Abstract
Aging is the process of system deterioration over time in the whole body. Stem cells are self-renewing and therefore have been considered exempt from the aging process. Earlier studies by Hayflick showed that there is an intrinsic limit to the number of divisions that mammalian somatic cells can undergo, and cycling kinetics and ontogeny-related studies strongly suggest that even the most primitive stem cell functions exhibit a certain degree of aging. Despite these findings, studies on the effects of aging on stem cell functions are inconclusive. Here we review the age-related properties of hematopoietic stem cells in terms of intrinsic and extrinsic alterations, proliferative potential, signaling molecules, telomere and telomerase, senescence and cancer issues, regenerative potential and other indications of stem cell aging are discussed in detail.
Keywords: Hematopoietic stem cells, Aging, Phenotype shift, Proliferative potential
Introduction: do stem cells age?
Stem cell therapy is the process of transplanting of un-differentiated cells into an organism with the aim of regenerating or replacing damaged tissue. Since stem cells can differentiate into multiple tissues in vitro and in vivo, they have attracted significant attention in the fields of cell and gene therapy. The best-known stem cell therapy to date is bone marrow transplantation (BMT) used to treat leukemia and other cancers as well as blood disorders.
Hematopoietic stem cells (HSCs) have been extensively investigated for several decades beginning with the seminal studies of Till & McCullough (1) and Moore & Metcalf (2), which reported that multipotent progenitors present in adult bone marrow were responsible for the lifelong production of blood.
Because HSCs are self-renewing and retain a lifelong capacity to produce blood cells, they have been considered exempt from the aging process. Earlier studies by Hayflick showed that there is an intrinsic limit to the number of cell divisions that mammalian somatic cells can undergo (3). The aging effect on stem cell function has drawn considerable research interest, but these studies have often resulted in conflicting conclusions. However, recent cycling kinetics and ontogeny-related studies strongly suggest a certain degree of aging, even with respect to the most primitive stem cell functions.
Similar to other somatic cells, stem cells undergo an aging process, with both intrinsic and extrinsic multi-factorial conditions affecting the degree of stem cell aging. In cell therapy intervention using bone marrow-derived HSCs and MSCs, donor age and in vitro cell aging issues are important for safety and therapeutic efficacy. Here, we summarize the current hematopoietic stem cell aging issues, including experimental data obtained by our group (4).
Aging from a developmental point of view
Although the term ‘aging’ may not seem appropriate for describing the period before an organism stops development and growth, aging is clearly a process that is initiated at the embryonic stage. Even during early development, beginning with the first zygotic cell division some loss of cell potential is evident.
In mice and humans, hematopoiesis begins in the yolk sac and aorta/gonad/mesonephros (AGM) region, followed by fetal liver and, finally, bone marrow, which becomes the chief production site throughout adult life (5). Stem cells are usually characterized by their self-renewal and multi-lineage differentiation capacity. However, even within the hematopoietic developmental hierarchy, cellular quality and proliferative potential decrease to a certain degree with age; thus, in HSCs, as in somatic cells, there is evidence for the aging phenomenon.
Blood cells are produced constantly from the bone marrow as a result of extensive HSC proliferation and differentiation. Homeostatic mechanisms regulate cell production and death within the hematopoietic system, thereby maintaining normal blood cell levels throughout life. While the balance between stem cell self-renewal and differentiation is tightly regulated to maintain blood production and sustain the HSC pool, stem cells are subject to exhaustion, both quantitatively and qualitatively, during the aging process.
Proliferative potential differences related to hematopoietic developmental hierarchy and stem cell pool size
Proliferative potential is often characterized by mean telomere restriction fragment lengths or human telomere reverse transcriptase activity, which are then used to extrapolate the replicative history and remaining replicative capacity of a cell population. A simple illustration of the relationship between telomere shortening and the level of embryonic development is provided by studies of neonates, which have shown that very low birth-weight pre-term neonates have significantly longer telomeres (p= 0.002) than low birth-weight preterm neonates (6).
Ontogeny-related changes in the proliferative potential of hematopoietic cells have been demonstrated in many studies. Studies of developmental regulation of telomerase activity during gestation using human fetal tissue have shown that telomere length shortening may be correlated with ontogeny-related alteration of hematopoiesis during the process of fetal development (7). For example, an analysis of telomere length during serial transplantation of murine HSCs showed that telomere shortening in HSCs was associated with aging in an animal model (8). In these studies, the telomere length of donor bone marrow cells decreased by ∼7 kb after just two rounds of transplantation, and cellular division capacity was reduced with serial transplantation. These results strongly support the concept that the maintenance of telomere length is critical for the replicative capacity of HSCs.
The proliferative potential and ability of primitive hematopoietic cells to produce CD34+ progenitor cells has also been shown to decrease with the age of the cell donor (9). In these studies, telomere length was studied in primitive hematopoietic cells isolated and purified from adult bone marrow, umbilical cord blood, and fetal liver, and cultured in cytokine-supplemented serum-free media. Telomere lengths in human bone marrow stem cells with the CD34+ CD38lo phenotype were found to be much shorter than those in cells from fetal liver or cord blood, but all cells showed a proliferation-associated loss of telomeric DNA (10). Many other ex vivo expansion trials designed for clinical applications have also experienced proliferation-associated loss of telomeric DNA and cellular senescence (11).
The stem cell pool expands rapidly after birth and then becomes relatively quiescent around a steady state (12). HSC pool dynamics could be affected by programmed cell death within the HSC population and HSC pool size might be feedback regulated. However, the pool size at normal steady state in the transplanted animal is not the same as that in the native state. This is because the functional dynamics of the experimental transplantation setting result in reconstitution kinetics that are different from those of normal steady state hematopoiesis (13, 14). The dynamics, regulation and function of HSCs between the bone marrow niche and the peripheral blood, and the effect of aging in this process are incompletely characterized and will require further study.
HSC pool size in mice varies by strain (15). Using adoptive transfer experiments, researchers have found that the functional HSC pool declines in old age and is correlated with the strain’s natural lifespan (16, 17). Long-lived strains, like B6 mice, have a larger HSC pool than the short-lived DBA strain.
In summary, from a developmental perspective, hematopoietic stem cell aging can be viewed as a process that starts in embryonic development, and can be characterized molecularly and functionally by changes in telomeric DNA length and HSC proliferative potential, respectively. In addition, the size of the HSC pool and the proliferative potential of HSCs are directly correlated with an organism’s lifespan.
Age-related alterations of hematopoietic stem cells
HSCs continuously provide blood components and maintain the immune system during an animal’s lifespan. The hematopoietic system utilizes a variety of homeostatic mechanisms to regulate cell production and cell death, and thus maintains a normal level of blood cells throughout life. HSCs sustain blood homeostasis through extensive proliferation and differentiation. Hayflick showed that normal mammalian somatic cells are intrinsically limited with regard to the number of cell divisions they can undergo prior to the onset of senescence (3). Studies on the effects of aging on primitive hematopoietic cell function have used a number of different experimental approaches and have often reached conflicting conclusions. Colony-forming unit-spleen (CFU-S) activity has been reported to either increase or decrease with aging (18–20). In serial transplantation assays, the age of donor mice was reported to have relatively little effect on engraftment (21). In contrast, competitive repopulation studies have demonstrated genetic regulation of HSC function, showing that decreases or increases in function with age were dependent upon the mouse strain. Recent studies in mouse (22), and human (23) systems suggest that the aging process might be delayed in HSCs due to the expression of telomerase activity. However, cycling kinetics (24), ontogeny-related studies (9), and ex vivo expansion trials (25) strongly suggest a certain degree of aging takes place even in the most primitive stem cell population. Alterations in the properties of HSCs during the aging process will ultimately affect the lymphocytes of the immune system. Such kinetic limitations on the stem cell reserve may be at least partly responsible for the clinical observations in elderly patients with immune dysregulation.
In summary, the phenomenon of HSC aging reflects a reduction in the size of the pluripotent hematopoietic stem cell pool, diminished self-renewal capacity, a decrease in differentiation and engraftment potential, and a lymphoid/myeloid lineage imbalance that results in immune dysfunction, immunosenescence, and increasing prevalence of myeloproliferative disease. In addition, the multidrug resistance (MDR) pump is up-regulated by aging in HSCs, presumably to protect cells from toxic materials that accumulate during the aging process (26). The p53 may play an important role in protecting HSCs from harmful environments such as oxidative stress and DNA damage by suppressing self-renewal and differentiation and preventing tumorigenesis during aging (27).
Reduction in the size and quality of the pluripotent HSC pool
An underlying HSC aging process is suggested by serial transplantation assays (21), which showed that although the number of HSCs increased, their proliferative capacity decreased (28, 29). Consistent with this, a general decline in HSC function was negatively correlated with lifespan and proliferative capacity (30).
From serial transplantation and ex vivo expansion studies, it is clear that HSCs age with repeated proliferation in vivo. Extensive proliferation of HSCs eventually exhausts the stem cell pool and reduces the quality of HSCs, limiting their self-renewal capacity, regenerative potential, multi-lineage potential and homing and engraftment potentials.
Wiesmann and Kim et al. showed in vitro expansion process will reduce the quality and homing efficiency of HSCs (31). Other study, using the gene expression analysis also identified HSC-specific genes that were up-and down-regulated by aging (32). Up-regulated genes associated with HSC aging were typically those involved in stress responses, inflammation and protein aggregation; the inflammatory markers P-selectin and clusterin, in particular, were significantly up-regulated. Prominent among down-regulated genes were those whose products contribute to genomic integrity and chromatin remodeling. The ability of HSCs to engraft in bone marrow is influenced by their homing abilities. Since cell adhesion molecules play a critical role in the engraftment of HSCs, aberrant up-regulation of the P-selectin gene might ultimately affect the ability of aged HSCs to home to bone marrow (32, 33), a question that warrants further study.
Phenotypic shift from lymphoid to myeloid lineage and manifestation of a leukemia pattern with aging
At a time during fetal development when hematopoiesis is beginning, a nascent immune system also begins to take form. Stem cells generate immune-related cells, such as common myelo-erythroid and lymphoid progenitors, which ultimately develop into the repertoire of differentiated immune cells that provide the body’s defense against foreign attack.
Several groups, including ours, have suggested that the patterns of myeloid and lymphoid cell production are changed during the aging progress. Rossi and co-workers used an animal model to investigate the cell-intrinsic alterations that underlie HSC aging. They purified long-term HSCs from young and old mice, and identified changes in age-related genes using microarray analysis. They found that lymphoid-specific gene expression and functions were down-regulated, while myeloid-related genes were up-regulated (33).
Recently Min and colleagues investigated the effects of aging on the transition from common lymphoid progenitors to pro-B cells, providing evidence for significant, age-related defects in the proliferative potential of early B cell precursors. In contrast, myeloid progenitor numbers and developmental potential do not decline with age, indicating that B lymphopoiesis is particularly sensitive to defects that accumulate during senescence (34).
The issue of whether alterations in HSCs with age are caused by changes in the intrinsic factors and properties of these cells is a subject of controversy. In an analysis of the clonal composition of HSCs for lymphoid or myeloid bias, Cho et al. showed that aging is associated with a marked shift in the clonal composition of HSCs: lymphoid-biased HSCs were lost, while myeloid-biased HSCs were enriched in the aged mouse (35). However, myeloid-biased HSCs from young and aged donors did not lose the capacity to generate lymphocytes. Accordingly, the authors suggested that changes in HSCs with aging occur due to alterations in HSC compartment and not by behavior.
This phenotypic shift from the lymphoid to the myeloid lineage tends to skew the distribution of aged HSCs and ultimately contributes to immune dysfunction and the decline of adaptive immunity in old age (Fig. 1 and Fig. 2). It also promotes a leukemia pattern, increasing the risk of developing myelogenous disease, leukemia and tumors. Consistent with this view, myeloid leukemia is accompanied by alterations in many genes related to HSC aging and leukemic transformation. Additionally, aged HSCs express elevated levels of genes involved in leukemic transformation (33). These data support the theory that the functional decline of aged HSCs and alterations in cellular compartments that produce more myeloid cells may be involved in myeloproliferative disease and immune deficiency-related diseases in the elderly population (36).
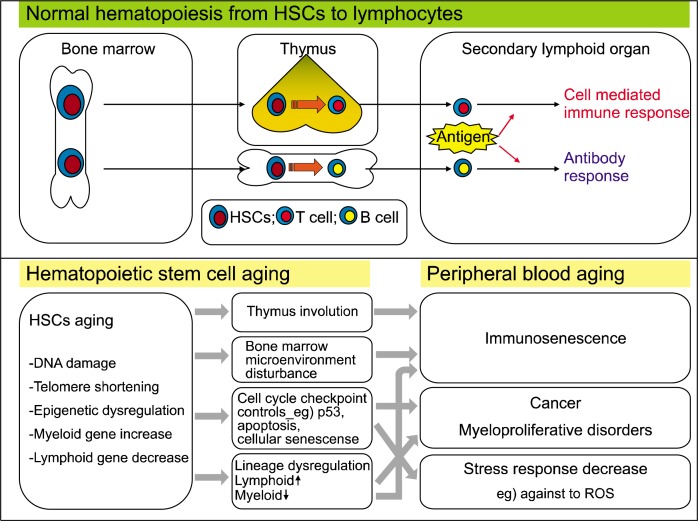
Fig. 1.
How do HSCs affect peripheral immunity? Lymphocytes in peripheral blood are generated from bone marrow HSCs. Aging of HSCs ultimately affects peripheral immunity through the accumulation of DNA damage, and epigenetic and lineage dysregulation.
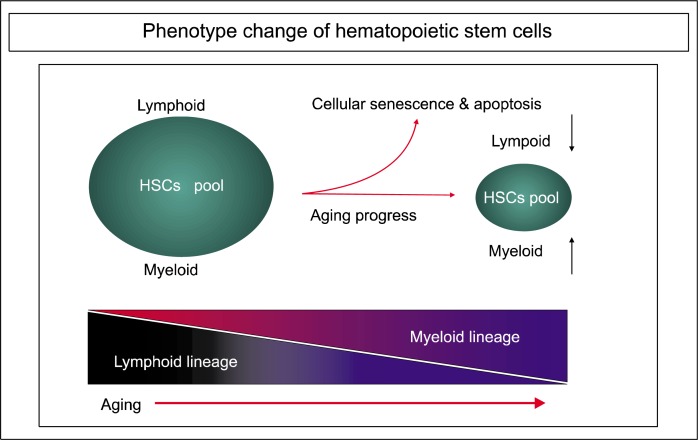
Fig. 2.
Change in HSC phenotype during the aging process. Since lymphoid genes are decreased and myeloid genes are increased by aging, the phenotypic shift from the lymphoid to the myeloid lineage of in aged HSCs ultimately contributes to immune dysfunction and the decline of adaptive immunity.
Notably, while pediatric leukemia tends to be lymphoid in origin, leukemias that develop in old age often originate in myeloid compartments, suggesting that the malignant capacity of hematopoietic progenitors mirror changes in the lineage potential of HSCs during aging (4, 33). This raises the possibility that differences in disease spectra arising at distinct times during ontogeny are influenced by or directly result from changes in the developmental potential of stem cells at a given stage. Consistent with this theory, a recent study showed that ectopic expression of the BCR-ABL oncogene, the causative agent of chronic myeloid leukemia in humans, led to the concurrent development of a myeloproliferative disease (MPD) and B cell leukemia in bone marrow cells of young mice. In contrast, expression of the BCR-ABL oncogene in marrow cells of old mice gave rise to MPD with little or no lymphoid involvement. Moreover, the observation that stem cell aging is coupled to elevated expression of numerous genes (e.g., Aml1, Pml and Eto) involved in the pathogenesis of myeloid leukemia suggests that up-regulation of such proto-oncogenes acts synergistically with the myeloid bias of aged stem cells to predispose toward myelogenous disease and leukemia. The issue of whether up-regulation of such proto-oncogenes in stem cells with age enhances their susceptibility to those types of cytogenetic rearrangements and translocations that promote the development of leukemia remains to be determined (37).
p53: regulation of HSCs in aging and cancer
The aging process brings a change in the system of hematopoietic stem cells (HSCs) and immunity against the age related disease like cancer. Aged HSCs are likely to express a proto-oncogenic phenotype, and are thus at risk of developing into tumor cells. At the outset, our body has mechanisms that protect against the accumulation of reactive oxygen species (ROS) and cancer. One such mechanism involves proteins that act in tumor suppressor-related pathways, such as p53 and p16INK4a. p53 is a major tumor suppressor that protects against the occurrence of cancer by directing mutated cells or cells with severely damaged DNA toward the apoptosis pathway (38, 39).
In HSCs, p53 continuously suppresses self-renewal and differentiation associated with aging; as a result, the proliferation and functionality of HSCs are decreased. Although tumor suppressor genes are altered in aged HSCs, which exhibit a reduction in tissue homeostasis and an aging phenotype, these cells are nonetheless able to avoid tumorigenesis. If p53 and p16INK4a in HSCs were down-regulated and normal regulation was lost, HSC proliferation would be increased and functionality would be altered, leading ultimately to formation of a cancer stem cell (27).
Tyner et al. (40) showed that p53 mutant mice display early aging-associated phenotypes and hematopoietic defects, such as a reduced stem cell pool and an HSC proliferative response throughout life, which lead to an inability to maintain a normal lifespan (41). The p53 hyper-morphic (p53+/m) mice are genetically predisposed toward a low rate of stem cell proliferation, which results in poor hematopoietic regenerative activity. These mice have fewer tumors, but showed an early aging-associated phenotype and a 23% reduction in median lifespan. Though lower HSC proliferation capacity may keep HSCs younger, it also results in poorer tissue regeneration. Thus, a balance between stem cell proliferation and regeneration is required to maximize an organism’s longevity. Altered p53 activity affects hematopoietic stem cell numbers and proliferation, supporting a model in which aging is caused by a decline in tissue stem cell regenerative function (41).
In summary, p53 might play an important role in protecting HSCs from harmful environmental stimuli, such as oxidative stress and DNA damage, by suppressing self-renewal and differentiation of HSCs and preventing tumorigenesis during the aging process. The p53 may also be a potential regulator of longevity and aging by regulating stem cell self-renewal (42).
Multidrug resistance pump vs. stem cell aging
The ATP-binding cassette (ABC) transporters are well-conserved membrane proteins involved in the trans-membrane transport of a variety of substrates. Among them, ABCB1 (MDR1/P-glycoprotein, P-gp), ABCC1 (multidrug resistance protein, MRP-1) and ABCG2 (breast cancer resistant protein, BCRP) have the ability to export chemotherapeutic compounds from cancer cells, thus conferring multidrug resistance (MDR) to these cells. These ABC transporters are highly expressed in HSCs.
Certain fluorescent dyes, such as rhodamine 123 (Rh-123), are substrates of ABC transporters, and can be used to assess pump activity. It has been demonstrated that re-tension of Rh-123 is low in the most primitive HSCs. While the Rh-123-low fraction provides long-term reconstitution, Rh-123-high fraction provides only short-term reconstitution after injection into lethally irradiated mice (43). The molecular basis of the dye-efflux phenotype of HSCs has been identified as ABCB1/P-gp. Hoechst-33342, another fluorescent substrate of P-gp, is used to isolate a very primitive stem cell-enriched fraction called the side population (SP) (44). Stem cells with the SP phenotype are known to express high levels of AGCG2/BCRP (45, 46).
P-gp function is up-regulated in mouse and human T lymphocytes from aged donors (47, 48). Our previous work (49) demonstrated that bone marrow donor age has a specific and pronounced effect on Rh-123 efflux in murine HSCs. As HSCs age, they are confronted by various environmental stresses, including reactive oxygen, heat shock and carcinogens. MDR transporter activity in HSCs may serve to pump out these toxic substances during the aging process to protect HSCs, and could thus be a mechanism for minimizing DNA damage due to xenobiotics and genotoxins (50).
In addition to its protective role, the MDR pump has also been proposed to contribute to cell quiescence, differentiation and self-renewal (51). ABC transporters may modulate the response of HSCs to differentiation factors present in the bone marrow and could potentially play a role in the pathogenesis and biology of stem cell-derived hematological malignancies, such as acute and chronic myeloid leukemia (52). Though the ABC transporters are clearly important in the stem cell aging process, there are many remaining questions about the role of ABC transporters in stem cell biology, hematopoiesis and the aging process.
Proteomic analysis of HSC aging
Hematopoiesis reflects a balance among self-renewal, proliferation and differentiation. The unique features of HSCs that account for their longevity and ability to strike the proper balance between selfrenewal and differentiation are not clearly identified. Ultimately, these properties are encoded in the transcriptional profile of stem cells (53). Transcriptional analyses have been performed to identify genes whose expression in HSC populations is associated with self-renewal and pluripotency (54, 55), and define differences in hematopoietic cell lines that occur during the differentiation process (56). Recent improvements in proteomics technology have made it possible to quantitatively assess individual proteins in stem cells.
Prall et al. (57) investigated age-related gene expression patterns in CD34+ cells and progenitors from new born, young and old donors. These studies identified age-related differences in the transcriptional levels of three distinct genes: KU70, a DNA repair gene and part of the DNA-dependent protein kinase complex, was negatively correlated with age; MGST1, a gene that protects against oxidative stress, progressively increased with age; and BIK, an apoptotic gene, was positively correlated with age (57).
Using HSCs purified from young and old mice, Chambers et al. (58) analyzed over 14,000 genes, identifying 1,500 that were age-induced and 1,600 that were age-repressed. Genes associated with the stress response, inflammation and protein aggregation were up-regulated, while those involved in the preservation of genomic integrity and chromatin remodeling were down-regulated. Their results are summarized in Table 1 and Table 2.
Table 1.
Factors related to HSC aging
| Cell | Factor | Fuction | Relevance to stem cell aging | Reference |
|---|---|---|---|---|
| HSCs | FOXO protein | Promoting cell cycle arrest, stress resistance or apoptosis Reducing age dependent disease |
Maintenance of HSCs compartment | Eric L Greer et al., 2005 Zuzana Tothova et al., 2007. |
| HOXB4 | Increasing HSC self-renewal | Increase HSCs expansion capacity without functional impairmnet | Jennifer Antonchuk et al., 2002. | |
| Notch1 | Regulating HSCs self renewal | Decrease | Barbara Varnum-Finney, 2000 | |
| Wnt signaling | Upregulating HOXB4 /Notch1 Critical for HSC homeostasis including regulation of HSC development |
May decrease in HSC? (Increase in some other stem cells) | Tannishtha Reya et al., 2003 Bryan D. White et al., 2007 Hongjun Liu et al., 2007 |
|
| c-Myc | Controlling the balance between HSC self renewal and differentiation, Stimulating tumorigenesis | Increase | Anne Wilson et al., 2004 Chi-Hwa Wu et al., 2007 |
|
| TNF-α | Interfere hematopoietic stem cells (HSCs) self renewal, expansion and hematopoiesis | Increase | Xiaoling Zhang1 et al., 2007 | |
| KU70 | DNA repair gene Part of DNA dependent protein kinase complex (DNA-PK) Telomerase maintenance |
Decrease | Wolf C. Prall et al., 2007. | |
| MGST1 | Microsomal glutathione S-transferase A gene protecing against oxidative stress |
Increase | Wolf C. Prall et al., 2007. | |
| BIK | Bcl2-interacting killer Pro apoptoic gene |
Increase | Wolf C. Prall et al., 2007. | |
| I-Kb kinase/NF-Kb cascade | Related to inflammation increase with aging | Increase | Stuart M. Chambers et al., 2007. |
Self-renewal-, proliferation- and lymphoid-related genes are mostly decreased by aging, whereas inflammation-, apoptosis-, and tumorigenesis-related genes are increased.
Table 2.
Representative gene profiles during HSC aging
| Function | Expression during aging | Reference |
|---|---|---|
| Protein folding/stress | Increase | Stuart M. Chambers et al., 2007. |
| Inflammation/proteolysis/cell-cell adhesion | Increase | |
| APP processing/Alzheimer | Increase | |
| Stress response/Senescence | Increase | |
| DNA repair | Decrease | |
| TGF β reglation/HSCs pool size change | Decrease | |
| Chromatic silencing | Decrease | |
| Deficiency/Mutation cause progeria | Decrease |
Genes that regulate DNA repair and HSC pool size are decreased by aging, and those involved in protein folding or stress response, inflammation, and senescence-related are increased.
Cell-intrinsic alterations underlie HSC aging. That these changes are directly related to the loss of immune function and an increased incidence of myeloid leukemia is well demonstrated by Rossi et al. (33). Their microarray analysis of age-related genes in HSCs purified from young and old mice revealed that, in aged HSCs, lymphoid genes are mostly down-regulated and myeloid genes are up-regulated. Among lymphoid genes, lymphoid leukemia genes, such as Bcl3, were up-regulated in old mice.
Collectively, these data define a sequence of events associated with hematopoietic stem cell aging that involves lymphoid/myeloid lineage skewing, immune dysfunction and immunosenescence and, ultimately, myeloproliferative diseases in the aged population.
Summary
Theoretically, both extrinsic and intrinsic molecular factors may influence HSC aging. HSCs are often referred to as a “Fountain of Youth”. We now know, however, that even HSCs are not exempt from the natural aging process. The functional HSC pool declines in old age and is directly correlated with lifespan in different mouse strains. A similar relationship likely exists in humans, as well.
Although HSCs, like all other cells and organisms, inevitably age, they have evolved exquisite mechanisms to protect themselves from toxic materials and maintain hematopoiesis throughout the lifetime of the organism, despite the fact that the size of the stem cell pool continuously decreases.
The increasing longevity of human populations has prompted a keen interest in molecular solutions to aging and age-related medical problems. Future studies that extend and integrate our understanding of stem cell aging will ultimately contribute to increasing the life expectancy of humans, and developing anti-aging therapies and regenerative medicines.
Acknowledgments
This study was supported by grants from the Korea Science and Engineering Foundation (R04-2003-000-10191-0) and Asan Institute for Life Sciences (#2000-133), Seoul, Korea.
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interest.
References
- 1.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 2.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 3.Hayflick L. The cell biology of human aging. N Engl J Med. 1976;295:1302–1308. doi: 10.1056/NEJM197612022952308. [DOI] [PubMed] [Google Scholar]
- 4.Kim M, Moon HB, Spangrude GJ. Major age-related changes of mouse hematopoietic stem/progenitor cells. Ann N Y Acad Sci. 2003;996:195–208. doi: 10.1111/j.1749-6632.2003.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 5.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich U, Schwab M, Griese EU, Fritz P, Klotz U. Telomeres in neonates: new insights in fetal hematopoiesis. Pediatr Res. 2001;49:252–256. doi: 10.1203/00006450-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Ulaner GA, Giudice LC. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod. 1997;3:769–773. doi: 10.1093/molehr/3.9.769. [DOI] [PubMed] [Google Scholar]
- 8.Allsopp RC, Cheshier S, Weissman IL. Telomere shortening accompanies increased cell cycle activity during serial transplantation of hematopoietic stem cells. J Exp Med. 2001;193:917–924. doi: 10.1084/jem.193.8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178:787–791. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelhardt M, Kumar R, Albanell J, Pettengell R, Han W, Moore MA. Telomerase regulation, cell cycle, and telomere stability in primitive hematopoietic cells. Blood. 1997;90:182–193. [PubMed] [Google Scholar]
- 12.Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Haan G, Van Zant G. Intrinsic and extrinsic control of hemopoietic stem cell numbers: mapping of a stem cell gene. J Exp Med. 1997;186:529–536. doi: 10.1084/jem.186.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol. 1997;7:805–808. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 15.De Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–1550. [PubMed] [Google Scholar]
- 16.Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 17.Kamminga LM, van Os R, Ausema A, Noach EJ, Weersing E, Dontje B, Vellenga E, de Haan G. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23:82–92. doi: 10.1634/stemcells.2004-0066. [DOI] [PubMed] [Google Scholar]
- 18.Micklem HS, Ford CE, Evans EP, Ogden DA, Papworth DS. Competitive in vivo proliferation of foetal and adult haematopoietic cells in lethally irradiated mice. J Cell Physiol. 1972;79:293–298. doi: 10.1002/jcp.1040790214. [DOI] [PubMed] [Google Scholar]
- 19.Albright JW, Makinodan T. Decline in the growth potential of spleen-colonizing bone marrow stem cells of long-lived aging mice. J Exp Med. 1976;144:1204–1213. doi: 10.1084/jem.144.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauch P, Botnick LE, Hannon EC, Obbagy J, Hellman S. Decline in bone marrow proliferative capacity as a function of age. Blood. 1982;60:245–252. [PubMed] [Google Scholar]
- 21.Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982;156:1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 23.Yui J, Chiu CP, Lansdorp PM. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood. 1998;91:3255–3262. [PubMed] [Google Scholar]
- 24.Phillips RL, Reinhart AJ, Van Zant G. Genetic control of murine hematopoietic stem cell pool sizes and cycling kinetics. Proc Natl Acad Sci U S A. 1992;89:11607–11611. doi: 10.1073/pnas.89.23.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nygren JM, Bryder D, Jacobsen SE. Prolonged cell cycle transit is a defining and developmentally conserved hemopoietic stem cell property. J Immunol. 2006;177:201–208. doi: 10.4049/jimmunol.177.1.201. [DOI] [PubMed] [Google Scholar]
- 26.Witkowski JM, Miller RA. Increased function of P-glyco-protein in T lymphocyte subsets of aging mice. J Immunol. 1993;150:1296–1306. [PubMed] [Google Scholar]
- 27.Gatza C, Moore L, Dumble M, Donehower LA. Tumor suppressor dosage regulates stem cell dynamics during aging. Cell Cycle. 2007;6:52–55. doi: 10.4161/cc.6.1.3667. [DOI] [PubMed] [Google Scholar]
- 28.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 29.De Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93:3294–3301. [PubMed] [Google Scholar]
- 30.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 31.Wiesmann A, Kim M, Georgelas A, Searles AE, Cooper DD, Green WF, Spangrude GJ. Modulation of hematopoietic stem/progenitor cell engraftment by transforming growth factor beta. Exp Hematol. 2000;28:128–139. doi: 10.1016/s0301-472x(99)00141-1. [DOI] [PubMed] [Google Scholar]
- 32.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol. 2006;176:1007–1012. doi: 10.4049/jimmunol.176.2.1007. [DOI] [PubMed] [Google Scholar]
- 35.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2008 doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 39.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 40.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 41.Dumble M, Gatza C, Tyner S, Venkatachalam S, Donehower LA. Insights into aging obtained from p53 mutant mouse models. Ann N Y Acad Sci. 2004;1019:171–177. doi: 10.1196/annals.1297.027. [DOI] [PubMed] [Google Scholar]
- 42.Gatza CE, Dumble M, Kittrell F, Edwards DG, et al. Does p53 affect organismal aging? J Cell Physiol. 2002;192:23–33. doi: 10.1002/jcp.10104. [DOI] [PubMed] [Google Scholar]
- 43.Spangrude GJ, Brooks DM, Tumas DB. Long-term re-population of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- 44.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 46.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 47.Witkowski JM, Gorgas G, Miller RA. Reciprocal expression of P-glycoprotein and TAP1 accompanied by higher expression of MHC class I antigens in T cells of old mice. J Gerontol A Biol Sci Med Sci. 1996;51:B76–B82. doi: 10.1093/gerona/51a.1.b76. [DOI] [PubMed] [Google Scholar]
- 48.Pilarski LM, Paine D, McElhaney JE, Cass CE, Belch AR. Multidrug transporter P-glycoprotein 170 as a differentiation antigen on normal human lymphocytes and thymocytes: modulation with differentiation stage and during aging. Am J Hematol. 1995;49:323–335. doi: 10.1002/ajh.2830490411. [DOI] [PubMed] [Google Scholar]
- 49.Kim M, Cooper DD, Hayes SF, Spangrude GJ. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood. 1998;91:4106–4117. [PubMed] [Google Scholar]
- 50.Smeets M, Raymakers R, Vierwinden G, Pennings A, van de Locht L, Wessels H, Boezeman J, de Witte T. A low but functionally significant MDR1 expression protects primitive haemopoietic progenitor cells from anthracycline toxicity. Br J Haematol. 1997;96:346–355. doi: 10.1046/j.1365-2141.1997.d01-2024.x. [DOI] [PubMed] [Google Scholar]
- 51.Good JR, Kuspa A. Evidence that a cell-type-specific efflux pump regulates cell differentiation in Dictyostelium. Dev Biol. 2000;220:53–61. doi: 10.1006/dbio.2000.9611. [DOI] [PubMed] [Google Scholar]
- 52.Raaijmakers MH. ATP-binding-cassette transporters in hematopoietic stem cells and their utility as therapeutical targets in acute and chronic myeloid leukemia. Leukemia. 2007;21:2094–2102. doi: 10.1038/sj.leu.2404859. [DOI] [PubMed] [Google Scholar]
- 53.Evans CA, Tonge R, Blinco D, Pierce A, Shaw J, Lu Y, Hamzah HG, Gray A, Downes CP, Gaskell SJ, Spooncer E, Whetton AD. Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility. Blood. 2004;103:3751–3759. doi: 10.1182/blood-2003-09-3294. [DOI] [PubMed] [Google Scholar]
- 54.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 55.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 56.Lian Z, Kluger Y, Greenbaum DS, Tuck D, Gerstein M, Berliner N, Weissman SM, Newburger PE. Genomic and proteomic analysis of the myeloid differentiation program. Blood. 2001;98:513–524. doi: 10.1182/blood.v98.3.513. [DOI] [PubMed] [Google Scholar]
- 57.Prall WC, Czibere A, Jäger M, Spentzos D, Libermann TA, Gattermann N, Haas R, Aivado M. Age-related transcription levels of KU70, MGST1 and BIK in CD34+ hematopoietic stem and progenitor cells. Mech Ageing Dev. 2007;128:503–510. doi: 10.1016/j.mad.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]