal transcribed spacer sequences. Journal of Clinical Microbiology. 2002;40(2):707–711. doi: 10.1128/JCM.40.2.707-711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown-Elliott BA, Wallace RJ., Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clinical Microbiology Reviews. 2002;15(4):716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schinsky MF, Morey RE, Steigerwalt AG, et al. Taxonomic variation in the Mycobacterium fortuitum third biovariant complex: description of Myobacterium boenickei sp. nov., Mycobacterium houstonense sp. nov., Mycobacterium neworleansense sp. nov. and Mycobacterium brisbanense sp. nov. and recognition of Mycobacterium porcinum from human clinical isolates. International Journal of Systematic and Evolutionary Microbiology. 2004;54(5):1653–1667. doi: 10.1099/ijs.0.02743-0. [DOI] [PubMed] [Google Scholar]
- 26.Mignard S, Flandrois J. A seven-gene, multilocus, genus-wide approach to the phylogeny of mycobacteria using supertrees. International Journal of Systematic and Evolutionary Microbiology. 2008;58(6):1432–1441. doi: 10.1099/ijs.0.65658-0. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamura M, Menoto H, Yugi H. Mycobacterium porcinum sp. nov., a porcine pathogen. International Journal of Systematic Bacteriology. 1983;33(2):162–165. [Google Scholar]
- 28.Adékambi T, Drancourt M. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. International Journal of Systematic and Evolutionary Microbiology. 2004;54(6):2095–2105. doi: 10.1099/ijs.0.63094-0. [DOI] [PubMed] [Google Scholar]
- 29.Diguimbaye-Djaibé C, Vincent V, Schelling E, et al. Species identification of non-tuberculous mycobacteria from humans and cattle of Chad. Schweizer Archiv für Tierheilkunde. 2006;148(5):251–256. doi: 10.1024/0036-7281.148.5.251. [DOI] [PubMed] [Google Scholar]
- 30.Guérin-Faublée V, Flandrois JP, Pichat C, Boschiroli ML, Lamy B. Mycobacterium bourgelatii sp. nov., a rapidly growing, non-chromogenic species isolated from the lymph nodes of cattle. International Journal of Systematic and Evolutionary Microbiology. 2013;63(part 12):4669–4674. doi: 10.1099/ijs.0.051979-0. [DOI] [PubMed] [Google Scholar]
- 31.Memery G, Mornet P, Camara A. Premiers cas authentiques de farcin du bceuf en Afrique occidentale franaise. Revue d’Elevage et de Medicine Veterinaire des Pays Tropicaux. 1958;11:11–16. [Google Scholar]
- 32.Awad FI, Karib AA. Studies on bovine farcy (nocardiosis) among cattle in the Sudan. Zentralblatt für Veterinärmedizin. 1958;5(3):265–272. [Google Scholar]
- 33.El-Nasri M. Some observation on bovine farcy. The Veterinary Record. 1961;73:370–372. [Google Scholar]
- 34.Mostafa IE. Studies on cattle nocardiosis (bovine farcy) in the Sudan. Sudan Journal of Veterinary Science and Animal Husbandry. 1962;7:1–9. [Google Scholar]
- 35.Mostafa IE. Studies of bovine farcy in the Sudan. II. Mycology of the disease. Journal of Comparative Pathology. 1967;77(2):231–237. doi: 10.1016/0021-9975(67)90015-1. [DOI] [PubMed] [Google Scholar]
- 36.Perpezat A, Destombes P, Mariat F. Histopathologic study of nocardiosis in oxen in Chad and biochemical characteristics of Nocardia farcinica . Revue d’Elevage et de Medicine Veterinaire des Pays Tropicaux. 1967;20(3):429–435. [PubMed] [Google Scholar]
- 37.El-Kareem MHA, Moustafa AA. Bovine nocardiosis, tuberculosis and other caseous infections at Omdurman central abattoir. Sudan Journal of Veterinary Science and Animal Husbandry. 1974;15:57–60. [Google Scholar]
- 38.Shigidi MTA, Mirghani T, Musa MT. Characterisation of Nocardia farcinica isolated from cattle with bovine farcy. Research in Veterinary Science. 1980;28(2):207–211. [PubMed] [Google Scholar]
- 39.Oyekunle MA, Ojo MO. Preliminary observations on bovine cutaneous nocardiosis and dermatophilosis in the subhumid climate of southern Nigeria. Revue d’Elevage et de Medecine Veterinaire des Pays Tropicaux. 1988;41(4):347–351. [PubMed] [Google Scholar]
- 40.Marchot PH, Amanfu W, Leroy PL. Bovine farcy in the Accra plains of Ghana. Revue d’Elevage et de Medecine Veterinaire des Pays Tropicaux. 1989;42(2):173–175. [PubMed] [Google Scholar]
- 41.Wallace RJ, Jr., Brown-Elliott BA, Brown J, et al. Polyphasic characterization reveals that the human pathogen Mycobacterium peregrinum type II belongs to the bovine pathogen species Mycobacterium senegalense . Journal of Clinical Microbiology. 2005;43(12):5925–5935. doi: 10.1128/JCM.43.12.5925-5935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong TC, Chan WF, Tsang WL, Yeung SH, Ip FK. Mycobacterium farcinogenes infection after total hip arthroplasty. Journal of Arthroplasty. 2005;20(5):684–687. doi: 10.1016/j.arth.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Mostafa IE. Bovine nocardiosis (cattle farcy): a review. The Veterinary Bulletin. 1966;36:189–193. [Google Scholar]
- 44.Olubunmi PA, Ayeni AO. A description of an outbreak of bovine nocardiosis in Western Nigeria. Journal of Animal Production Research. 1983;3:127–138. [Google Scholar]
- 45.Hamid ME. Identification of bovine farcy by simple lipid analysis and rapid biochemical tests [M.S. thesis] Khartoum, Sudan: University of Khartoum; 1988. [Google Scholar]
- 46.Al Janabi BM, Branagan D, Danskin D. The trans stadial transmission of the bovine farcy organism, Nocardia farcinica, by the ixodid Amblyomma variegatum (Fabricius, 1794) Tropical Animal Health and Production. 1975;7(4):205–209. [Google Scholar]
- 47.Cowan ST, Steel KJ. Cowan and Steel’s Manual for the Identification of Medical Bacteria. New York, NY, USA: Cambridge University Press; 1974. [Google Scholar]
- 48.Gordon RE, Mihm JM. Identification of Nocardia caviae (Erikson) nov comb. Annals of the New York Academy of Sciences. 1962;98:628–636. [Google Scholar]
- 49.Mordarska H, Mordarski M, Goodfellow M. Chemotaxonomic characters and classification of some nocardioform bacteria. Journal of General Microbiology. 1972;71(1):77–86. doi: 10.1099/00221287-71-1-77. [DOI] [PubMed] [Google Scholar]
- 50.Ridell M, Goodfellow M, Minnikin DE, Minnikin SM, Hutchinson IG. Classification of Mycobacterium farcinogenes and Mycobacterium senegalense by immunodiffusion and thin-layer chromatography of long-chain components. Journal of General Microbiology. 1982;128(6):1299–1307. doi: 10.1099/00221287-128-6-1299. [DOI] [PubMed] [Google Scholar]
- 51.El Sanousi SM, Tag El Din MH. On the aetiology of bovine farcy in the Sudan. Journal of General Microbiology. 1986;132(6):1673–1675. doi: 10.1099/00221287-132-6-1673. [DOI] [PubMed] [Google Scholar]
- 52.Hamid ME, Mohamed GE, Abu-Samra MT, El-Sanousi SM, Barri ME. Bovine farcy: a clinico-pathological study of the disease and its aetiological agent. Journal of Comparative Pathology. 1991;105(3):287–301. doi: 10.1016/s0021-9975(08)80197-1. [DOI] [PubMed] [Google Scholar]
- 53.Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) Catalogue Microorganisms. Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH; 2012. http://www.dsmz.de/catalogues/details/culture/DSM. [Google Scholar]
- 54.Hamid ME, Goodfellow M. Rapid growth and increased biomass yield of Mycobacterium farcinogenes and some related taxa in broth and agar media. Zentralblatt für Veterinärmedizin B. 1995;42(7):397–404. doi: 10.1111/j.1439-0450.1995.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 55.Hamid ME, Goodfellow M. In vitro antimicrobial susceptibility of bovine farcy organisms. Revue d’Elevage et de Medicine Veterinaire des Pays Tropicaux. 1997;50:5–9. [Google Scholar]
- 56.Hamid ME. Classification and identification of actinomycetes associated with bovine farcy [Ph.D. thesis] Tyne and Wear, UK: University of Newcastle; 1994. [Google Scholar]
- 57.Wellington EMH, Williams ST. Preservation of actinomycete inoculum in frozen glycerol. Microbios Letters. 1977;6(23-24):151–157. [Google Scholar]
- 58.Laneelle G, Asselineau J, Chamoiseau G. Presence de mycosides C′ (formes simplifiees de mycoside C) dans les bacteries isolees de bovins atteints du farcin. FEBS Letters. 1971;19(2):109–111. doi: 10.1016/0014-5793(71)80490-8. [DOI] [PubMed] [Google Scholar]
- 59.Marin LML, Laneelle M, Prome D, Daffe M. Structures of the glycopeptidolipid antigens of two animal pathogens: Mycobacterium senegalense and Mycobacterium porcinum . European Journal of Biochemistry. 1993;215(3):859–866. doi: 10.1111/j.1432-1033.1993.tb18103.x. [DOI] [PubMed] [Google Scholar]
- 60.Besra GS, Gurcha SS, Khoo K-H, et al. Characterization of the specific antigenicity of representatives of M. senegalense and related bacteria. Zentralblatt für Bakteriologie. 1994;281(4):415–432. doi: 10.1016/s0934-8840(11)80328-2. [DOI] [PubMed] [Google Scholar]
- 61.Lopez Marin LM, Laneelle MA, Prome D, Daffe M, Laneelle G, Prome J-C. Glycopeptidolipids from Mycobacterium fortuitum. A variant in the structure of C-mycosides. Biochemistry. 1991;30(43):10536–10542. doi: 10.1021/bi00107a024. [DOI] [PubMed] [Google Scholar]
- 62.Besra GS, McNeil MR, Brennan PJ. Characterization of the specific antigenicity of Mycobacterium fortuitum . Biochemistry. 1992;31(28):6504–6509. doi: 10.1021/bi00143a021. [DOI] [PubMed] [Google Scholar]
- 63.Minnikin DE, Minnikin SM, Parlett JH, Goodfellow M. Mycolic acid patterns of some rapidly-growing species of Mycobacterium . Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene A. 1985;259(4):446–460. doi: 10.1016/s0176-6724(85)80076-6. [DOI] [PubMed] [Google Scholar]
- 64.Luquin M, Ausina V, Lopez-Calahorra F, et al. Evaluation of practical chromatographic procedures for identification of clinical isolates of mycobacteria. Journal of Clinical Microbiology. 1991;29(1):120–130. doi: 10.1128/jcm.29.1.120-130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goodfellow M, Maldonado LA. The families Dietziaceae, Gordoniaceae, Nocardiaceae and Tsukamurellaceae. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The Prokaryotes. 3rd edition. Vol. 3. New York, NY, USA: Springer; 2006. pp. 843–888. (Archaea and Bacteria: Firmicutes, Actinomycetes). [Google Scholar]
- 66.Lanéelle MA, Launay A, Spina L, et al. A novel mycolic acid species defines two novel genera of the Actinobacteria, Hoyosella and Amycolicicoccus . Microbiology. 2012;158(part 3):843–855. doi: 10.1099/mic.0.055509-0. [DOI] [PubMed] [Google Scholar]
- 67.Minnikin DE, Alshamaony L, Goodfellow M. Differentiation of Mycobacterium, Nocardia and related taxa by thin layer chromatographic analysis of whole organism methanolysates. Journal of General Microbiology. 1975;88(1):200–204. doi: 10.1099/00221287-88-1-200. [DOI] [PubMed] [Google Scholar]
- 68.Lechevalier MP, Horan AC, Lechevalier HA. Lipid composition in the classification of nocardiae and mycobacteria. Journal of Bacteriology. 1971;105(1):313–318. doi: 10.1128/jb.105.1.313-318.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanetsuna F, Bartoli A. A simple chemical method to differentiate Mycobacterium from Nocardia . Journal of General Microbiology. 1972;70(2):209–212. doi: 10.1099/00221287-70-2-209. [DOI] [PubMed] [Google Scholar]
- 70.Mostafa IE. The relationship between bovine farcy and tuberculosis. The Veterinary Record. 1967;81(3):74–76. doi: 10.1136/vr.81.3.74. [DOI] [PubMed] [Google Scholar]
- 71.Magnusson M, Mariat F. Delineation of Nocardia farcinica by delayed type skin reactions on guinea pigs. Journal of General Microbiology. 1968;51(1):151–158. doi: 10.1099/00221287-51-1-151. [DOI] [PubMed] [Google Scholar]
- 72.Ridell M, Norlin M. Serological study of Nocardia by using mycobacterial precipitation reference systems. Journal of Bacteriology. 1973;113(1):1–7. doi: 10.1128/jb.113.1.1-7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ridell M. Taxonomic study of Nocardia farcinica using serological and physiological characters. International Journal of Systematic Bacteriology. 1975;25(2):124–132. [Google Scholar]
- 74.Ridell M. Immunodiffusion analysis of Mycobacterium farcinogenes, Mycobacterium senegalense and some other mycobacteria. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1981;(supplement 2):235–241. [Google Scholar]
- 75.Ridell M. Immunodiffusion analyses of Mycobacterium farcinogenes, Mycobacterium senegalense and some other mycobacteria. Journal of General Microbiology. 1983;129(3):613–619. doi: 10.1099/00221287-129-3-613. [DOI] [PubMed] [Google Scholar]
- 76.El Sanousi SM, Salih MA, Mousa MT, Tag El Din MH, Ali SA. Further studies on the properties of the aetiology of bovine farcy isolated from Sudanese cattle. Revue d’Elevage et de Medecine Veterinaire des Pays Tropicaux. 1979;32(2):135–141. [PubMed] [Google Scholar]
- 77.El Hussein HA, Hamid ME. Evaluation of ELISA in the serodiagnosis of bovine farcy. Tropical Animal Health and Production. 2009;41(4):617–622. doi: 10.1007/s11250-008-9232-4. [DOI] [PubMed] [Google Scholar]
- 78.Hamid ME, Ridell M, Minnikin DE, Goodfellow M. Serotaxonomic analysis of glycolipids from Mycobacterium chelonae-M. fortuitum complex and bovine farcy strains. Zentralblatt für Bakteriologie. 1998;288(1):23–34. doi: 10.1016/s0934-8840(98)80094-7. [DOI] [PubMed] [Google Scholar]
- 79.Mukhtar EAM, Alwali AA, El Eragi AMS, Hamid ME, El Hussein HA. Attenuated Mycobacterium farcinogenes strain A24 as a protective vaccine candidate against bovine farcy. Journal of Animal and Veterinary Advances. 2005;4:269–273. [Google Scholar]
- 80.Boddinghaus B, Rogall T, Flohr T, Blocker H, Bottger EC. Detection and identification of mycobacteria by amplification of rRNA. Journal of Clinical Microbiology. 1990;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirschner P, Springer B, Vogel U, et al. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. Journal of Clinical Microbiology. 1993;31(11):2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ridell M, Goodfellow M, Abdulle MA. Identification of actinomycetes isolated from cases of bovine farcy in the Sudan. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene A. 1985;259(1):43–50. doi: 10.1016/s0176-6724(85)80006-7. [DOI] [PubMed] [Google Scholar]


