Abstract
Background
GWAS have consistently revealed that LDLR locus variability influences LDL-cholesterol in general population. Severe LDLR mutations are responsible for familial hypercholesterolemia (FH). However, most primary hypercholesterolemias are polygenic diseases. Although Cis-regulatory regions might be the cause of LDL-cholesterol variability; an extensive analysis of the LDLR distal promoter has not yet been performed. We hypothesized that genetic variants in this region are responsible for the LDLR association with LDL-cholesterol found in GWAS.
Methods
Four-hundred seventy-seven unrelated subjects with polygenic hypercholesterolemia (PH) and without causative FH-mutations and 525 normolipemic subjects were selected. A 3103 pb from LDLR (-625 to +2468) was sequenced in 125 subjects with PH. All subjects were genotyped for 4 SNPs (rs17242346, rs17242739, rs17248720 and rs17249120) predicted to be potentially involved in transcription regulation by in silico analysis. EMSA and luciferase assays were carried out for the rs17248720 variant. Multivariable linear regression analysis using LDL-cholesterol levels as the dependent variable were done in order to find out the variables that were independently associated with LDL-cholesterol.
Results
The sequencing of the 125 PH subjects did not show variants with minor allele frequency ≥ 10%. The T-allele from g.3131C > T (rs17248720) had frequencies of 9% (PH) and 16.4% (normolipemic), p < 0.00001. Studies of this variant with EMSA and luciferase assays showed a higher affinity for transcription factors and an increase of 2.5 times in LDLR transcriptional activity (T-allele vs C-allele). At multivariate analysis, this polymorphism with the lipoprotein(a) and age explained ≈ 10% of LDL-cholesterol variability.
Conclusion
Our results suggest that the T-allele at the g.3131 T > C SNP is associated with LDL-cholesterol levels, and explains part of the LDL-cholesterol variability. As a plausible cause, the T-allele produces an increase in LDLR transcriptional activity and lower LDL-cholesterol levels.
Keywords: LDL-cholesterol, Hypercholesterolemia, Variant, Gene regulation, Polygenic, LDLR
Background
Hypercholesterolemia due to high plasma levels of low-density lipoprotein cholesterol (LDL-C) is the main risk factor for cardiovascular diseases. The individual concentration of LDL-C depends on the interplay between genetic and environmental factors. Severe primary hypercholesterolemias are often monogenic disorders, such as familial hypercholesterolemia (FH) associated with mutations in coding regions of LDLR, APOB, or PCSK9 genes [1-4]. However, no functional defects are detected in these genes in most subjects with primary hypercholesterolemia, albeit, in comparison with FH, a milder phenotype and lower family penetrance are usually present. Actually, most of these subjects receive a diagnosis of polygenic hypercholesterolemia (PH), and it is believed that common genetic variants in the population are responsible for this phenotype [5]. It has been recently published that a substantial number of subjects with FH without a known mutation have increased the number of polymorphisms associated with higher LDL-C levels, therefore they might have a polygenic cause [6,7].
Several Genome Wide Association Studies (GWAS) carried out during the past decade have revealed that variability at the LDLR locus is associated with LDL-C levels [8-11]. These GWAS clearly signal the LDLR as a candidate gene for PH. As an example, the minor allele of the rs6511720 polymorphism in LDLR was associated with a reduction of 6.99 mg/dl in total cholesterol levels [12] and this variant has been included in the LDL-C gene score proposed by Talmud, however its functionality is still unknown [6].
Cis-regulatory sequences control when, where, and with what intensity genes are expressed [13]. Genetic variation in these regions that influence gene expression are widespread in the human genome and are responsible for most of the inter-individual variability of normal phenotypes, but also for complex and polygenic diseases [14-18]. Various rare mutations in the promoter region of LDLR have been characterized in subjects with FH [19-22]. However, whether or not common genetic variants in the regulatory region of LDLR are associated with non-FH primary hypercholesterolemia had not been studied previously and is the hypothesis of the present study.
Methods
Study subjects
The PH group was composed of 477 unrelated subjects recruited at two lipid clinics and a large car factory involved in a prospective study of cardiovascular health named Aragon Workers’ Health Study (AWHS). The three centers are located in north-eastern Spain, and the population groups involved share the same genetic background. Participants were selected according to the following criteria, absence of prior cardiovascular disease, LDL-C ≥ 90th percentile of a Spanish reference population [23], triglyceride levels ≤ 200 mg/dl, BMI < 30 kg/m2, and no mutations in the LDLR, APOB or PCSK9 genes. Secondary causes of hypercholesterolemia were excluded in all subjects by clinical history and blood tests. A normolipemic group of 525 unrelated healthy individuals was selected from the AWHS with LDL-C ≤ 90th percentile and TG ≤ 200 mg/dl. Exclusion criteria for controls were the same as those for PH cases, except that they were not tested for FH-causing mutations and had not received prior treatment with hypolipidemic drugs. All subjects provided written consent to a protocol approved by the ethical review boards of the two participating hospitals and the Comité Ético de Investigación Clínica de Aragón, CEICA, for AWHS subjects.
Clinical and laboratory characteristics
All subjects were assessed for family history of early-onset coronary heart disease, clinical history, medication use, demographic characteristics, adiposity measures, and cardiovascular risk factors. Fasting blood for biochemical profiles was drawn after ≥ 4 weeks without hypolipidemic drug treatment.
Subjects were categorized as never smokers and current or former smokers. Hypertension was defined as systolic Blood Pressure (BP) ≥ 140 mm Hg, diastolic BP ≥ 90 mm Hg, or current use of antihypertensive medication. Diabetes was defined as a fasting glucose level ≥ 126 mg/dL on at least two occasions or treatment with anti-diabetic agents.
Blood glucose, High-density lipoprotein cholesterol (HDL-C), cholesterol and triglyceride levels were determined by standard methods. LDL-C was estimated as total cholesterol – HDL-C–TG/5. Apolipoprotein (apo) B and lipoprotein(a) were determined in the lipid clinics by using immunoturbidimetry (Unimate 3, Roche, Basel, Switzerland) and by the nephelometry analyzer IMMAGE 800 (Beckman Coulter, USA) in AWHS subjects.
Genetic analyses
DNA was isolated from EDTA blood samples following standard procedures. A molecular diagnosis of FH was ruled out in all hypercholesterolemic subjects from the lipid clinics by using the LIPOchip® genetic diagnostic platform [24]. The LIPOchip® platform (v3 to v9.1) contains the most frequent Spanish mutations and CNVs in LDLR, APOB and 4 in PCSK9. According to the LIPOchip® protocol, the coding sequences, the intron-exon boundaries as well as the promoter of the LDLR and part of the APOB exon 26 were sequenced in those individuals with negative results.
The variability of the regulatory region of the LDLR gene was studied in 125 PH subjects. To this end, a 3.103 Kb fragment of the LDLR gene [ENSG:00000130164], including the promoter (from -625 to +2478, considering the A nucleotide from ATG as +1) was sequenced (Additional file 1: Table S1).
In order to predict possible transcription changes due to the presence of a variant, known SNPs located in the 3 Kb fragment upstream of the transcription initiation site were analyzed with both MatInspector and CHIP Mapper [25]. These in silico analyses reported four SNPs, namely rs17242346, rs17242739, rs17248720 and rs17249120, potentially involved in the LDLR regulation. We genotyped these polymorphisms, located in the LDLR 5’ promoter, by iPlex technology based on a MassARRAY® platform (Sequenom®, Valencia, Spain) in all the PH (n = 477) and control (n = 525) subjects. The reproducibility of this method was confirmed by obtaining consistent genotypes in 16 replicated samples.
Electrophoretic mobility shift assays
Preparation of nuclear extracts from sub-confluent HepG2 cell cultures and Electrophoretic Mobility Shift Assays (EMSAs) were done as reported by Riancho et al. [26]. The gel bands were analyzed in an ODYSSEY infrared imaging system (Li-Cor Biosciences, Lincoln, NE). The relative affinity of each allele was analyzed by competing it with increasing amounts of the unlabelled allele that had shown stronger binding. The bands were quantified and the affinities were calculated by representing the inverse of band intensity versus the excess of unlabelled oligonucleotide. A higher slope indicates lower oligonucleotide-protein affinity. The experiment was performed in triplicate to confirm results.
Plasmid construct
A fragment of 2457 bp of the LDLR promoter from c.-2,335 to c.67 + 36 was amplified by Polymerase Chain Reaction (PCR) from the DNA of individuals carrying the g.3131C > T (rs17248720) variants in homozygosis, g.3131C and g.3131 T alleles. The oligonucleotides introduce a SacI and NheI restriction site on the 5’ and 3’ end of the amplified fragment, respectively. The PCR product was digested with SacI and NheI and cloned into the digested luciferase reporter gene vector pGL3-Basic (Promega, Madison, WI). The identity of the LDLR promoter within the cloned DNA fragment, the C or T variant allele, and the absence of other polymorphisms or mutations were verified by sequencing of the resulting plasmid.
Cell culture and transient transfection
HepG2 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin and transfected with a jetPEI™-Hepatocyte. As a positive control and for normalization pGL3-Basic and pRL-TK plasmids (Promega, Madison, WI) were used. In the first experiment we added either g.3131C_LDLR-pGL3 or g.3131T_LDLR-pGL3. Cells were processed and Luciferase assays performed as previously described in De Castro-Orós et al. [21].
Statistical analyses
Data are presented as means ± Standard Deviation (SD) for continuous variables (medians and interquartile ranges for variables with a skewed distribution) and as frequencies or percentages for categorical variables. Differences in mean values were assessed by using unpaired t-tests or the Mann–Whitney U-test, as appropriate. Categorical variables were compared using χ2 test. Genotype prevalence was estimated as observed proportions. First, all subjects were distributed into quintiles according to the LDL-C levels; these groups were composed of 199 individuals. Second, the quintile distribution was made in control subjects and in each group 105 subjects were included. Quintiles distribution of T-carriers and T-allele frequency for rs17248720 SNP in the LDLR gene were calculated in control subjects according to LDL-C levels, p-value was calculated by Chi-square test corrected by Bonferroni. Bivariate logistic regression analysis was used to determine the odds ratios (OR) and 95% confidence intervals (CI) of LDLR genotypes in cases versus controls. Regression coefficients (β) and multivariate logistic regression with the forward selection based on likelihood ratios was used to examine the effect of LDLR genotypes on LDL-C. Variables shown in Table 1 showing P values < 0.20 in the bivariate analysis were introduced in the model. All analyses were performed with the Statistical Package for the Social Sciences software, version 16.0 (SPSS Inc., Chicago, IL), with significance set at P < 0.05.
Table 1.
Characteristics of the hypercholesterolemic and normolipemic groups
| Polygenic hypercholesterolemia (n=477) | Normolipemics (n=525) | P value* | |
|---|---|---|---|
|
Men (%) |
57.4 |
90.9 |
< 0.001 |
|
Age (years) |
51 (41-56) |
51 (42-54) |
0.004 |
|
BMI (kg/m
2
) |
26 (24-28) |
27 (25-29) |
< 0.001 |
|
Waist circumference (cm) |
92 (84-98) |
95 (88-101) |
< 0.001 |
|
Smoking status (%) |
|
|
0.019 |
| Active smokers |
24.8 |
37.3 |
|
| Non-smokers |
49.9 |
37.0 |
|
| Past smokers |
25.3 |
25.7 |
|
|
Hypertension (%) |
20.7 |
20.2 |
0.885 |
|
Laboratory variables (mg/dL) |
|
|
|
| Fasting glucose |
91 (84-98) |
94 (87-102) |
< 0.001 |
| Total cholesterol |
290 ± 42 |
202 ± 32 |
< 0.001 |
| Triglycerides |
108 (83-137) |
100 (72-136) |
0.019 |
| HDL-cholesterol |
53 (46-65) |
53 (47-61) |
0.026 |
| LDL-cholesterol |
207 (186-231) |
127 (107-147) |
< 0.001 |
| Lipoprotein(a) |
29 (13-63) |
19 (8-51) |
< 0.001 |
| Apolipoprotein B | 148 ± 27 | 97 ± 22 | < 0.001 |
Data shown for quantitative variable when normal distribution are means ± SD or median and interquartile range for variables with non-normal distribution. Data shown for qualitative variable are percentages. *Calculated by unpaired t-test or Mann–Whitney U–test, as appropriate.
Results
Table 1 shows the clinical and biochemical characteristics of study subjects. By study design, PH subjects disclosed higher levels of total cholesterol, LDL-C, and apo B than controls, while triglycerides were similar between groups. The sequencing of 3.103 Kb of the relevant LDLR promoter region was carried out in 125 PH subjects and only six (rs12981050, rs17242353, rs57217136, rs59281581, rs60173709 and rs6511720) appeared with frequencies for the minor allele between 0.012 and 0.085 (Additional file 2: Table S2).
We also analyzed four polymorphisms revealed by prediction software [25,27] to be located in potential regulatory elements in the distal promoter. The minor allele frequencies of these variants are shown in Table 2. All the analyzed polymorphisms were compatible with Hardy-Weinberg expectations. The analyzed SNPs rs17242346, rs17242739 and rs17249120 presented a minor allele frequency below 10% and only the minor allele of the rs17242346 was more frequent in the control group. One of the four variants, rs17248720, showed highly significant (p < 0.00001) allelic distribution differences between the PH and normolipemic populations. In fact, when the frequency was calculated separately within the three hypercholesterolemic populations, all showed minor allele frequencies significantly lower than those in the control group.
Table 2.
Polymorphisms analyzed in PH and normolipemic populations by iPlex technology
| |
Polygenic hypercholesterolemia |
Controls |
|
|||
|---|---|---|---|---|---|---|
| Polymorphisms | Zaragoza N=252 | Barcelona N=127 | AWHS N=98 | Total N=477 | AWHS N=525 | P value |
|
rs17242346 (g.3724G>A) |
0.107 |
0.071 |
0.122 |
0.100 |
0.080 |
0.113 |
|
rs17242739 (g.3627C>A) |
0.000 |
0.008 |
0.000 |
0.000 |
0.000 |
0.280 |
|
rs17248720 (g.3131C>T) |
0.090 |
0.083 |
0.102 |
0.090 |
0.160 |
< 0.0001 |
| rs17249120 (g.4440G>A) | 0.004 | 0.000 | 0.020 | 0.003 | 0.010 | 0.082 |
AWHS, Aragon Workers’ Health Study. Zaragoza, Barcelona and AWHS were the three hypercholesterolemic populations analyzed. Variables were compared using χ2 test. Genotype prevalences were estimated as observed proportions.
When control groups were distributed in quintiles (Q) according to LDL-C levels the Q2 showed more T-allele carriers than the Q3, Q4 and Q5 (0.371 > 0.352 > 0.267 > 0.200) (p < 0.05) (Table 3). When all study subjects were distributed in quintiles we observed that this frequency was higher in Q3 than Q4 and Q5 with an almost significant p-value (Additional file 3: Table S3).
Table 3.
LDL cholesterol quintile distribution of frequencies of genotypes CT and TT and T-allele frequency for rs17248720 polymorphism in LDLR gene in Control subjects
| Q1 | Q2 | Q3 | Q4 | Q5 | p | |
|---|---|---|---|---|---|---|
|
LDL-C |
86 (77.5-95.0) |
112 (107-116) |
127 (124-131) |
143 (139-147) |
163 (155-168) |
|
|
(mg/dl) | ||||||
|
CT+TT genotype |
0.324 |
0.371 |
0.352 |
0.267 |
0.200 |
0.046 |
| T-allele frequency | 0.176 | 0.195 | 0.195 | 0.143 | 0.109 |
LDL-C, LDL cholesterol measured by median and interquartiles. p: p-value was calculated by Chi-square test and corrected by Bonferroni.
Table 4 shows the results of multivariable linear regression analysis using LDL-C levels as the dependent variable. The variables that were independently associated with LDL-C were lipoprotein(a) concentration, LDLR g.3131C > T, and age, in this order, explaining together 10% of its variability in men. The results were similar when all study subjects were considered and did not substantially differ when triglycerides, HDL-C, fasting glucose, and waist circumference were forced into the model. The age- and gender-adjusted OR of disclosing PH for subjects bearing the LDLR g.3131 T allele was 0.478 (IC 0.342-0.667, p < 0.001).
Table 4.
Variables independently associated with LDL-cholesterol concentration by multivariate lineal regression analysis*
| Variables | Standardized coefficient (β) | P | Adjusted R 2 |
|---|---|---|---|
| Men |
|
|
|
| Lipoprotein(a), mg/dL |
0.190 |
< 0.001 |
0.039 |
|
LDLR g.3131C > T, yes/no |
-0.179 |
< 0.001 |
0.070 |
| Age, years |
0.177 |
< 0.001 |
0.100 |
| All study subjects |
|
|
|
| Age, years |
0.236 |
< 0.001 |
0.054 |
| Lipoprotein(a), mg/dL |
0.172 |
< 0.001 |
0.083 |
| LDLR g.3131C > T, yes/no | -0.146 | < 0.001 | 0.103 |
*Variables allowed to enter the model were those with P values < 0.20 in the bivariate analysis.
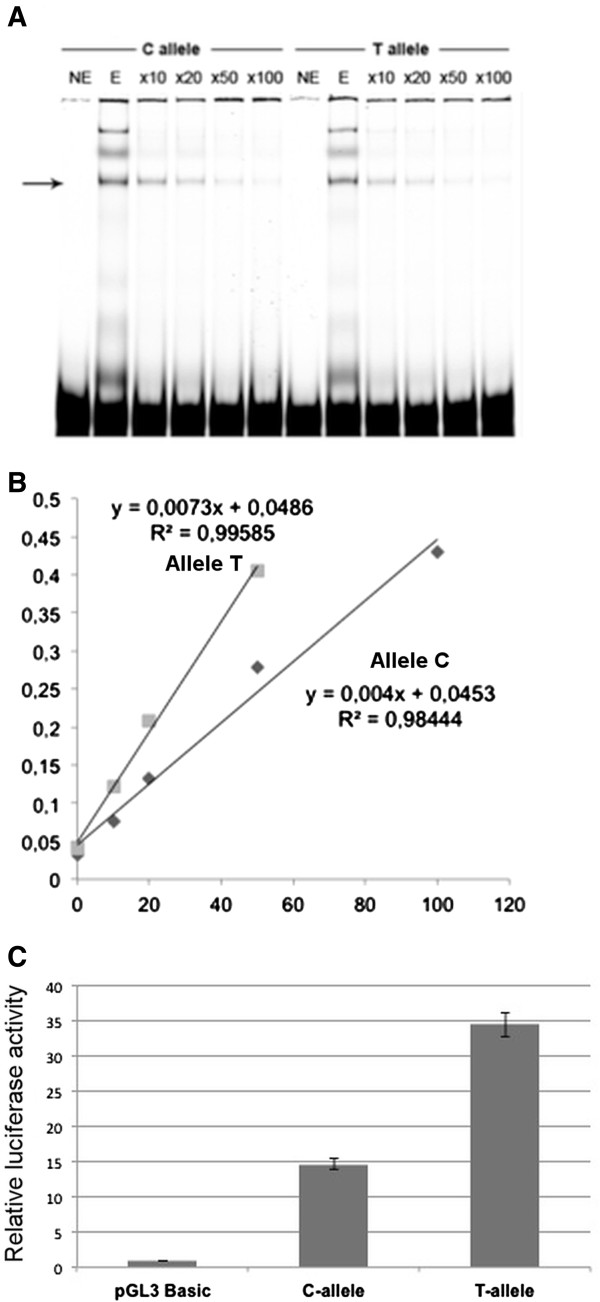
To find out whether the C to T change alters the ability of the polymorphism region to bind transcription factors, we performed an EMSA using oligonucleotides specific for each variant. The results (Figure 1A) showed noticeable differences in affinity for nuclear proteins between the two alleles. After the binding was competed with increasing amounts of an unlabelled oligonucleotide corresponding to allele C, the inverse of the intensity of the major band was plotted against the excess of unlabelled oligonucleotide. The result (Figure 1B) indicated that the T allele was easily displaced from the protein-DNA complex (slope 0.0073 vs 0.004).
Figure 1.
Functional assays for g.3131C > T SNP (rs17248720). A) EMSA assay carried out with probes containing the C or T allele for the g.3131 SNP (rs17248720) in the LDLR gene. NE, no HepG2 extract added. E, nuclear extract from HepG2 was added, ×10, ×20, ×50 and ×100 mean 10, 20, 50 and 100 times excess of unlabelled C-allele oligonucleotide. B) The inverse of band densities from the EMSA shown in Figure 1A were plotted against the excess of unlabelled allele C oligonucleotide. C) Influence of g.3131C > T (rs17248720) variant on LDLR promoter strength in transient transfection assay in FBS supplemented media. Fragments containing, none, either C or T allele were cloned in the promoterless plasmid pGL3-Basic and transfected to FBS supplemented media cultures of HepG2. Measurements of luciferase activity were assayed in cell extracts 36 h after transfection. The results were expressed as the media of three experiments carried out in triplicate considering pGL3-Basic expression as value 1.
In order to test if the different affinity between variants influences the transcription of the LDLR gene, two constructs carrying both variants of SNP g.3131C > T were transfected in HepG2 cells. In comparison with cells transfected with the g.3131C allele, those having the g.3131 T construct showed a significant increase of 2.5 times (p < 0.01) of transcriptional activity (Figure 1C).
Discussion
In this study we used the novel approach of searching for common polymorphisms located in the 5’UTR region of the LDLR that might be involved in the pathogenesis of primary hypercholesterolemia because of their possible influence in the transcription of the LDLR gene. The major conclusions drawn from this study are that, the LDLR g.3131C > T polymorphism in the 5’ region of the LDLR is responsible for a major effect on LDL-C concentration because of the increase in the transcriptional activity of the LDLR gene, and also indicates that the LDLR variation is associated with PH.
It has been shown that 58.9% of the variants involved in regulation changes are located within the first 500 bp upstream of the transcription start site, but 12.8% are more than 1 kb upstream and show transcription factor switching, with each allele having a higher affinity for a different transcription factor [15,28]. As the LDLR promoter is well-conserved in most of its positions among chimps and humans, the sequencing of a 3103 pb fragment, from -625 to +2478 of the ENSG00000130164 reference, showed only 6 variants, all located within the first and second introns of the LDLR gene and with ≤ 8.5% minor allele frequency.
Simultaneously to the sequencing of the LDLR promoter we analyzed four polymorphisms that appeared to be relevant in the regulation of LDLR expression according to the bioinformatics analysis. We found that only the variant g.3131C > T had a different distribution of genotypes between a control group of 525 individuals carefully selected among a working population of 1137 subjects and a group of 477 hypercholesterolemic individuals without known FH-causing mutations (p < 0.00001). A lower frequency of the minor T allele of this SNP was observed in the total hypercholesterolemic population and also in each of the three subgroups taken separately. Surprisingly, the T-allele is also the ancestral one, as it has been observed by sequence comparison between 20 eutherian mammals, and, according to our results, this allele would have a protective effect against hypercholesterolemia.
The g.3131C > T is placed in a large linkage disequilibrium (LD) block. In this work we had sequenced this LD region in 125 subjects, including the rs6511720 SNP, and we have calculated the LD between these SNPs (r2 = 0.211 and D’ = 0.487) not observing an association in this group.
A limitation to the study would be the diagnosis with the LIPOchip® in the PH subjects as non-carriers of FH causal mutations. It would be remotely possible to have missed a variant located in a region not sequenced with this platform, although coefficients for specificity and sensitivity are 99.7% and 99.9%, respectively [29]. Another limitation to the study is the different gender distribution in the PH and control groups. However, regression analyses in men and in the overall population showed a similar independent inverse association of the LDLR g.3131C > T variant with LDL-C that, together with the lipoprotein(a) concentration and age, explained ≈ 10% of LDL-C variability. The increase of the ancestral (and minor) allele frequency in populations with a lower concentration of LDL-C comparing to PH group and in replication cohorts suggests that T-allele of the g.3131C > T variant in the LDLR gene protects against hypercholesterolemia. This observation might be explained by the lower affinity of the g.3131 T variant for some repressors of LDLR gene transcription, and as a consequence, it would increase the transcriptional activity of the LDLR gene comparing with the wild-type allele (Figure 1).
Taking together the genetic analyses and the functional assays, our results suggest that the ancestral and minor allele T at g.3131C > T is associated with LDL-C levels and explains part of the LDL-C variability. This variant is located in a regulatory element, and the shift of nucleotide C to T produce a change in the affinity for transcription factors as well as an increase of 2.5 times in the transcriptional activity of the LDLR gene explaining the protective effect of the T allele. Further studies aimed to expand knowledge on the regulatory elements in the distal region of the LDLR promoter are warranted. Future studies should also consider the effect of this variant on the LDL-C response to lipid-lowering drugs or lifestyle changes.
The association of the rs17248720 polymorphism with hypercholesterolemia confirms previous observations from GWAS suggesting that non-coding variants located at the LDLR locus are associated with blood lipid levels, including LDL-C [8,9,12,13]. While studies attempt to find new loci implicated in the hereditability of lipid levels, the strongest locus associated with LDL-C continues to be that containing the LDLR gene [10].
Conclusions
We have identified a variant located in the distal promoter of the LDLR gene, g.3131C > T (rs17248720) that would explain part of the variability o the LDL cholesterol levels. The ancestral T-allele at this site plays a protective role against hypercholesterolemia by changing the affinity of this region for transcription factors leading to an increase of 2.5 times in the transcriptional activity of the LDLR gene.
Abbreviations
Apo: Apolipoprotein; AWHS: Aragon Workers Health Study; BMI: Body mass index; BP: Blood pressure; EMSA: Electrophoretic mobility shift assay; FH: Familial hypercholesterolemia; GWAS: Genome wide association studies; HDL-C: HDL cholesterol; LD: Linkage disequilibrium; LDL-C: LDL cholesterol; PH: Polygenic hypercholesterolemia; SNPs: Single nucleotide polymorphisms; TG: Triglycerides.
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
IDCO carried out the genetic studies, the sequence alignment, participated in functional studies and statistical analysis and drafted the manuscript. JPL and SR developed the in silico analysis and the functional studies. RMG, ML, ML, MC and JAC participated in the acquisition of data and samples. ER, JCRR, FC and MP conceived of the study and participated in its design and coordination. All authors have been involved in revising critically for important intellectual content and have given final approval of the version to be published.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Primers designed for sequencing a 3.103 Kb fragment from the -625 to +2478 position in the LDLDR gene.
Frequencies of variants in the regulatory region of LDLR found in 125 PH subjects by sequencing.
LDL cholesterol quintile distribution of frequencies of genotypes CT and TT and T-allele frequency for rs17248720 polymorphism in LDLR gene in all study subjects.
Contributor Information
Isabel De Castro-Orós, Email: idecastro.iacs@aragon.es.
Javier Pérez-López, Email: javier.perez@unican.es.
Rocio Mateo-Gallego, Email: rmateo.iacs@aragon.es.
Soraya Rebollar, Email: soraya_4730@hotmail.com.
Marta Ledesma, Email: meledesma@cnic.es.
Montserrat León, Email: mleon@unizar.es.
Montserrat Cofán, Email: mcofan@clinic.ub.es.
Jose A Casasnovas, Email: jacasas@posta.unizar.es.
Emilio Ros, Email: eros@clinic.ub.es.
Jose C Rodríguez-Rey, Email: josecarlos.rodriguez@unican.es.
Fernando Civeira, Email: civeira@unizar.es.
Miguel Pocoví, Email: mpocovi@unizar.es.
Acknowledgements
Grants from the Spanish Ministry of Economy and Competitiveness PI12/00637, PI01/01087, PI12/01231, PI12/01703, RIC RD12/0042/0055, IPT-2011-0817-010000, Aragon Workers’ Health Study (AWHS) and CIBERobn. AWHS is an initiative of CNIC and IACS, Spain. RIC and CIBERobn are initiatives of ISCIII, Spain.
References
- Goldstein J, Hobbs HH, Brown MS. In: The Metabolic and Molecular Bases of Inherited Disease. 8. Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, editor. New York: Mc Graw-Hill; 2001. Familial hypercholesterolemia; pp. 2863–2914. [Google Scholar]
- Civeira F. International Panel on Management of Familial Hypercholesterolemia. Guidelines for the diagnosis and management of heterozygous familial hypercholesterolemia. Atherosclerosis. 2004;7:55–68. doi: 10.1016/j.atherosclerosis.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Innerarity TL, Weisgraber KH, Arnold KS, Mahley RW, Krauss RM, Vega GL, Grundy SM. Familial defective apolipoprotein B-100, low density lipoproteins with abnormal receptor binding. Proc Natl Acad Sci USA. 1987;7:6919–6923. doi: 10.1073/pnas.84.19.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;7:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia, new insights in pathogenesis and treatment. J Clin Invest. 2003;7:1795–1803. doi: 10.1172/JCI200318925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmud PJ, Shah S, Whittall R, Futema M, Howard P, Cooper JA, Harrison SC, Li K, Drenos F, Karpe F, Neil HA, Descamps OS, Langenberg C, Lench N, Kivimaki M, Whittaker J, Hingorani AD, Kumari M, Humphries SE. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia, a case–control study. Lancet. 2013;7:1293–1301. doi: 10.1016/S0140-6736(12)62127-8. [DOI] [PubMed] [Google Scholar]
- De Castro-Orós I, Pocoví M, Civeira F. The fine line between familial and polygenic hypercholesterolemia. Clin Lipidol. 2013;7:303–306. doi: 10.2217/clp.13.20. [DOI] [Google Scholar]
- Sandhu MS, Waterworth DM, Debenham SL, Wheeler E, Papadakis K, Zhao JH, Song K, Yuan X, Johnson T, Ashford S, Inouye M, Luben R, Sims M, Hadley D, McArdle W, Barter P, Kesäniemi YA, Mahley RW, McPherson R, Grundy SM, Bingham SA, Khaw KT, Loos RJ, Waeber G, Barroso I, Strachan DP, Deloukas P, Vollenweider P, Wareham NJ. Well come Trust Case Control Consortium et al. LDL-cholesterol concentrations, a genome-wide association study. Lancet. 2008;7:483–491. doi: 10.1016/S0140-6736(08)60208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, Voight BF, Bonnycastle LL, Jackson AU, Crawford G, Surti A, Guiducci C, Burtt NP, Parish S, Clarke R, Zelenika D, Kubalanza KA, Morken MA, Scott LJ, Stringham HM, Galan P, Swift AJ, Kuusisto J, Bergman RN, Sundvall J, Laakso M. et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2008;7:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, Aulchenko YS, Zhang W, Yuan X, Lim N, Luan J, Ashford S, Wheeler E, Young EH, Hadley D, Thompson JR, Braund PS, Johnson T, Struchalin M, Surakka I, Luben R, Khaw KT, Rodwell SA, Loos RJ, Boekholdt SM, Inouye M, Deloukas P, Elliott P, Schlessinger D, Sanna S. et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010;7:2264–2276. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna S, Li B, Mulas A, Sidore C, Kang HM, Jackson AU, Piras MG, Usala G, Maninchedda G, Sassu A, Serra F, Palmas MA, Wood WH 3rd, Njølstad I, Laakso M, Hveem K, Tuomilehto J, Lakka TA, Rauramaa R, Boehnke M, Cucca F, Uda M, Schlessinger D, Nagaraja R, Abecasis GR. Fine mapping of five loci associated with low-density lipoprotein cholesterol detects variants that double the explained heritability. PLoS Genet. 2011;7:e1002198. doi: 10.1371/journal.pgen.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;7:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley-Hunt R, Bernard V, Wasserman WW. Identification of cis-regulatory sequence variations in individual genome sequences. Genome Med. 2011;7:65. doi: 10.1186/gm281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DE, Lander ES. On the allelic spectrum of human disease. Trends Genet. 2001;7:502–510. doi: 10.1016/S0168-9525(01)02410-6. [DOI] [PubMed] [Google Scholar]
- Rockman MV, Wray GA. Abundant raw material for cis-regulatory evolution in humans. Mol Biol Evol. 2002;7:1991–2004. doi: 10.1093/oxfordjournals.molbev.a004023. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos JA. The genomics of gene expression. Genomics. 2004;7:449–457. doi: 10.1016/j.ygeno.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ. Genomic sources of regulatory variation in cis and in trans. CMLS Cell Mol Life Sci. 2005;7:1779–1783. doi: 10.1007/s00018-005-5064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampín S, Rodríguez-Rey JC. Functional analysis of regulatory single-nucleotide polymorphisms. Curr Opin Lipidol. 2007;7:194–198. doi: 10.1097/MOL.0b013e3280145093. [DOI] [PubMed] [Google Scholar]
- Mozas P, Galetto R, Albajar M, Ros E, Pocoví M, Rodríguez-Rey JC. A mutation (-49C > T) in the promoter of the low density lipoprotein receptor gene associated with familial hypercholesterolemia. J Lipid Res. 2002;7:13–18. [PubMed] [Google Scholar]
- Mozas P, Castillo S, Tejedor D, Reyes G, Alonso R, Franco M, Saenz P, Fuentes F, Almagro F, Mata P, Pocoví M. Molecular characterization of familial hypercholesterolemia in Spain, identification of 39 novel and 77 recurrent mutations in LDLR. Hum Mutat. 2004;7:187. doi: 10.1002/humu.9265. [DOI] [PubMed] [Google Scholar]
- De Castro-Orós I, Pampín S, Bolado-Carrancio A, De Cubas A, Palacios L, Plana N, Puzo J, Martorell E, Stef M, Masana L, Civeira F, Rodríguez-Rey JC, Pocoví M. Functional analysis of LDLR promoter and 5′ UTR mutations in subjects with clinical diagnosis of familial hypercholesterolemia. Hum Mutat. 2011;7:868–872. doi: 10.1002/humu.21520. [DOI] [PubMed] [Google Scholar]
- Bourbon M, Duarte MA, Alves AC, Medeiros AM, Marques L, Soutar AK. Genetic diagnosis of familial hypercholesterolaemia, the importance of functional analysis of potential splice-site mutations. J Med Genet. 2009;7:352–357. doi: 10.1136/jmg.2007.057000. [DOI] [PubMed] [Google Scholar]
- Gómez-Gerique JA, Gutiérrez-Fuentes JA, Montoya MT, Porres A, Rueda A, Avellaneda A, Rubio MA. Lipid profile of the Spanish population, the DRECE (diet and risk of cardiovascular disease in Spain) study. DRECE study group. Med Clin (Barc) 1999;7:730–735. [PubMed] [Google Scholar]
- Palacios L, Grandoso L, Cuevas N, Olano-Martín E, Martinez A, Tejedor D, Stef M. Molecular characterization of familial hypercholesterolemia in Spain. Atherosclerosis. 2012;7:137–142. doi: 10.1016/j.atherosclerosis.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Marinescu VD, Kohane IS, Riva A. MAPPER, a search engine for the computational identification of putative transcription factor binding sites in multiple genomes. BMC Bioinformatics. 2005;7:79. doi: 10.1186/1471-2105-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riancho JA, Vázquez L, García-Pérez MA, Sainz J, Olmos JM, Hernández JL, Pérez-López J, Amado JA, Zarrabeitia MT, Cano A, Rodríguez-Rey JC. Association of ACACB polymorphisms with obesity and diabetes. Mol Genet Metab. 2011;7:670–676. doi: 10.1016/j.ymgme.2011.08.013. [DOI] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector, new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;7:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Birney E, Dunham I, Gree ED, Gunter C, Snyder M. ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;7:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor D, Castillo S, Mozas P, Jiménez E, López M, Tejedor MT, Artieda M, Alonso R, Mata P, Simón L, Martínez A, Pocoví M. Spanish Familial Hypercholesteroelmia Group 2005. Reliable low-density DNA array based on allele-specific probes for detection of 118 mutations causing familial hypercholesterolemia. Clin Chem. 2005;7(51):1137–1144. doi: 10.1373/clinchem.2004.045203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers designed for sequencing a 3.103 Kb fragment from the -625 to +2478 position in the LDLDR gene.
Frequencies of variants in the regulatory region of LDLR found in 125 PH subjects by sequencing.
LDL cholesterol quintile distribution of frequencies of genotypes CT and TT and T-allele frequency for rs17248720 polymorphism in LDLR gene in all study subjects.