Abstract
Background: Plasma phospholipid concentrations of trans-palmitoleic acid (trans-16:1n−7), a biomarker of dairy fat intake, are inversely associated with incident type 2 diabetes in 2 US cohorts.
Objective: The objective was to investigate whether the intake of trans-16:1n−7 in particular, or dairy fat in general, is associated with glucose tolerance and key factors determining glucose tolerance.
Design: A cross-sectional investigation was undertaken in 17 men and women with nonalcoholic fatty liver disease and 15 body mass index (BMI)- and age-matched controls. The concentrations of trans-16:1n−7 and 2 other biomarkers of dairy fat intake, 15:0 and 17:0, were measured in plasma phospholipids and free fatty acids (FFAs). Liver fat was estimated by computed tomography–derived liver-spleen ratio. Intravenous-glucose-tolerance tests and oral-glucose-tolerance test (OGTT) and hyperinsulinemic-euglycemic clamps were performed to assess β-cell function and hepatic and systemic insulin sensitivity.
Results: In multivariate analyses adjusted for age, sex, and BMI, phospholipid 17:0, phospholipid trans-16:1n−7, FFA 15:0, and FFA 17:0 were inversely associated with fasting plasma glucose, the area under the curve for glucose during an OGTT, and liver fat. Phospholipid trans-16:1n−7 was also positively associated with hepatic and systemic insulin sensitivity. None of the biomarkers were associated with β-cell function. The associations between dairy fat intake and glucose tolerance were attenuated by adjusting for insulin sensitivity or liver fat, but strengthened by adjusting for β-cell function.
Conclusion: Although we cannot rule out reverse causation, these data support the hypothesis that dairy fat improves glucose tolerance, possibly through a mechanism involving improved hepatic and systemic insulin sensitivity and reduced liver fat. This trial was registered at clinicaltrials.gov as NCT01289639.
INTRODUCTION
In 2 recent studies, Mozaffarian et al (1, 2) reported an inverse association between the plasma phospholipid trans-palmitoleic acid (trans-16:1n−7) content and the risk of type 2 diabetes mellitus in 2 US cohorts. trans-16:1n−7 cannot be synthesized by the human body, and is present in the diet almost exclusively in the meat and milk from ruminants. On the basis of work by Hotamisligil et al (3, 4), Mozaffarian et al hypothesized that trans-16:1n−7 found in ruminant fat may stimulate fat oxidation or inhibit de novo lipogenesis in the liver, thereby lowering hepatic fat content and improving hepatic insulin sensitivity and glucose tolerance. As a result, individuals with high baseline plasma concentrations of trans-16:1n−7 were hypothesized to have a lower risk of developing type 2 diabetes. Whereas their overall hypothesis of an inverse association of plasma phospholipid trans-16:1n−7 concentrations and type 2 diabetes risk was confirmed in their studies, several important questions remained. First, trans-16:1n−7 is an established biomarker for high-fat dairy product intake (5), and other components in dairy fat may partly explain the inverse relation with type 2 diabetes risk. It is important to note that in their studies, the inverse association between trans-16:1n−7 in plasma phospholipids and type 2 diabetes risk persisted after adjustment for full-fat dairy product intake. This may suggest that endogenous regulation of phospholipid composition rather than diet composition may be linked to diabetes risk. Another explanation may be that full-fat dairy product intake, as assessed by food-frequency questionnaire, explains only a small portion of the variation in plasma phospholipid trans-16:1n−7, possibly related to the fact that assessment of full-fat dairy product consumption by food-frequency questionnaire, as in these studies, is a poor measure of actual intakes (6). This may also be because the content of trans-16:1n−7 in dairy products varies widely depending on the dairy cow feed (7). Second, because the studies by Mozaffarian et al were large observational studies, subjects were not carefully metabolically phenotyped. Measures of metabolic health included only fasting glucose and insulin and insulin resistance as estimated by HOMA-IR.
Here, we aimed to test the hypothesis that trans-16:1n−7 intake in particular, and dairy fat intake in general, is associated with glucose tolerance and with major determinants of glucose tolerance, including liver fat, hepatic and systemic insulin sensitivity, and β-cell function. To this end, we conducted a cross-sectional analysis of the relation between trans-palmitoleic acid concentrations and the concentrations of 2 other established biomarkers for dairy fat intake, pentadecanoic acid (15:0) and heptadecanoic acid (17:0) (8), in both the plasma phospholipid and the free fatty acid (FFA)4 fraction. Subjects were carefully phenotyped by measuring oral glucose tolerance, hepatic and systemic insulin sensitivity, liver fat, and β-cell function. We present data from 17 subjects with nonalcoholic fatty liver disease (NAFLD) and 15 age- and BMI-matched controls—a study population that has wide heterogeneity in liver fat, glucose tolerance, and insulin sensitivity.
SUBJECTS AND METHODS
Study design and subjects
This cross-sectional study compared 17 subjects with diagnosed NAFLD with 15 controls matched for age and BMI. All subjects gave written informed consent to participate, and the study was approved by the Human Subjects Review Committees at the VA Puget Sound Health Care System and the University of Washington.
This was a secondary analysis of a study originally designed, powered, and conducted to assess the relation between liver fat and insulin sensitivity. The sample-size target was 32 subjects (16 in each group) to provide 90% power to detect a between-group difference of 50% in systemic insulin sensitivity.
Subjects underwent an initial screening visit that included assessment of medical history, physical examination, and fasting blood tests. Medical records of case subjects were evaluated to determine eligibility. Case subjects were defined as having NAFLD based on either a liver biopsy within the past 3 y meeting criteria for >5% fatty infiltration or the presence of elevated liver enzymes in conjunction with fatty liver by ultrasonography or computed tomography scan, both in the absence of other causes for liver dysfunction. Twelve of the 17 case subjects had biopsy samples available for review. These were reviewed by a single pathologist and scored for fat, inflammation, presence of ballooning, and fibrosis by using the Nonalcoholic Steatohepatitis Clinical Research Network criteria (9). Six met histological criteria for nonalcoholic steatohepatitis (NASH) and 6 had steatosis without NASH. Exclusion criteria for cases included cirrhosis on liver biopsy, significant weight loss (>5%) since liver biopsy, other causes of elevated liver enzymes, or serum glutamic pyruvic transaminase (sGPT) >5 times the upper limit of normal (laboratory normal range: 0–39 U/L). Controls were recruited by advertisement and fliers from the Seattle area. They were required to have normal liver enzymes and no history of liver disease. Additional exclusion criteria for both cases and controls included self-reported alcohol intake >20 g/d, hepatitis C antibody positivity, hepatitis B surface antigen positivity, iron saturation >55%, serum creatinine >1.4 mg/dL in men and >1.3 mg/dL in women, hematocrit <33%, pregnancy or lactation, any serious medical condition, or use of any of the following medications that could affect study outcome measures: corticosteroids, estrogens at doses higher than standard replacement therapy, tamoxifen, amiodarone, accutane, sertraline, atypical antipsychotics, antiretrovials, niacin, gemfibrozil, fenofibrate, medications to treat diabetes, ursodeoxycholic acid, betaine, and milk thistle. A total of 34 subjects were studied, but fatty acid composition data were available for only 32 subjects (17 cases and 15 controls).
Study procedures
Subjects were told to fast for 10 to 12 h before undergoing the study procedures. For each participant, all study procedures were performed within a period of 2 wk. Plasma samples were placed immediately on ice and processed in a refrigerated centrifuge at 4°C, and aliquots were frozen at −70°C until assayed. Samples for FFAs were drawn into tubes containing the lipolysis inhibitor tetrahydrolipstatin (orlistat), placed immediately on ice, processed within 30 min, and then flash frozen.
Oral-glucose-tolerance test
A standard 75-g oral-glucose-tolerance test (OGTT) was performed after the subjects fasted overnight fast. Seventy-five grams of glucose were consumed within 5 min, and blood samples were drawn at −10, −5, −1, 10, 20, 30, 60, 90, and 120 min relative to the start of glucose ingestion. Glucose tolerance status was determined by fasting and 2-h OGTT glucose concentrations according to American Diabetes Association guidelines (10).
Intravenous-glucose-tolerance test
An intravenous-glucose-tolerance test (IVGTT) was performed on a separate day to assess insulin secretion and intravenous glucose tolerance. One intravenous line was established in an antecubital or forearm vein for administration of glucose, and a second was placed in the opposite arm for blood sampling. The sampling arm was wrapped in a heating pad to “arterialize” the blood samples. Glucose (11.4 g/m2) was injected over 60 s, and blood samples were drawn at −10, −5, −1, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, and 30 min relative to the start of the glucose injection.
Hyperinsulinemic euglycemic clamp
A 2-step hyperinsulinemic euglycemic clamp with 6,6 2d glucose isotope label to estimate endogenous glucose production was performed on a separate day. Subjects were admitted the night before and fed a standardized dinner from 1900 to 2000 that consisted of 7 kcal/kg body wt (50% of energy from carbohydrate, 30% from fat, and 20% from protein). An intravenous catheter was placed in an antecubital or forearm vein for administration of glucose and insulin, and a second catheter was placed in the opposite arm for blood sampling. The sampling arm was wrapped in a heating pad to “arterialize” the blood. A blood sample was obtained at 0400 to assess blood glucose and background concentrations of 6,6 2d glucose. At 0500, a primed (200 mg/m2 over 5 min), continuous (2 mg/m2 per minute) infusion of 6,6 2d glucose was started and continued throughout the clamp procedure. Basal blood samples were drawn every 15 min during the last half hour of the 3-h basal equilibration period. At 0800, a low-dose insulin infusion (20 mU/m2 per minute) was started and continued for 3 h. Subsequently a primed, continuous high-dose insulin infusion (160 mU/m2 per minute × 5 min then 80 mU/m2 per minute) was continued for another 2 h. Blood glucose was measured every 5 min by using a handheld blood analyzer (iStat System; Abbott Laboratories), and a variable rate infusion of 20% dextrose enriched with 2% 6,6 2d glucose was titrated to maintain the blood glucose concentration at 5 mmol/L (90 mg/dL). Samples were drawn for glucose and insulin every 30 min throughout the clamp. Samples for glucose, insulin, and 6,6 2d glucose were drawn every 15 min during the final half hour of the basal and low-dose and high-dose insulin periods.
Body-composition analyses
Body fat mass and lean mass were determined by using dual-energy X-ray absorptiometry (Lunar Prodigy; GE Medical Systems). Unenhanced computed tomography scan images were obtained on a General Electric Discovery HD750 computed tomography scanner. From these images, intraabdominal and abdominal subcutaneous fat areas were measured at the top of the iliac crest and quantified by using the Tomovision program SliceOMatic V4.3. A minimum of 2 readings per slice were made spaced ≥24 h apart and then averaged. All intraabdominal fat and subcutaneous fat measures were made by one trained technologist with an intraobserver CV of <7% for intraabdominal fat and of <3% for subcutaneous fat.
Liver fat was estimated by measuring the density ratio between the liver and spleen by computed tomography in Hounsfield units (liver-spleen ratio), which was previously correlated with liver fat quantification by magnetic resonance spectroscopy (11). A liver-spleen ratio <1 is consistent with fatty liver. Ten separate areas equally distributed throughout the liver and spleen were identified, taking care to avoid blood vessels and bile ducts, and the Hounsfield units were averaged. In subjects with more than one slice through the liver and spleen, the liver-spleen ratio for each slice was averaged.
Assessment of habitual dietary intakes
Twenty-five of the 32 participants completed a 3-d dietary record detailing all food and drink consumed over 2 weekdays and 1 weekend day. A dietitian reviewed the dietary record with the participant, and questions were clarified. Diet composition was assessed by using the Nutrition Data System for Research, developed by the Nutrition Coordinating Center, University of Minnesota (Minneapolis, MN).
Laboratory analyses
Fasting plasma measures of metabolic and liver health
Total cholesterol and triglyceride concentrations in fasting plasma were measured by enzymatic assays on a Roche Double Modular P Analytics automated analyzer. HDL cholesterol was similarly assessed after precipitation of apolipoprotein B–containing particles by using dextran sulfate Mg2+. LDL cholesterol was calculated by using the Friedewald equation (12). The LDL relative flotation rate was calculated as the fraction number of the major peak of LDL divided by the total number of fractions. The relative flotation rate of each plasma sample calculated by this procedure is highly reproducible with a CV <2%. Plasma glucose was measured by the glucose oxidase method. Plasma insulin was measured by an automated electrochemiluminescence immunoassay on a Cobas 6000 analyzer with a Cobas e601 module (Roche). High-sensitivity C-reactive protein was measured by nephelometry (Siemens). Plasma total adiponectin was measured by radioimmunoassay (Millipore Inc). Concentrations of 6,6 2d glucose were measured by gas chromatography–mass spectrometry as previously described (13, 14). Liver enzymes (serum glutamic oxaloacetic transaminase, sGPT, and serum γ-glutamyl transferase) were measured by enzymatic assays and glycated hemoglobin by a turbidimetric inhibition immunoassay, all on a Roche Cobas 6000 analyzer.
Measurement of plasma biomarkers of dairy fat intake
Dairy fat intake was assessed by measuring the contents of 15:0, 17:0, and trans-16:1n−7 in plasma phospholipids and the FFA fraction; 15:0, 17:0, and trans-16:1n−7 are validated biomarkers of dairy fat intake (5, 8). Although there is evidence that the plasma phospholipids and FFA compositions reflect the fat composition of the diet, the exact temporal relations are less well established (15). The existing data suggest that the plasma phospholipid composition changes relatively quickly and may reflect intakes over the past ∼3 wk (15). Fewer data are available for FFAs, but given that fasting FFAs are derived largely from stored fat in triglycerides, it seems plausible to hypothesize that the FFA composition reflects longer-term dietary intakes, possibly covering several months to years. Total lipids were extracted from tetrahydrolipstatin-treated plasma by using the method of Folch (16). Plasma phospholipids were separated from other lipids by 1-dimensional thin-layer chromatography (17). Fatty acid methyl ester samples were prepared by direct trans-esterification with the method of Lepage and Roy (18) and separated by using gas chromatography (Agilent 5890 Gas Chromatograph with FID detector and ChemSation software; Supelco fused-silica 100-m capillary column SP-2560; initial 160°C for 16 min, ramp 3.0°C/min to 240°C, hold for 15 min). Fatty acid composition was expressed as weight percentage of the total. The assay generates data on 46 fatty acids. Interassay CVs in our laboratory are 4.0% for 15:0, 1.3% for 17:0, and 2.6% for trans-16:1n−7. Identification, precision, and accuracy were continuously evaluated by using both model mixtures of known fatty acid methyl esters and established in-house control pools.
Calculations
OGTT data
The total area under the curve for glucose and insulin, and the AUC for incremental glucose and insulin above basal from 0 to 120 min during the OGTT, were calculated by using the trapezoidal method. The insulinogenic index was calculated as the change in insulin divided by the change in glucose from 0 to 30 min. The oral DI was calculated as the insulinogenic index × 1/fasting insulin.
IVGTT data
The acute insulin response to glucose (AIRg) was calculated from the IVGTT as the AUC insulin response above basal from 0 to 10 min. AIRg was adjusted for insulin sensitivity measured by the clamp method to estimate β-cell function. The glucose disappearance constant, a measure of intravenous glucose tolerance, was calculated as the slope of the natural log of glucose from 10 to 30 min during the IVGTT.
Clamp data
Isotopic steady state concentrations were achieved during the final 30 min of the basal and low- and high-dose insulin periods of the clamp. The rate of glucose appearance was calculated by using Steele's steady state equations (19). Whole-body insulin sensitivity at both low and high insulin infusion rates was calculated as the glucose infusion rate/lean body mass and adjusted for steady state insulin concentrations. The hepatic insulin resistance index was calculated as basal hepatic glucose production × fasting plasma insulin. The intravenous disposition index (IV DI) was calculated as the product of AIRg and insulin sensitivity during the low-dose clamp.
Statistical analyses
All statistical analyses were conducted with SPSS for Macintosh (version 20; IBM Corporation). Normal distribution was confirmed by checking histograms and normal plots and by conducting Shapiro-Wilk tests. Variables that were not consistent with a normal distribution were log transformed before statistical analyses, or appropriate nonparametric tests were used, as detailed below. Baseline characteristics and habitual dietary intakes of the 2 groups (NAFLD compared with controls) were compared by using t tests or Mann-Whitney U tests (the latter for BMI, sex, alcohol intake, liver enzymes, high-sensitivity C-reactive protein, fasting glucose, fasting insulin, glucose tolerance status, adiponectin, triglycerides, all foods and food categories, and fiber and alcohol intakes). The fatty acid compositions of phospholipids and FFAs of controls compared with NAFLD subjects were similarly compared by using t tests or Mann-Whitney U tests (the latter for phospholipid cis-17:1n−7, trans-18:1n−9, trans-18:1n−8, cis-18:1n−8, cis-18:1n−5, and cis-24:1n−9 and FFA 14:1n−5, cis-18:1n−7, and 24:1n−9). Subjects with missing data for any given endpoint or covariate were excluded from that analysis. We conducted multiple linear regression analyses with the following dependent variables: fasting glucose, oral glucose tolerance (AUC glucose, incremental AUC glucose), systemic insulin sensitivity (at low- and high-level infusion of insulin), hepatic insulin resistance, liver fat (liver-spleen ratio), and β-cell function (AIRg, IV DI, and oral disposition index). Fasting glucose, systemic insulin sensitivity (at low- and high-level infusion of insulin), hepatic insulin resistance, and all 3 measures of β-cell function were natural log-transformed before regression analyses. Independent variables were plasma phospholipid 15:0, phospholipid 17:0, phospholipid trans-16:1n−7, FFA 15:0, FFA 17:0, and FFA trans-16:1n−7 (each separately). For all analyses, we computed 3 models: one crude unadjusted model (model 1); a model adjusted for age, sex, and BMI (model 2); and a model additionally adjusted for liver-spleen ratio (model 3). For AIRg, we also computed a fourth model that adjusted for insulin sensitivity (low-level insulin infusion) in addition to the matching variables (age, sex, and BMI) and liver fat. We also computed additional models in which we adjusted for hepatic insulin resistance, systemic insulin sensitivity, or β-cell function to assess to what extent the association between biomarkers of dairy fat intake and glucose tolerance (AUC glucose) may be mediated by these variables. Because we were analyzing a data set based on a case-control study as a cohort, we ran an additional model adjusted for case-control status (NAFLD compared with controls) in addition to age, sex, and BMI to test whether any observed associations are present independent of case-control status. Finally, we tested whether any observed associations between biomarkers of dairy fat intake and the metabolic endpoints may be attributable to confounding by other dietary variables by performing multiple regression analyses adjusted for each dietary variable separately in addition to age, sex, and BMI. The dietary variables tested included total fat, fruit, vegetables, whole grains, nuts, fish, coffee, PUFAs, fiber, fructose, and sugar-sweetened beverages (defined as the sum of soda, fruit juices and drinks, and energy and sports drinks). Because 15:0, 17:0, and trans-16:1n−7 in plasma phospholipids and FFAs are measures of the same exposure, dairy fat intake, we did not consider these 6 analyses as independent; therefore, we did not adjust our analyses for multiple testing. All 6 analyses therefore need to be interpreted together, and consideration needs to be given to the fact that an individual significant finding in the absence of significant associations for the other 5 biomarkers may be a result of an inflated overall α-error. P values <5% were considered statistically significant.
RESULTS
Subject characteristics
Subject characteristics for the 32 participants who completed all study procedures are shown in Table 1. Of the women, 4 of 6 in the control group and all in the NAFLD group were postmenopausal. The 2 groups were well matched for BMI and age. Notable differences existed in the liver-spleen ratio (P < 0.001); a lower ratio indicated more liver fat in the NAFLD group. Two cases had liver-spleen ratios of slightly >1 (1.09 and 1.10), which indicates borderline liver fat content. Both were kept in the NAFLD group because we had a biopsy-confirmed diagnosis of NAFLD in one and of NASH in the other. As expected, the NAFLD group also had higher plasma concentrations of the liver enzymes serum glutamic oxaloacetic transaminase and sGPT (P < 0.001), with a trend toward higher serum γ-glutamyl transferase concentrations (P = 0.058). In addition, their fasting glucose and insulin concentrations were higher, and their fasting triglycerides tended to be higher. Total fat mass was well matched in women, but tended to be lower in men with NAFLD than in men in the control group (P = 0.056).
TABLE 1.
Subject characteristics1
| Control group(n = 15) | NAFLD group(n = 17)2 | P value3 | |
| Age (y) | 51.3 ± 7.94 | 51.0 ± 7.8 | 0.905 |
| BMI (kg/m2) | 33.0 (6.3)5 | 31.7 (5.2) | 0.331 |
| Sex (M/F) | 9/6 | 12/5 | 0.628 |
| Liver-spleen ratio | 1.19 ± 0.15 | 0.71 ± 0.21 | <0.001 |
| Fat mass (% of total body mass) | |||
| Men | 35.2 ± 4.5 | 31.4 ± 4.1 | 0.056 |
| Women | 49.3 ± 5.4 | 47.0 ± 4.0 | 0.443 |
| Intraabdominal fat area (cm2) | |||
| Men | 183 ± 56 | 186 ± 90 | 0.944 |
| Women | 166 ± 62 | 216 ± 54 | 0.025 |
| Subcutaneous fat area (cm2) | |||
| Men | 381 ± 86 | 294 ± 68 | 0.194 |
| Women | 526 ± 207 | 477 ± 137 | 0.662 |
| Waist-to-hip ratio | |||
| Men | 0.98 ± 0.06 | 0.99 ± 0.05 | 0.706 |
| Women | 0.88 ± 0.07 | 0.94 ± 0.07 | 0.176 |
| Systolic blood pressure (mm Hg) | 126 ± 16 | 128 ± 13 | 0.630 |
| Diastolic blood pressure (mm Hg) | 81 ± 10 | 81 ± 7 | 0.986 |
| sGOT (U/L) | 20 (11) | 33 (14) | <0.001 |
| sGPT (U/L) | 26 (15) | 62 (52) | <0.001 |
| sGGT (U/L) | 26 (38) | 49 (46) | 0.058 |
| Glycated hemoglobin (%) | 5.67 ± 0.26 | 5.65 ± 0.22 | 0.870 |
| High-sensitivity C-reactive protein (mg/L) | 2.0 (6.1) | 2.9 (3.9) | 0.882 |
| Fasting glucose (mg/dL) | 94.3 (14.6) | 107.3 (17.9) | 0.011 |
| Fasting insulin (pmol/L) | 54.0 (62.8) | 105.6 (81.1) | 0.023 |
| Glucose tolerance (n) | 0.132 | ||
| Normal glucose tolerance | 5 | 4 | |
| Impaired fasting glucose | 3 | 2 | |
| Impaired glucose tolerance | 5 | 2 | |
| IFG + IGT | 1 | 7 | |
| Type 2 diabetes mellitus | 1 | 2 | |
| Fasting total adiponectin (μg/mL) | |||
| Men | 6.2 (5.7) | 7.1 (7.7) | 0.464 |
| Women | 11.1 (17.3) | 11.9 (4.1) | 0.792 |
| Fasting total triglycerides (mg/dL) | 97 (68) | 156 (150) | 0.082 |
| Fasting total cholesterol (mg/dL) | 191 ± 38 | 192 ± 31 | 0.876 |
| Fasting LDL cholesterol (mg/dL) | 121 ± 32 | 116 ± 23 | 0.667 |
| Fasting HDL cholesterol (mg/dL) | 45 ± 13 | 40 ± 12 | 0.248 |
| LDL relative flotation rate | 0.27 ± 0.02 | 0.26 ± 0.03 | 0.127 |
| Phospholipid 15:0 (% of total) | 0.18 ± 0.04 | 0.15 ± 0.03 | 0.047 |
| Phospholipid 17:0 (% of total) | 0.42 ± 0.07 | 0.37 ± 0.06 | 0.038 |
| Phospholipid trans-16:1n−7 (% of total) | 0.20 ± 0.05 | 0.15 ± 0.03 | 0.004 |
| Free fatty acid 15:0 (% of total) | 0.23 ± 0.05 | 0.19 ± 0.04 | 0.019 |
| Free fatty acid 17:0 (% of total) | 0.45 ± 0.09 | 0.40 ± 0.06 | 0.138 |
| Free fatty acid trans-16:1n−7 (% of total) | 0.15 ± 0.03 | 0.13 ± 0.03 | 0.175 |
IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NAFLD, nonalcoholic fatty liver disease; sGGT, serum γ-glutamyl transferase; sGOT, serum glutamic oxaloacetic transaminase; sGPT, serum glutamic pyruvic transaminase.
n = 15 for intraabdominal fat, subcutaneous fat, fasting insulin, and fatty acid composition of the free fatty acid fraction.
Significant difference set at P < 0.05 (independent-samples t tests for normally distributed variables or Mann-Whitney U test).
Mean ± SD (all such values).
Median; IQR in parentheses (all such values).
Four of 6 plasma biomarkers of dairy fat intake were significantly lower in participants with NAFLD than in controls (Table 1). This was consistent with significantly lower dairy product (P = 0.038) and butter (P = 0.029) intakes and with a trend toward lower full-fat cheese intake (P = 0.085) in those individuals with NAFLD than in controls for whom habitual dietary intake data from 3-d dietary records were available (Table 2). Other significant differences in habitual dietary intakes between these groups were greater intakes of poultry (P = 0.021) and fructose (P = 0.014), but a lower intake of SFAs (0.037) in participants with NAFLD. Subjects with NAFLD also tended to consume more sugary beverages (P = 0.075) and PUFAs (P = 0.097). Of note, the macronutrient composition, fiber content, and intake of alcohol and most major food groups including grains, fruit and vegetables, fish and meat (other than poultry), eggs, and sweets were very similar in subjects with NAFLD than in controls. With the exception of phospholipid 20:0 and FFA 14:0, which were the only other fatty acids showing statistically significant differences between control and NAFLD subjects in addition to the dairy fat biomarkers, the fatty acid compositions of plasma phospholipids and FFAs were remarkably similar (see Supplementary Tables 1 and 2 under “Supplemental data” in the online issue).
TABLE 2.
Composition of the participants’ habitual diets, based on a 3-d dietary record
| Control group(n = 11) | NAFLD1 group(n = 14) | P value2 | |
| Foods (servings/d) | |||
| Total dairy products | 4.4 ± 2.03 | 2.8 ± 2.1 | 0.038 |
| 4.9 (3.4)4 | 2.5 (2.4) | ||
| Reduced-fat milk | 0.7 ± 0.7 | 0.7 ± 1.3 | 0.244 |
| 0.4 (0.8) | 0.2 (0.9) | ||
| Full-fat milk | 0.1 ± 0.3 | 0.2 ± 0.6 | 0.851 |
| 0.0 (0.0) | 0.0 (0.1) | ||
| Reduced-fat cheese | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.851 |
| 0.0 (0.1) | 0.0 (0.2) | ||
| Full-fat cheese | 0.9 ± 0.7 | 0.4 ± 0.4 | 0.085 |
| 0.6 (1.1) | 0.3 (0.7) | ||
| Cream | 0.1 ± 0.2 | 0.2 ± 0.4 | 0.979 |
| 0.0 (0.1) | 0.0 (0.1) | ||
| Butter | 1.9 ± 1.8 | 0.8 ± 1.6 | 0.029 |
| 1.2 (2.3) | 0.1 (1.2) | ||
| Yogurt | 0.0 ± 0.1 | 0.2 ± 0.2 | 0.244 |
| 0.0 (0.0) | 0.0 (0.3) | ||
| Dairy-based desserts | 0.6 ± 0.9 | 0.2 ± 0.3 | 0.317 |
| 0.3 (0.7) | 0.0 (0.5) | ||
| Total fruit | 0.3 (1.3) | 1.5 (2.1) | 0.183 |
| Total vegetables | 2.7 (2.6) | 3.3 (4.0) | 0.291 |
| Total grains | 5.8 (6.2) | 7.5 (4.8) | 0.609 |
| Refined grains | 3.7 (3.3) | 3.9 (3.3) | 0.936 |
| Partially whole grains | 0.0 (1.4) | 0.0 (1.2) | 0.893 |
| Whole grains | 0.7 (2.5) | 1.3 (1.7) | 0.609 |
| Nuts | 0.0 (0.3) | 0.0 (1.1) | 0.809 |
| Fish | 0.0 (0.7) | 0.7 (2.0) | 0.120 |
| Red meat (beef, pork, lamb, game) | 1.3 (4.1) | 2.2 (1.7) | 0.687 |
| Processed meat | 0.7 (1.3) | 0.0 (0.6) | 0.202 |
| Poultry | 0.4 (1.0) | 1.9 (4.2) | 0.021 |
| Eggs | 0.7 (1.3) | 0.5 (1.0) | 0.609 |
| Coffee | 0.1 (1.0) | 1.7 (2.1) | 0.183 |
| Total sweets | 0.4 (1.3) | 0.8 (1.4) | 0.267 |
| Sugary beverages (soda, fruit juices, fruit drinks, energy drinks) | 0.0 (0.0) | 0.5 (1.5) | 0.075 |
| Alcoholic beverages | 0.0 (1.1) | 0.0 (0.1) | 0.609 |
| Nutrients | |||
| Energy (kcal/d) | 1929 ± 755 | 2335 ± 618 | 0.152 |
| Total protein (% of energy) | 16.9 ± 2.8 | 17.1 ± 3.1 | 0.908 |
| Total carbohydrates (% of energy) | 42.3 ± 6.6 | 42.9 ± 6.1 | 0.802 |
| Fructose (% of energy) | 5.4 ± 1.8 | 8.2 ± 3.1 | 0.014 |
| Fiber (g/1000 kcal) | 10.2 (5.7) | 9.9 (3.5) | 0.936 |
| Total fat (% of energy) | 39.8 ± 4.9 | 39.4 ± 7.3 | 0.858 |
| SFA (% of energy) | 15.3 ± 2.7 | 12.5 ± 3.4 | 0.037 |
| MUFA (% of energy) | 14.7 ± 1.5 | 14.9 ± 3.0 | 0.824 |
| PUFA (% of energy) | 6.6 ± 2.5 | 8.7 ± 3.2 | 0.097 |
| Alcohol (% of energy) | 0.0 (5.3) | 0.0 (1.5) | 0.893 |
| Glycemic index (100 = bread) | 84.4 ± 6.3 | 81.6 ± 4.3 | 0.186 |
NAFLD, nonalcoholic fatty liver disease.
Significant difference set at P < 0.05 (independent-samples t tests for normally distributed variables or Mann-Whitney U test).
Mean ± SD (all such values).
Median; IQR in parentheses (all such values).
Data from all 32 participants were available for fasting glucose, AUC glucose, incremental AUC glucose, the liver-spleen ratio (liver fat), and AIRg. Data on systemic insulin sensitivity were missing from 2 subjects, data on hepatic insulin sensitivity and IV DI were missing from 4 subjects, and data on oral DI were missing from 5 subjects. Phospholipid composition data were available from all 32 subjects, whereas FFA composition data were available from 30 subjects.
Relation between dairy fat biomarkers and measures of glucose tolerance
In a first step, we assessed the relation between biomarkers of dairy fat intake, fasting glucose, and oral glucose tolerance. Three of the 6 biomarkers of dairy fat intake were inversely associated with fasting glucose concentrations in a crude univariate model and with 4 of the 6 biomarkers after adjustment for BMI, sex, and age (Table 3, Figure 1A). Adjustment for liver-spleen ratio (a measure of liver fat content; model 3) barely attenuated the association between dairy fat intake and fasting glucose concentrations.
TABLE 3.
Multiple linear regression analyses of the relation between biomarkers of dairy fat intake and fasting glucose concentrations and oral glucose tolerance, as measured by the AUC for glucose during a 2-h oral-glucose-tolerance test1
| Oral glucose tolerance |
|||
| Model | Fasting glucose(n = 32)2 | Glucose AUC(n = 32)2 | Incremental glucose AUC(n = 32)2 |
| ln(mg/dL) | mg/dL · min | ||
| PL 15:0 | |||
| 1 | −1.816 (−2.899, −0.733)* | −33,873 (−63,876, −3869)* | −10,011 (−32,457, 12,435) |
| 2 | −1.841 (−2.913, −0.768)* | −34,272 (−63,883, −4661)* | −9,940 (−32,755, 12,874) |
| 3 | −1.833 (−2.980, −0.686)* | −28,202 (−58,821, 2417) | −4,202 (−27,365, 18,961) |
| PL 17:0 | |||
| 1 | −0.703 (−1.371, −0.035)* | −22,850 (−39,035, −6665)* | −13,462 (−25,220, −1703)* |
| 2 | −0.798 (−1.452, −0.144)* | −23,941 (−39,542, −8340)* | −13,259 (−25,126, −1392)* |
| 3 | −0.834 (−1.592, −0.075)* | −21,060 (−39,010, −3110)* | −10,109 (−23,628, 3410) |
| PL t-16:1n−7 | |||
| 1 | −1.036 (−2.081, 0.008) | −26,160 (−52,809, 489) | −12,775 (−32,030, 6481) |
| 2 | −1.310 (−2.416, −0.204)* | –36,018 (−63,213, −8824)* | −18,683 (−39,220, 1853) |
| 3 | −1.358 (−2.639, −0.077)* | −29,916 (−60,997, 1166) | −12,350 (−35,529, 10,828) |
| FFA 15:0 | |||
| 1 | −1.179 (−2.043, −0.316)* | −31,701 (−52,064, −11,338)* | −16,085 (−31,143, −1027)* |
| 2 | −1.086 (−2.077, −0.096)* | −32,426 (−55,447, −9405)* | −17,995 (−35,134, −856)* |
| 3 | −1.016 (−2.116, 0.084) | −27,342 (−52,392, −2293)* | −14,120 (−32,748, 4508) |
| FFA 17:0 | |||
| 1 | −0.474 (−1.077, 0.129) | −18,610 (−32,147, −5074)* | −12,200 (−21,589, −2811)* |
| 2 | −0.536 (−1.186, 0.113) | −18,071 (−33,126, −3016)* | −10,957 (−21,841, −72)* |
| 3 | −0.466 (−1.203, 0.271) | −14,255 (−31,029, 2519) | −8,283 (−20,428, 3862) |
| FFA t-16:1n−7 | |||
| 1 | −1.384 (−3.056, 0.289) | −31,900 (−72,816, 9017) | −13,833 (−42,632, 14,967) |
| 2 | −1.230 (−3.328, 0.867) | −35,252 (−86,061, 15,558) | −18,945 (−55,190, 17,300) |
| 3 | −1.006 (−3.189, 1.176) | −25,086 (−75,933, 25,762) | −12,072 (−48,554, 24,409) |
Data are unadjusted β coefficients (95% CIs). Model 1, unadjusted; model 2, adjusted for age, sex, and BMI; model 3, adjusted as for model 2 plus liver-spleen ratio, a measure of liver fat content. *Statistically significant association, P < 0.05. FFA, free fatty acid; PL, phospholipid.
n = 32 for PL analyses and n = 30 for FFA analyses.
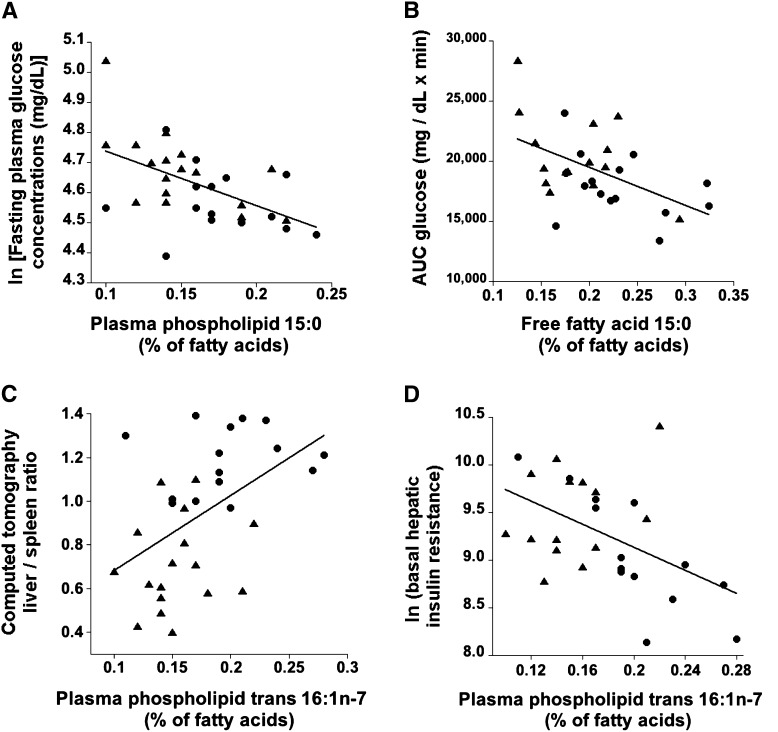
FIGURE 1.
Bivariate associations in subjects with nonalcoholic fatty liver disease (▴) and controls (•) between biomarkers of dairy fat intake and fasting glucose concentrations (A; r = −0.530, P = 0.002; n = 32); glucose tolerance (B), as assessed by the total AUC for glucose during a 2-h frequently sampled oral-glucose-tolerance test (r = −0.516, P = 0.004; n = 30); liver fat content (C), as assessed by the liver-spleen ratio in a computed tomography scan (liver-spleen ratio is inversely associated with liver fat mass: r = 0.488, P = 0.005; n = 32); and hepatic insulin resistance (D), as assessed by the basal hepatic glucose production rate (mg/min) × fasting insulin concentration (pmol/L) (r = −0.497, P = 0.007; n = 28).
Four of the 6 biomarkers were inversely associated with the AUC for glucose in the crude models (model 1) and 5 of 6 after adjustment for BMI, sex, and age (model 2; Table 3, Figure 1B). Three of 6 biomarkers (17:0 in both phospholipids and FFAs and 15:0 in FFA) were inversely associated with the incremental AUC for glucose (Table 3). For the associations of biomarkers of dairy fat intake with both total and incremental AUC for glucose, adjustment for liver fat content led to a substantial attenuation in the strength of the association, often rendering the associations nonsignificant (Table 3). However, the associations between the AUC for glucose and phospholipid 17:0 and FFA 15:0 remained statistically significant even after adjustment for liver fat content in addition to age, sex, and BMI. Similar to the adjustment for liver fat content, adjustment of model 2 (adjusted for BMI, sex, and age) for hepatic insulin resistance or systemic insulin sensitivity strongly attenuated the association between biomarkers of dairy fat intake and the AUC for glucose (see Supplementary Table 3 under “Supplemental data” in the online issue). In contrast, adjustment for pancreatic β-cell function (AIRg) strengthened the association between dairy fat intake and the AUC for glucose (see Supplementary Table 3 under “Supplemental data” in the online issue).
Relation between dairy fat biomarkers and liver fat content and hepatic and systemic insulin sensitivity
Glucose tolerance is largely determined by systemic insulin sensitivity and pancreatic β-cell function. We therefore investigated the relation between dairy fat intake and systemic and hepatic insulin sensitivity and liver fat—a determinant of hepatic insulin sensitivity. The trans-16:1n−7 content in plasma phospholipids was positively associated with systemic insulin sensitivity (during both low-level and high-level insulin infusion), both in the crude univariate model and in the model adjusted for BMI, sex, and age (Table 4). These associations were attenuated and were no longer statistically significant after adjustment for liver fat (model 3). Four of the 6 biomarkers of dairy fat intake were robustly and inversely associated with liver fat (ie, positively with the liver-spleen ratio) in the model adjusted for age, sex, and BMI (model 2 in Table 4;Figure 1C). Only phospholipid trans-16:1n−7 was inversely associated with hepatic insulin resistance in the unadjusted model and after adjustment for BMI, sex, and age (Table 4;Figure 1D). The association between phospholipid trans-16:1n−7 and hepatic insulin resistance was slightly attenuated and became nonsignificant after further adjustment for liver fat (Table 4).
TABLE 4.
Multiple linear regression analyses of the relation between biomarkers of dairy fat intake and the liver-spleen ratio, a measure of liver fat content (inverse association), hepatic insulin resistance, and systemic insulin sensitivity1
| Systemic insulin sensitivity |
||||
| Model | Liver-spleen ratio (liver fat content)(n = 32)2 | Hepatic insulin resistance(n = 28) | Low-level insulin infusion(n = 30)3 | High-level insulin infusion(n = 30)3 |
| ln(mg/min · pmol/L) | ln(mg · min−1 · kg lean mass−1 per pmol/L insulin) | |||
| PL 15:0 | ||||
| 1 | 2.58 (−0.24, 5.39) | −2.46 (−8.41, 3.48) | 0.84 (−10.7, 12.4) | 0.60 (−10.7, 11.9) |
| 2 | 2.39 (−0.61, 5.40) | −1.21 (−7.09, 4.67) | −0.60 (−12.5, 11.3) | −0.78 (−12.3, 10.7) |
| 3 | NA | 0.18 (−5.72, 6.08) | −3.24 (−15.1, 8.59) | −3.28 (−14.8, 8.20) |
| PL 17:0 | ||||
| 1 | 2.00 (0.50, 3.50)* | −3.04 (−6.16, 0.09) | 5.33 (−0.79, 11.4) | 4.99 (−1.02, 11.0) |
| 2 | 2.13 (0.58, 3.69)* | −2.68 (−5.84, 0.47) | 5.93 (−0.36, 12.2) | 5.72 (−0.36, 11.8) |
| 3 | NA | −1.76 (−5.35, 1.82) | 4.38 (−2.86, 11.6) | 4.31 (−2.71, 11.3) |
| PL t-16:1n−7 | ||||
| 1 | 3.39 (1.13, 5.66)* | −6.24 (−10.6, −1.84)* | 10.3 (1.38, 19.1)* | 10.04 (1.35, 18.7)* |
| 2 | 3.56 (0.94, 6.18)* | −5.95 (−10.8, −1.09)* | 11.2 (1.09, 21.2)* | 10.97 (1.27, 20.7)* |
| 3 | NA | −4.91 (−10.5, 0.67) | 9.06 (−2.45, 20.6) | 9.12 (−2.00, 20.2) |
| FFA 15:0 | ||||
| 1 | 2.35 (0.30, 4.41)* | −3.08 (−7.41, 1.26) | 8.91 (0.67, 17.2)* | 8.83 (0.82, 16.9)* |
| 2 | 2.55 (0.11, 4.99)* | −2.21 (−6.81, 2.39) | 7.24 (−2.20, 16.7) | 6.89 (−2.24, 16.0) |
| 3 | NA | −0.95 (−5.76, 3.85) | 5.02 (−4.97, 15.0) | 4.79 (−4.89, 14.5) |
| FFA 17:0 | ||||
| 1 | 1.33 (−0.03, 2.68) | −1.16 (−4.00, 1.67) | 1.53 (−4.16, 7.2) | 1.44 (−4.11, 6.99) |
| 2 | 1.80 (0.29, 3.30)* | −0.74 (−3.65, 2.17) | 1.15 (−5.02, 7.3) | 1.10 (−4.86, 7.06) |
| 3 | NA | 0.38 (−2.73, 3.49) | −1.27 (−7.86, 5.3) | −1.19 (−7.57, 5.19) |
| FFA t-16:1n−7 | ||||
| 1 | 3.01 (−0.85, 6.88) | −6.41 (−13.9, 1.10) | 12.0 (−3.11, 27.0) | 12.51 (−2.08, 27.1) |
| 2 | 3.20 (−1.91, 8.31) | −4.79 (−13.7, 4.12) | 7.95 (−11.1, 27.0) | 8.14 (−10.2, 26.5) |
| 3 | N/A | −3.19 (−12.1, 5.73) | 4.50 (−14.6, 23.6) | 4.90 (−13.5, 23.3) |
Data are unadjusted β coefficients (95% CIs). Model 1, unadjusted; model 2, adjusted for age, sex, and BMI; model 3, adjusted as for model 2 plus liver-spleen ratio, a measure of liver fat content. *Statistically significant association, P < 0.05. FFA, free fatty acid; NA, not available; PL, phospholipid.
n = 32 for PL analyses and n = 30 for FFA analyses.
n = 30 for PL analyses and n = 28 for FFA analyses.
Relation between dairy fat biomarkers and β-cell function
No association was found between any of the 6 biomarkers of dairy fat intake and β-cell function (Table 5). Adjustment for insulin sensitivity (as assessed during low-level insulin infusion) in addition to BMI, sex, age, and liver fat content had very little effect on the relation between dairy fat intake and AIRg (data not shown).
TABLE 5.
Multiple linear regression analyses of the relation between biomarkers of dairy fat intake and measures of pancreatic β-cell function1
| Model | AIRg(n = 32) | Oral DI(n = 27) | IV DI(n = 28) |
| ln(pmol/L) | ln[(mg/dL)−1] | ln(mg · min−1 · kg lean mass−1) | |
| PL 15:0 | |||
| 1 | 0.91 (−9.80, 11.6) | 2.68 (−4.23, 9.60) | 1.43 (−10.2, 13.1) |
| 2 | 2.12 (−9.27, 13.5) | 2.73 (−4.95, 10.4) | 1.68 (−11.0, 14.3) |
| 3 | 3.46 (−8.60, 15.5) | 0.88 (−6.92, 8.68) | 1.21 (−12.3, 14.7) |
| PL 17:0 | |||
| 1 | 3.97 (−1.87, 9.80) | 3.51 (−0.10, 7.12) | 6.28 (0.31, 12.3)* |
| 2 | 4.35 (−1.82, 10.5) | 3.82 (−0.24, 7.87) | 6.24 (−0.45, 12.9) |
| 3 | 6.81 (−0.05, 13.7) | 2.72 (−1.89, 7.33) | 7.40 (−0.35, 15.2) |
| PL t-16:1n−7 | |||
| 1 | −3.22 (−12.5, 6.04) | 3.51 (−2.18, 9.21) | 0.63 (−9.14, 10.4) |
| 2 | −1.67 (−12.4, 9.08) | 4.34 (−2.65, 11.3) | 1.24 (−10.6, 13.0) |
| 3 | −0.18 (−12.6, 12.2) | 1.84 (−6.10, 9.78) | 0.47 (−13.3, 14.2) |
| FFA 15:0 | |||
| 1 | −3.27 (−11.5, 4.94) | 3.16 (−2.00, 8.33) | 0.47 (−8.25, 9.17) |
| 2 | −2.32 (−12.1, 7.45) | 3.75 (−2.30, 9.79) | 2.15 (−7.90, 12.2) |
| 3 | −1.41 (−12.2, 9.41) | 2.06 (−4.39, 8.50) | 1.82 (−9.21, 12.9) |
| FFA 17:0 | |||
| 1 | 1.82 (−3.49, 7.14) | 1.09 (−2.21, 4.38) | 3.90 (−1.43, 9.22) |
| 2 | 2.29 (−3.89, 8.35) | 1.30 (−2.48, 5.07) | 4.05 (−1.99, 10.1) |
| 3 | 3.76 (−3.07, 10.6) | −0.08 (−4.14, 3.97) | 4.47 (−2.39, 11.3) |
| FFA t-16:1n−7 | |||
| 1 | −6.19 (−20.9, 8.56) | −1.01 (−10.6, 8.56) | 0.28 (−15.1, 15.6) |
| 2 | −3.49 (−22.9, 15.9) | −1.82 (−14.3, 10.7) | 4.82 (−14.7, 24.4) |
| 3 | −2.22 (−22.6, 18.2) | −5.14 (−17.4, 7.11) | 4.33 (−16.3, 24.9) |
Data are unadjusted β coefficients (95% CIs). Model 1, unadjusted; model 2, adjusted for age, sex, and BMI; model 3, adjusted as for model 2 plus liver-spleen ratio, a measure of liver fat content. *Statistically significant association, P < 0.05. AIRg, acute insulin response to glucose; DI, disposition index; FFA, free fatty acid; IV DI, intravenous disposition index; PL, phospholipid.
One potential concern was that the association between dairy fat intake and glucose tolerance, insulin sensitivity, and liver fat may be explained by reverse causation, ie, patients with NAFLD may be actively avoiding fatty foods, including dairy fat. However, on the basis of the dietary records available, the NAFLD and control groups did not differ (P = 0.86) in reported mean (±SD) total fat intake: 39.4% ± 7.3% of total energy intake in the NAFLD group (n = 14) compared with 39.8% ± 4.9% in the control group (n = 11). Furthermore, adjustment for total fat intake in the multiple regression models in addition to age, sex, and BMI (model 2) had little effect on the β-coefficients. Specifically, the β-coefficients describing the association between biomarkers of dairy fat intake and metabolic health-related endpoints changed by a mean (±SD) of 1.4 ± 1.7% when total fat intake was included in the 19 models (model 2 in Tables 3 and 4) that indicated a statistically significant association. We also addressed this issue by adjusting for case-control status in these statistically significant multiple regression models (model 2 in Tables 3 and 4), in addition to age, sex, and BMI. Because case-control status was strongly correlated with the liver-spleen ratio (Spearman's correlation coefficient r = −0.816, P < 0.001), adjustment for this variable had an effect on the relation between biomarkers of dairy fat intake, liver fat, glucose tolerance, and insulin sensitivity similar to that of adjustment for the liver-spleen ratio. As in those models (Table 3), phospholipid 15:0 remained significantly associated with fasting glucose concentrations (β = −1.553; 95% CI: −2.685, −0.422; P = 0.009) and the phospholipid 17:0 content remained significantly associated with the AUC for glucose (β = −18,204; 95% CI: −34,822, −1587; P = 0.033), even after adjustment for case-control status. Other observed associations, however, were strongly attenuated and no longer statistically significant after adjustment for case-control status. This was particularly the case for associations between biomarkers of dairy fat intake and the liver-spleen ratio and the different measures of hepatic and systemic insulin sensitivity.
Finally, we assessed whether any associations between dairy fat intake, liver fat, glucose tolerance, and insulin sensitivity may be confounded by dietary variables other than fat. In additional analyses built on the 19 statistically significant associations (model 2), as shown in Tables 3 and 4, inclusion of most foods into the model had very little effect on the β-coefficients describing the relation between a biomarker of dairy fat intake and an endpoint related to metabolic health. Specifically, the mean (±SD) changes in the β-coefficient when model 2 was adjusted for specific foods were as follows: fruit, −2.4 ± 15.5%; vegetables, −0.9 ± 6.9%; whole grains, +0.8 ± 2.8%; nuts, +1.2 ± 5.4%; fish, −8.5 ± 4.6%; coffee, +1.4 ± 18.4%; and fiber, +1.7 ± 8.3%. The only dietary variables that substantially (ie, by more than 10%) and consistently reduced the estimates were dietary PUFA content (−12.3 ± 7.5%), fructose intake (−11.0 ± 8.0%), and the consumption of sugar-sweetened beverages (−15.4 ± 12.7%).
DISCUSSION
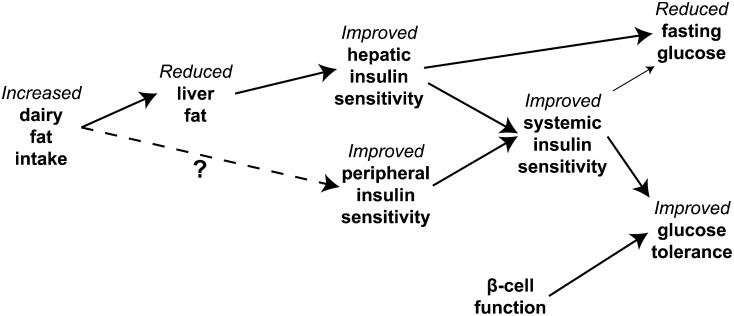
In this cross-sectional clinical investigation, we found strong and consistent associations between established biomarkers of dairy fat intake and measures of metabolic health. As we had hypothesized, the consumption of greater amounts of dairy fat was associated with lower fasting glucose concentrations, better glucose tolerance in response to a standardized OGTT, higher systemic and hepatic insulin sensitivity, and less liver fat. The fact that the relation between biomarkers of dairy fat intake and glucose tolerance was attenuated when we adjusted for liver fat or measures of systemic or hepatic insulin sensitivity, but not β-cell function, suggests that the relation between dairy fat and glucose tolerance may be largely mediated by greater insulin sensitivity because of reduced liver fat content in individuals consuming more dairy fat, as proposed in Figure 2.
FIGURE 2.
The mechanism through which higher intakes of dairy fat in general, or trans-palmitoleic acid in particular, may affect fasting glucose concentrations and glucose tolerance include a reduced liver fat content leading to improved hepatic and systemic insulin sensitivity.
Our results are consistent with the previous work our hypothesis was based on (1–4). The work conducted in the Hotamisligil laboratory in transgenic mice suggests that cis-palmitoleic acid may be an endogenous regulator of hepatic fat metabolism, insulin sensitivity, and glucose tolerance (3, 4). The subsequent work by Mozaffarian et al (1, 2) extended this work to dietary sources of palmitoleic acid and observed an intriguing inverse association between trans-16:1n−7 in fasting plasma phospholipids and the prospective risk of type 2 diabetes in 2 US cohorts. The authors hypothesized that trans-16:1n−7 may have effects on hepatic fat content, insulin sensitivity, and glucose tolerance similar to those of cis-palmitoleic acid. However, data on liver fat, insulin sensitivity, or glucose tolerance beyond fasting measures of glucose and insulin were not available in their study. Our finding therefore complements the human observational data by Mozaffarian nicely, which suggests that dietary dairy fat in general, or trans-16:1n−7 in particular, may improve glucose tolerance by decreasing liver fat and improving hepatic and systemic insulin sensitivity.
It is important to note that although Hotamisligil, Mozaffarian, and colleagues focused on trans-16:1n−7, it remains unclear whether trans-16:1n−7 is indeed the active component. For example, phytanic acid is another minor fatty acid in dairy fat that is a potent agonist of peroxisome proliferator–activated receptor α (20)—a transcription factor in the liver that plays an important role in regulating liver fat oxidation. It is possible that trans-16:1n−7 acts synergistically with phytanic and possibly other fatty acids to stimulate hepatic β-oxidation and/or inhibit de novo lipogenesis. It is also possible that trans-16:1n−7 or other dairy fatty acids act on other tissues such as the muscle or adipose tissue to improve insulin sensitivity.
Limitations of this study include the small sample size and the cross-sectional and observational nature of the project. Thus, we were unable to determine cause and effect. Most importantly, we cannot rule out reverse causation, which remains a concern even though total fat intake did not differ between the NAFLD and control groups, and adjustment for total fat content had no effect on the association between biomarkers of dairy fat intake and endpoints related to metabolic health. It is important to note that most of the associations were greatly attenuated and no longer statistically significant when adjusted for case-control status. On the one hand, this may indicate that the observed associations were the result of a systematic bias such as reverse causation, triggered by reduced dairy fat intake in the NAFLD group as a consequence of their diagnosis. On the other hand, adjustment for case-control status may have such a profound effect on the associations because case-control status was very strongly associated with liver fat content, and liver fat content is in the hypothesized pathway between dairy fat intake and improved insulin sensitivity and glucose tolerance. Whereas the data on the subjects’ habitual diets do not suggest that those with NAFLD consumed less total fat than did controls, and because adjustment for total fat intake had little effect on the models, it appears unlikely that our findings are largely attributable to reverse causation. However, we cannot conclusively rule out that possibility. At the same time, it is important to note that some associations between dairy fat biomarkers and fasting glucose and glucose tolerance (AUC for glucose) remained statistically significant even after adjustment for case-control status. The fact that adjustment for PUFA, fructose, and sugar-sweetened beverage intakes consistently attenuated the estimates by 10–20% suggests that the observed association between dairy fat intake and metabolic health may have been partly a result of other dietary characteristics in individuals with low dairy fat intake, notably an increased intake of sugar-sweetened beverages and fructose and PUFAs. This is consistent with the fact that participants with NAFLD tended to have higher intakes of these foods than did controls (Table 2). However, it is important to note that the association between dairy fat intake and endpoints related to metabolic health commonly remained statistically significant (or close to significant) even after adjustment for PUFA, fructose, or sugar-sweetened beverage intakes. As in any observational study, it is important to point out that our findings may have been affected by residual and unmeasured confounding by other factors. Finally, our results may not be generalizable given that all subjects in this study were overweight or obese and that we specifically recruited individuals with NAFLD and matching controls. The relation between trans-16:1n−7 and dairy fat on the one hand and liver fat and metabolic health on the other hand may be different in leaner individuals and even in the general obese population. Related to this is a possible selection bias in the control group that may have caused us to underestimate the true association between dairy fat and metabolic health. Controls who volunteer for case-control studies tend to be healthier and more health conscious than the general population, and healthy behaviors are associated with a lower intake of dairy fat in the United States (7). Whereas it is unclear whether this was the case in this particular study, such an effect would attenuate any association between dairy fat intake and improved metabolic health. The study is strengthened by the fact that the participants were very well phenotypically characterized. Most NAFLD cases were confirmed by liver biopsy, and controls were well matched by BMI and age. The matching helped to disentangle the otherwise tight association between BMI on the one hand and liver fat, insulin resistance, and glucose intolerance on the other hand. The metabolic assessments used state-of-the-art measurements of systemic and hepatic insulin sensitivity with the hyperinsulinemic euglycemic clamp. Furthermore, dairy fat intake was assessed by an objective, validated set of biomarkers rather than by diet record or food-frequency questionnaire. Last, the fact that our hypothesis was based on a set of previous studies in mice and humans that together with our data provide consistent results makes it less likely that the observed associations are chance findings.
We conclude that the plasma concentrations of established biomarkers of dairy fat intake, including trans-16:1n−7, are robustly associated with lower fasting glucose concentrations, greater oral glucose tolerance, reduced liver fat, and greater hepatic and systemic insulin sensitivity but not with pancreatic β-cell function. Together with previously published data by Mozaffarian et al (1, 2), our results suggest that dairy fat may have beneficial effects on liver fat and lead to improved hepatic and systemic insulin sensitivity and improved glucose tolerance. Because of the potential for reverse causation, and other limitations of this and previous observational studies, this hypothesis needs to be confirmed in a randomized controlled trial.
Supplementary Material
Acknowledgments
We are grateful to the study participants for their contribution and time. We also thank the nursing staff at the University of Washington Clinical Research Center and Tiffany Speron, Sherree Miller, George Ioannou, and Jeff Maki for assistance with the study. None of the funding agencies had any influence over the design or conduct of the study or the interpretation and publication of the findings.
The authors’ responsibilities were as follows—MK, JEN, MMY, KVK, and KMU: designed the research; MK, JEN, SM, HSC, XS, and KMU: conducted the research; MK, CD, and KMU: analyzed the data and performed the statistical analyses; and MK and KMU: wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript. MK has received compensation for travel and conference fees and speaker's honoraria from the Global Dairy Platform and the Dairy Research Institute. None of the other authors had any conflicts of interest.
Footnotes
Abbreviations used: AIRg, acute insulin response to glucose; FFA, free fatty acid; IV DI, intravenous disposition index; IVGTT, intravenous-glucose-tolerance test; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OGTT, oral-glucose-tolerance test; sGPT, serum glutamic pyruvic transaminase.
REFERENCES
- 1.Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GS. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med 2010;153:790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab 2005;1:107–19. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008;134:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 2007;86:929–37. [DOI] [PubMed] [Google Scholar]
- 6.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 7.Kratz M, Baars T, Guyenet S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur J Nutr 2013;52:1–24. [DOI] [PubMed] [Google Scholar]
- 8.Wolk A, Vessby B, Ljung H, Barrefors P. Evaluation of a biological marker of dairy fat intake. Am J Clin Nutr 1998;68:291–5. [DOI] [PubMed] [Google Scholar]
- 9.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(suppl 1):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longo R, Ricci C, Masutti F, Vidimari R, Croce LS, Bercich L, Tiribelli C, Dalla Palma L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol 1993;28:297–302. [PubMed] [Google Scholar]
- 12.Friedewald WT, Levy J, Fredrickson DS. Estimation of the concentration of low-density-lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003;285:E906–16. [DOI] [PubMed] [Google Scholar]
- 14.Yarasheski KE, Cade WT, Overton ET, Mondy KE, Hubert S, Laciny E, Bopp C, Lassa-Claxton S, Reeds DN. Exercise training augments the peripheral insulin-sensitizing effects of pioglitazone in HIV-infected adults with insulin resistance and central adiposity. Am J Physiol Endocrinol Metab 2011;300:E243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 2008;47:348–80. [DOI] [PubMed] [Google Scholar]
- 16.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 17.Schlierf G, Wood P. Quantitative determination of plasma free fatty acids and triglycerides by thin-layer chromatography. J Lipid Res 1965;6:317–9. [PubMed] [Google Scholar]
- 18.Lepage G, Roy CL. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20. [PubMed] [Google Scholar]
- 19.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research - principles and practice of kinetic analyses. Hoboken, NJ: John Wiley & Sons, Inc, 2005. [Google Scholar]
- 20.Hellgren LI. Phytanic acid—an overlooked bioactive fatty acid in dairy fat? Ann N Y Acad Sci 2010;1190:42–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.