Abstract
Background: Effects of alcohol consumption on health and disease are complex and involve a number of cellular and metabolic processes.
Objective: We examined the association between alcohol consumption habits and metabolomic profiles.
Design: We conducted a cross-sectional study to explore the association of alcohol consumption habits measured by using a questionnaire with serum metabolites measured by using untargeted mass spectrometry in 1977 African Americans from the Jackson field center in the Atherosclerosis Risk in Communities Study. The whole sample was split into a discovery set (n = 1500) and a replication set (n = 477). Alcohol consumption habits were treated as an ordinal variable, with nondrinkers as the reference group and quartiles of current drinkers as ordinal groups with higher values. For each metabolite, a linear regression was conducted to estimate its relation with alcohol consumption habits separately in both sets. A modified Bonferroni procedure was used in the discovery set to adjust the significance threshold (P < 1.9 × 10−4).
Results: In 356 named metabolites, 39 metabolites were significantly associated with alcohol consumption habits in both discovery and replication sets. In general, alcohol consumption was associated with higher levels of most metabolites such as those in amino acid and lipid pathways and with lower levels of γ-glutamyl dipeptides. Three pathways, 2-hydroxybutyrate-related metabolites, γ-glutamyl dipeptides, and lysophosphatidylcholines, which are considered to be involved in inflammation and oxidation, were associated with incident cardiovascular diseases.
Conclusions: To our knowledge, this is the largest metabolomic study thus far conducted in nonwhites. Metabolomic biomarkers of alcohol consumption were identified and replicated. The results lend new insight into potential mediating effects between alcohol consumption and future health and disease.
INTRODUCTION
Benefits of moderate alcohol consumption for the prevention of heart disease have been reported in observational studies (1, 2), but evidence from clinical trials has been lacking (1). In contrast, harms associated with heavy alcohol use are well established (3, 4). Regardless of whether the effects are beneficial or harmful, physiologic effects of alcohol consumption on health and disease are complex and involve a number of cellular and metabolic processes (5). Only a few studies have explored the effects of alcohol consumption habits on health and disease in African Americans, most of which concluded that associations of alcohol consumption with health and disease may not be consistent with those observed in whites (6–8).
Metabolomics hold promise for novel biomarker discovery and development of an improved understanding of underlying exposure-disease relations. Metabolomic approaches have rarely been used in alcohol-related research (5). Therefore, we explored cross-sectional associations of multiple named metabolites quantified by an untargeted high-throughput mass spectrometry–based protocol with alcohol consumption habits and, in addition, longitudinal associations of selected alcohol-related metabolites with future cardiovascular diseases in a well-characterized, population-based sample of African Americans from the Atherosclerosis Risk in Communities (ARIC)4 Study.
SUBJECTS AND METHODS
Study design and population
The ARIC Study consists of a prospective cohort designed to identify causes and outcomes of cardiovascular disease in 15,792 individuals from 4 communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD). Detailed descriptions of its study design, objectives, and procedures have been published elsewhere (9). ARIC Study participants underwent interviews, fasting venipuncture, and anthropometric measurements at baseline and follow-up examinations. Trained interviewers ascertained basic demographic data, medical histories, and information about personal diet habits.
Self-reported alcohol consumption habits were ascertained at the baseline examination by means of an interviewer-administered dietary food-frequency questionnaire. Participants were asked whether they currently drank alcohol beverages and, if not, whether they ever drank alcohol beverages. Current drinkers were asked how often they usually drank wine, beer, or hard liquor, and the amount of alcohol consumed (in g/wk) was calculated with the assumption that 4 oz wine was equal to 10.8 g, 12 oz beer was equal to 13.2 g, and 1.5 oz liquor was equal to 15.1 g ethanol. For a drinker who reported <1 drink/wk, alcohol consumption was recorded as 0 g/wk. The level of alcohol consumption in ARIC participants was general low (10). In this study, current drinkers were further subclassified into 4 groups by quartiles with corresponding names as follows: rare drinkers (<26.0 g/wk), light drinkers (26.0–59.9 g/wk), low-moderate drinkers (60.0–135.5 g/wk), and moderate-high drinkers (>135.5 g/wk) (10, 11).
Participants provided all of their medications for transcription by field-center staff. Race was self-reported. BMI (in kg/m2) was calculated as weight divided by the square of height and measured by field-center staff. The estimated glomerular filtration rate (eGFR) was calculated by using the Chronic Kidney Disease Epidemiology Collaboration equation (12). Cigarette smoking status was self-reported and categorized as a current and noncurrent smoker.
See Supplementary Material under “Supplemental data” in the online issue for detailed information about clinical endpoints. Definitions of prevalent and incident hypertension and heart failure (HF) were described previously (13, 14). Briefly, hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking antihypertensive medication, and HF was defined by a clinical diagnosis or medication use for HF. Prevalent coronary heart disease (CHD) was defined by evidence of previous myocardial infarction (MI) by an electrocardiogram at baseline, self-reported history of physician-diagnosed MI, or a previous coronary reperfusion procedure (9). The ARIC definition of hospitalized MI includes events that meet one of the following criteria: 1) an evolving diagnostic electrocardiography pattern, 2) a diagnostic electrocardiography pattern and abnormally elevated cardiac enzymes (≥2 times normal), 3) cardiac pain and abnormal enzymes, 4) cardiac pain and slightly elevated enzymes (between upper limits of normal and twice upper limits of normal) and an evolving ST-T pattern or diagnostic electrocardiography pattern, and 5) abnormally elevated enzymes and an evolving ST-T pattern (15). A hospitalized incident of MI was defined as one in a patient for whom the medical record either stated that there was no previous history of MI or did not contain any reference to a previous history of MI (15). Incident hypertension was ascertained at each follow-up examination (3, 6, and 9 y after the baseline visit) by blood pressure measurement and medication use. Incident HF and incident CHD were determined by contacting participants annually and surveying discharge lists from local hospitals and death certificates for potential cardiovascular events until 31 December 2008.
Serum metabolomic profiles were measured in a subsample of ARIC African American participants at the baseline examination who were randomly selected from the Jackson, MS, field center, consented for genetic research, provided dietary quality data, and fasted ≥8 h before the baseline exam (n = 1977). Metabolomic profiling was completed in June 2010 by using fasting serum samples that had been stored at −80°C since collection at the baseline examination in 1987–1989. An untargeted, gas chromatography– and liquid chromatography– mass spectrometry–based metabolomic quantification protocol was used to detect and analyze samples by Metabolon Inc (16, 17). Summary assay procedures have been described in previous work (14). This untargeted approach identifies and quantifies named compounds with unknown chemical identities as well as additional unnamed compounds that do not currently have a chemical standard. Only named compounds (n = 356) were included in the current study. Local institutional review boards approved the ARIC protocol, and all subjects gave written informed consent.
Statistical analysis
On the basis of both practical and theoretical considerations, the 356 named metabolites were divided into 3 groups according to their percentages of values that were missing or below the detection limit (m/bdl) for that metabolite in all participants (n = 1977). Group 1 contained metabolites (n = 308) with <50% of observations with m/bdl values. Levels of group 1 metabolites were analyzed as a continuous variable with m/bdl values replaced by the lowest detected level. Group 2 contained metabolites (n = 29) that had a moderate number of m/bdl values (50–80% of observations). For group 2 metabolites, we considered m/bdl values as category 1, measured (ie, non-m/bdl) values below the median as category 2, and measured values above the median as category 3. Group 3 contained metabolites (n = 19) that had >80% of observations with m/bdl values, and we considered m/bdl values as category 1, whereas measured values were considered as category 2. During data analysis, levels of group 2 and 3 metabolites were analyzed as ordinal variables by using the previously mentioned categories in each group.
Data are presented as means (±SEs) for continuous variables and numbers (percentages) for categorical variables. Baseline characteristics were compared by using the chi-square test for categorical variables and 2-sample t test for continuous variables. The alcohol consumption volume was treated as an ordinal variable in all analyses (nondrinker: 0; rare drinker: 1; light drinker: 2; low-moderate drinker: 3; and moderate-high drinker: 4). In current drinkers, the type of alcohol beverage predominantly consumed was defined when the amount of ethanol of one type of beverage (wine, beer, or liquor) corresponded to two-thirds or more of the total amount of ethanol consumed (7, 11, 18).
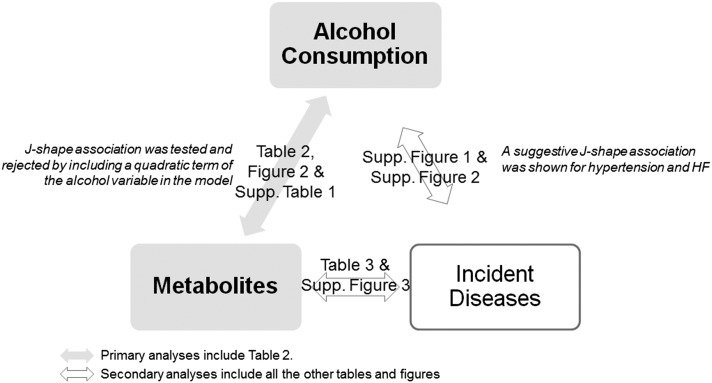
A schematic of analyses in this study is presented in Figure 1. For the primary analysis, in all participants with metabolomic data, 1500 randomly selected participants were included in the discovery set, whereas the remaining 477 participants were included in the replication set. Linear regression analyses were conducted to estimate the relation of alcohol consumption habits with each metabolite in both discovery and replication sets. Adjustments include age, sex, BMI, current cigarette smoking status, and eGFR at baseline. β coefficients in linear models represented the change in SD units of each metabolite in group 1 or change in the categorical unit of each metabolite in groups 2 and 3 for each unit difference in the ordinal grouping of alcohol consumption (eg, light compared with low-moderate drinkers). The corresponding P-trend value across alcohol ordinal groups was also calculated. Linearity in associations of alcohol consumption with metabolite levels was examined and not rejected by comparing means across alcohol groups. With the introduction of a quadratic term of the ordinal alcohol variable in the previously mentioned linear regressions (data not shown), a possible J-shaped association was tested and rejected.
FIGURE 1.
Analysis schematic. HF, heart failure; Supp., Supplemental.
Statistical significance was prespecified with an experiment-wise α = 0.05 (2 tailed). A modified Bonferroni procedure was used in the discovery set to consider correlations in metabolites and correct for multiple comparisons of metabolites (19, 20). This adjustment took into account the full correlation matrix of metabolites and used the mean correlation in the metabolites in the formula, in which the new α level for the kth hypothesis for k = 1, 2, …, K was readjusted for each individual metabolite according to
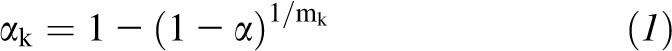 |
where
 |
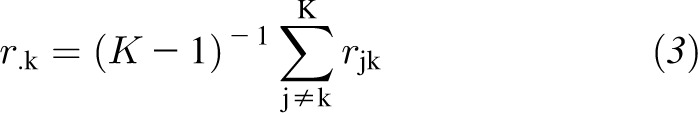 |
and rjk is the correlation coefficient between jth and kth metabolites. When the average of correlation coefficients was zero, this adjustment was equivalent to the Bonferroni procedure, and when the average of correlation coefficients was one, adjusted and unadjusted P values were the same. For each metabolite level, the significance level for a 2-tailed test was set as 1.9 × 10−4 in the discovery set and 0.05 in the replication set.
In a secondary analysis, all participants were included (by combining discovery and replication sets) to assess additional details of alcohol-related associations, including that for the association between alcohol beverage types and metabolites as well as the sex-stratified analyses of alcohol relations with sex-steroid metabolites. An ANCOVA was conducted with a categorical independent variable (the type of alcohol beverage) and dependent group 1 metabolite variable, with adjustment for age, sex, BMI, current cigarette smoking status, and eGFR. A composite metabololomic score (MetScore) was created by summing the quartile ranks of alcohol-related metabolites belonging to the same metabolic subpathway (eg, γ-glutamyl dipeptide). The association between the MetScore and established inflammation biomarker was assessed by partial correlations, with incident hypertension (interval-censoring data) by using Weibull parametric models and incident CHD and HF (right-censoring data) by using Cox proportional hazards regressions. For all analyses of alcohol-related associations, nondrinkers were treated as the reference group. A sensitivity analysis of the alcohol association with metabolites was done by adding the medication use of diuretics as a confounding variable in the model in all prevalent HF-free and CHD-free participants (n = 1823). All statistical analyses were performed with SAS version 9.2 software (SAS Institute).
RESULTS
Demographic characteristics for both discovery and replication samples are presented in Table 1. In general, this sample of African Americans was middle aged and had a high prevalence of hypertension. As expected, participants from discovery and replication sets were similar. Compared with men, women had higher BMI and eGFR and were less likely to be current alcohol drinkers, current cigarette smokers, or have prevalent CHD.
TABLE 1.
Distribution of participant characteristics at the baseline visit in both discovery and replication sets in African Americans in the ARIC Study1
| Discovery set (n = 1500) | Replication set (n = 477) | |
| Age (y) | 52.90 ± 5.82 | 52.83 ± 5.6 |
| M [n (%)] | 543 (36.2) | 159 (33.3) |
| BMI (kg/m2) | 29.69 ± 6.0 | 29.55 ± 6.1 |
| eGFR (mL · min−1 · 1.73 m−2) | 103.97 ± 18.4 | 105.44 ± 17.6 |
| Current cigarette smoker [n (%)] | 426 (28.4) | 143 (30.0) |
| Prevalent hypertension [n (%)] | 798 (53.2) | 252 (52.8) |
| Prevalent heart failure [n (%)] | 78 (5.2) | 19 (4.0) |
| Prevalent coronary heart disease [n (%)] | 60 (4.0) | 14 (2.9) |
| Current alcohol drinker [n (%)] | 446 (29.7) | 135 (28.3) |
| Rare drinkers | 110 (7.3) | 34 (7.1) |
| Light drinkers | 110 (7.3) | 37 (7.8) |
| Low-moderate drinkers | 105 (7.0) | 39 (8.2) |
| Moderate-high drinkers | 121 (8.1) | 25 (5.2) |
| Wine drinkers | 20 (1.3) | 10 (2.1) |
| Beer drinkers | 181 (12.1) | 52 (10.9) |
| Hard liquor drinkers | 111 (7.4) | 32 (6.7) |
| No preference drinkers | 130 (8.7) | 45 (9.4) |
Alcohol consumption habit was treated as an ordinal variable in all analyses (nondrinker: 0; rare drinker: 1; light drinker: 2; low-moderate drinker: 3; and moderate-high drinker: 4). Percentages for categorical variables are shown in parentheses. Baseline characteristics were compared by using the chi-square test for categorical variables and 2-sample t test for continuous variables. No significant difference of characteristics between discovery and replication sets, P < 0.05. ARIC, Atherosclerosis Risk in Communities; eGFR, estimated glomerular filtration rate.
Mean ± SE (all such values of continuous variables).
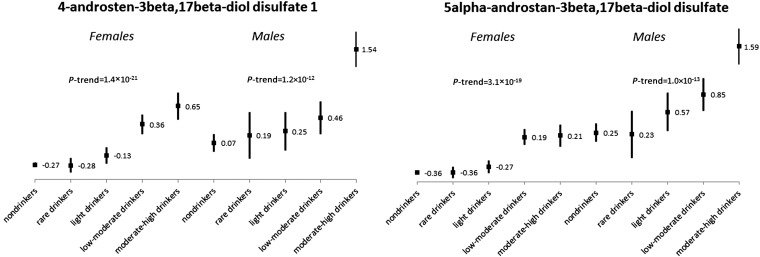
In the 356 named metabolites (147 metabolites in lipid metabolism, 88 metabolites in amino acid, 42 metabolites in xenobiotics, 29 metabolites in peptide, 16 metabolites in carbohydrate, 14 metabolites in nucleotide, 12 metabolites in cofactors and vitamins, and 8 metabolites in energy-metabolism pathways), 39 metabolites (37 group 1 metabolites and 2 group 2 metabolites) were identified in the discovery sample and replicated in the replication sample as significantly associated with alcohol consumption habits (Table 2). Alcohol consumption habits showed consistent associations with metabolite levels across discovery and replication sets. In general, greater alcohol consumption was associated with higher levels of a majority of metabolites, such as those in amino acid and lipid pathways, and with lower levels in γ-glutamyl dipeptides. With consideration of those metabolites associated most strongly with alcohol consumption, each unit increase in the alcohol ordinal group was associated with a 0.27-SD higher level of 4-androsten-3β and 17β-diol disulfate 1 and with 0.18-SD lower levels of γ-glutamyl valine and γ-glutamyl leucine. Sex-stratified results of sex steroids are presented in Figure 2, with the exclusion of subjects who were taking exogenous hormone-replacement medication (n = 1783), and results showed consistent findings between men and women.
TABLE 2.
Metabolites significantly associated with alcohol consumption volume in discovery and replication samples in African Americans in the ARIC Study1
| Discovery set |
Replication set |
|||||
| Pathway | Metabolite | Subpathway or brief description | β ± SE | P | β ± SE | P |
| Amino acid | 2-Aminobutyrate | Considered to be in AHB-related subpathway | 0.10 ± 0.02 | 1.68 × 10−5 | 0.13 ± 0.04 | 1.34 × 10−3 |
| AHB | — | 0.10 ± 0.02 | 2.81 × 10−6 | 0.13 ± 0.04 | 2.27 × 10−3 | |
| α-Hydroxyisovalerate | — | 0.15 ± 0.02 | 3.31 × 10−12 | 0.24 ± 0.04 | 6.99 × 10−9 | |
| 2-Hydroxyisobutyrate | — | 0.10 ± 0.02 | 3.51 × 10−6 | 0.10 ± 0.04 | 7.48 × 10−3 | |
| α-Hydroxyisocaproate | — | 0.11 ± 0.02 | 3.44 × 10−7 | 0.12 ± 0.04 | 1.92 × 10−3 | |
| 2-Hydroxy-3-methylvalerate2 | — | 0.12 ± 0.02 | 2.02 × 10−11 | 0.07 ± 0.03 | 4.19 × 10−2 | |
| 5-oxoproline | A product of γ-glutamyl dipeptides | −0.17 ± 0.02 | 6.68 × 10−15 | −0.11 ± 0.04 | 6.67 × 10−3 | |
| Indolelactate | Involved in tryptophan metabolism | 0.08 ± 0.02 | 1.90 × 10−5 | 0.15 ± 0.04 | 7.18 × 10−4 | |
| Lipid | Docosapentaenoate (n−3 docosapentaenoic acid; 22:5n−3) | Unsaturated fatty acids | 0.13 ± 0.02 | 1.24 × 10−9 | 0.13 ± 0.05 | 3.59 × 10−3 |
| Palmitoleate (16:1n−7) | — | 0.20 ± 0.02 | 2.00 × 10−20 | 0.19 ± 0.04 | 4.78 × 10−7 | |
| Adrenate (22:4n−6) | — | 0.17 ± 0.02 | 1.35 × 10−15 | 0.16 ± 0.04 | 1.39 × 10−4 | |
| Dihomolinoleate (20:2n−6) | — | 0.17 ± 0.02 | 1.11 × 10−14 | 0.15 ± 0.04 | 1.47 × 10−4 | |
| 10-Heptadecenoate [17:1n−Cyclo(leu-pro)7] | — | 0.14 ± 0.02 | 2.45 × 10−11 | 0.14 ± 0.04 | 1.90 × 10−4 | |
| Eicosenoate (20:1n−Cyclo(leu-pro)9 or 11) | — | 0.19 ± 0.02 | 1.06 × 10−16 | 0.12 ± 0.04 | 8.87 × 10−4 | |
| Oleate (18:1n−Cyclo(leu-pro)9) | — | 0.16 ± 0.02 | 2.10 × 10−12 | 0.12 ± 0.04 | 2.06 × 10−3 | |
| Myristoleate (14:1n−Cyclo(leu-pro)5) | — | 0.10 ± 0.02 | 1.70 × 10−5 | 0.11 ± 0.04 | 3.43 × 10−3 | |
| Palmitate (16:0) | — | 0.12 ± 0.02 | 1.73 × 10−8 | 0.11 ± 0.04 | 4.95 × 10−3 | |
| Myristate (14:0) | — | 0.10 ± 0.02 | 7.41 × 10−6 | 0.10 ± 0.04 | 1.47 × 10−2 | |
| Stearidonate (18:4n−3) | — | 0.12 ± 0.02 | 5.64 × 10−8 | 0.11 ± 0.04 | 1.03 × 10−2 | |
| 5-Hete | — | 0.14 ± 0.02 | 5.57 × 10−11 | 0.16 ± 0.04 | 1.62 × 10−4 | |
| 5-Hepe | — | 0.14 ± 0.02 | 5.87 × 10−11 | 0.09 ± 0.04 | 3.23 × 10−2 | |
| 1-Palmitoleoyl-glycerophosphocholine | Belong to lysophosphatidylcholines subpathway | 0.09 ± 0.02 | 5.98 × 10−5 | 0.10 ± 0.04 | 2.47 × 10−2 | |
| 1-Stearoyl-glycerophosphoethanolamine | — | 0.12 ± 0.02 | 4.63 × 10−8 | 0.09 ± 0.04 | 2.23 × 10−2 | |
| 1-Pentadecanoyl-glycerophosphocholine | — | −0.10 ± 0.02 | 2.69 × 10−6 | −0.12 ± 0.04 | 2.95 × 10−3 | |
| 2-Arachidonoyl-glycerophosphoethanolamine | — | 0.10 ± 0.02 | 3.90 × 10−6 | 0.14 ± 0.04 | 1.01 × 10−3 | |
| 4-Androsten-3β,17β-diol disulfate 1 | Sex steroids | 0.27 ± 0.02 | <10−20 | 0.25 ± 0.03 | 3.55 × 10−13 | |
| 5α-Androstan-3β,17β-diol disulfate | — | 0.24 ± 0.02 | <10−20 | 0.26 ± 0.04 | 9.10 × 10−12 | |
| Isovalerate | A food additive | 0.08 ± 0.02 | 1.37 × 10−4 | 0.10 ± 0.04 | 1.46 × 10−2 | |
| Peptide | Leucylleucine | Dipeptides | 0.10 ± 0.02 | 2.65 × 10−6 | 0.10 ± 0.05 | 4.61 × 10−2 |
| Cyclo(leu-pro)2 | — | 0.12 ± 0.02 | 5.45 × 10−12 | 0.09 ± 0.03 | 4.11 × 10−3 | |
| γ-Glutamyl valine | Metabolized in γ-glutamyl dipeptides subpathway | −0.18 ± 0.02 | 1.03 × 10−15 | −0.15 ± 0.04 | 1.70 × 10−4 | |
| γ-Glutamyl phenylalanine | — | −0.16 ± 0.02 | 3.77 × 10−12 | −0.14 ± 0.04 | 5.22 × 10−4 | |
| γ-Glutamyl leucine | — | −0.18 ± 0.02 | 3.22 × 10−16 | −0.14 ± 0.04 | 5.43 × 10−4 | |
| γ-Glutamyl isoleucine | — | −0.17 ± 0.02 | 8.72 × 10−15 | −0.12 ± 0.04 | 1.84 × 10−3 | |
| γ-Glutamyl tyrosine | — | −0.12 ± 0.02 | 8.70 × 10−8 | −0.10 ± 0.04 | 1.24 × 10−2 | |
| γ-Glutamyl glutamate | — | −0.17 ± 0.02 | 2.97 × 10−15 | −0.13 ± 0.04 | 1.27 × 10−3 | |
| γ-Glutamyl alanine | — | −0.14 ± 0.02 | 6.05 × 10−12 | −0.11 ± 0.04 | 9.12 × 10−3 | |
| Energy | Malate | Involved in Krebs cycle | 0.11 ± 0.02 | 9.74 × 10−7 | 0.11 ± 0.04 | 1.07 × 10−2 |
| Xenobiotics | Piperine | Found in black pepper (21) | 0.13 ± 0.02 | 1.49 × 10−9 | 0.21 ± 0.04 | 1.15 × 10−6 |
Group 2 metabolites with the percentage of values that were missing or below the detection limit from 50% to 80%. We considered these missing or below the detection limit values as category 1. For the measured values (non–missing or below the detection limit), we considered values below the median as category 2 and values above the median as category 3. These categories formed an ordinal variable, and it was used during data analysis. Alcohol consumption habit was treated as an ordinal variable in all analyses (nondrinker: 0; rare drinker: 1; light drinker: 2; low-moderate drinker: 3; and moderate-high drinker: 4). Data were calculated by using general linear models after adjustment for age, sex, BMI, kidney function measured by using the estimated glomerular filtration rate, and current smoking status. AHB, 2-hydroxybutyrate; ARIC, Atherosclerosis Risk in Communities.
FIGURE 2.
Mean (±SE) of 2 sex steroids by sex and alcohol consumption habits in Atherosclerosis Risk in Communities African Americans who were not taking exogenous sex-hormone therapy (n = 1783).
See Supplementary Table 1 under “Supplemental data” in the online issue for the adjusted mean amounts by different types of alcohol beverage of the 37 group 1 metabolites significantly related to alcohol consumption. The directions of the difference in metabolite levels between nondrinkers (as a reference group) and consumers of different alcohol beverage types were generally consistent in magnitude. For most metabolites, liquor and beer consumers had larger differences in metabolite levels (compared with those of nondrinkers), whereas wine and nonpreference consumers had smaller differences. However, for a few amino acids (eg, 2-hydroxyisobutyrate and 5-oxoproline), the difference in levels in wine consumers from those in nondrinkers were in the opposite direction compared with changed levels in other drinkers. All 39 alcohol-related metabolites retained significance in the sensitivity analysis with an extra confounding variable of diuretics use in prevalent CHD-free and HF-free participants (all P < 1.5 × 10−5).
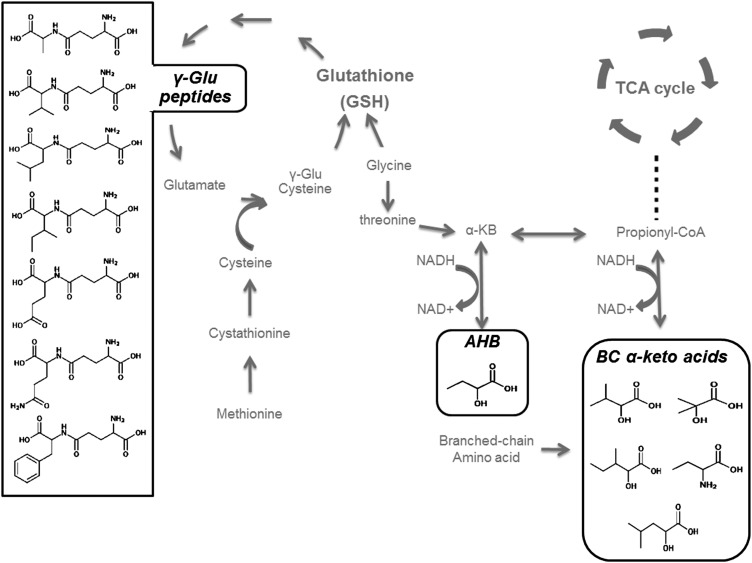
On the basis of structural and metabolic similarities, we identified several subpathways of metabolites, which are shown in Table 2. Compounds 2-aminobutyrate, α-hydroxyisovalerate, 2-hydroxyisobutyrate, α-hydroxyisocaproate, and 2-hydroxy-3-methylvalerate are structurally similar to 2-hydroxybutyrate (AHB), and they are related to the branched-chain amino acid metabolism (Figure 3). These metabolites, including AHB, were considered to be in an AHB-related subpathway and were combined into a composite metabolomic score (MetScore_AHB). Compounds 1-palmitoleoyl-glycerophosphocholine, 1-stearoyl-glycerophosphoethanolamine, 1-pentadecanoyl-glycerophosphocholine, and 2-arachidonoyl-glycerophosphoethanolamine belong to a subpathway of lysophosphatidylcholines, which are a class of intermediate phospholipids in the metabolism of lipids. These compounds were combined into a composite metabolomic score (MetScore_LysoPC). Quartile ranks for 1-pentadecanoyl-glycerophosphocholine were reversed because it was the only identified and replicated lysophosphatidylcholine inversely associated with alcohol consumption. Compounds γ-glutamyl valine, γ-glutamyl phenylalanine, γ-glutamyl leucine, γ-glutamyl isoleucine, γ-glutamyl tyrosine, γ-glutamyl glutamate, and γ-glutamyl alanine were metabolized in a γ-glutamyl dipeptide subpathway, and these compounds were combined into a composite metabolomic score (MetScore_GLU). Metabolomic scores of these 3 groups of metabolites (ie, MetScore_GLU, MetScore_AHB, and MetScore_LysoPC) showed significant associations both with established biomarker of inflammation and multiple-incident cardiovascular diseases (Table 3).
FIGURE 3.
Metabolic pathways featuring γ-Glu dipeptides and AHB. The metabolites in the γ-glutamyl peptide pathway (in the left box) were negatively related to alcohol consumption; 2-hydroxybutyrate and branched-chain α-keto acids (in the right bottom boxes, AHB-related metabolites) were positively related to alcohol consumption. BC, branched chain; AHB, 2-hydroxybutyrate; TCA, tricarboxylic acid; γ-Glu, γ-glutamyl.
TABLE 3.
Associations of MetScore_GLU, MetScore_AHB, and MetScore_LysoPC with the prevalent biomarker of inflammation as well as incident cardiovascular diseases in African Americans in the ARIC Study1
| MetScore_GLU |
MetScore_AHB |
MetScore_LysoPC |
||||
| Values | P | Values | P | Values | P | |
| Prevalent biomarker of inflammation (n = 1911)2 | ||||||
| White blood cells (×1000/mm3) | −0.13 | 1.2 × 10−8 | 0.08 | 3.6 × 10−4 | 0.06 | 0.01 |
| Incident cardiovascular diseases3 | ||||||
| Hypertension (n = 896) | 0.99 | 0.07 | 1.02 | 0.03 | 1.01 | 0.73 |
| Coronary heart disease (n = 1903) | 0.98 | 0.03 | 1.04 | 0.03 | 1.07 | 0.03 |
| Heart failure (n = 1850) | 0.96 | 1.0 × 10−6 | 1.02 | 0.12 | 1.04 | 0.16 |
Five metabolites structurally similar to 2-hydroxybutyrate and related to the branched-chain amino acid metabolism, including 2-hydroxybutyrate, were considered to be in a 2-hydroxybutyrate–related subpathway and were combined into a composite metabolomic score MetScore_AHB. Compounds belonging to a subpathway of lysophosphatidylcholines were combined into a composite metabolomic score MetScore_LysoPC. Compounds γ-glutamyl valine, γ-glutamyl phenylalanine, γ-glutamyl leucine, γ-glutamyl isoleucine, γ-glutamyl tyrosine, γ-glutamyl glutamate, and γ-glutamyl alanine were metabolized in a γ-glutamyl dipeptide subpathway and were combined into a composite metabolomic score MetScore_GLU. The association between metabolomic scores and disease biomarkers was assessed by using partial correlations, with incident hypertension (interval-censoring data) by using Weibull parametric models and with incident coronary heart disease and heart failure (right-censoring data) by using Cox proportional hazards regressions. All analyses were adjusted for age, sex, BMI, and kidney function measured by using the estimated glomerular filtration rate. P < 0.05 was significant. ARIC, Atherosclerosis Risk in Communities; MetScore_AHB, a composite metabolomic score for AHB and metabolites in an AHB-related pathway; MetScore_GLU, a composite metabolomic score for metabolites in γ-glutamyl dipeptide pathway; MetScore_LysoPC, a composite metabolomic score for phospholipids.
All values are Pearson's partial correlation coefficients (γ).
All values are HRs.
Associations of alcohol consumption volume and type of alcohol beverages with these incident cardiovascular diseases did not reach significance (see Supplementary Figures 1 and 2, respectively, under “Supplemental data” in the online issue). When we analyzed the shape of associations between quartile levels of alcohol-related metabolites and these incident cardiovascular diseases, no obvious J-shape association was shown (see Supplementary Figure 3 under “Supplemental data” in the online issue).
DISCUSSION
With the use of a mass spectrometry–based metabolomic profiling platform, we conducted the largest nonwhite metabolomic study in a well-characterized, population-based sample of African Americans and evaluated associations of alcohol consumption habits with the fasting serum metabolome. Unique metabolomic biomarkers of alcohol consumption were identified and replicated. A MetScore that consisted of summary information from a priori defined subpathways was associated with biomarker of inflammation and multiple incident cardiovascular diseases. To our knowledge, these data suggest new aspects of important roles inflammation and oxidation play in the association of alcohol consumption habits with these metabolites and perhaps with disease.
The γ-glutamyl dipeptides were significantly and inversely associated with alcohol consumption habits. The γ-glutamyl dipeptides are the products of the transpeptidation from γ-glutamyl transpeptidase (22) and are biosynthesized through a reaction with γ-glutamyl cysteine synthetase (23). Serum levels of γ-glutamyl dipeptides are indicative of the production of glutathione (23), which is a major endogenous antioxidant (Figure 3). Chronic alcohol abuse decreases glutathione levels via oxidative stress (24). Previous studies have shown that decreased glutathione is a risk factor for multiple chronic diseases (25, 26), but little is known about γ-glutamyl dipeptides and human disease. In our study sample, a MetScore_GLU showed inverse associations with the baseline biomarker of inflammation and longitudinal associations with lower risk of incident CHD and HF over decades of follow-up.
AHB-related metabolites were positively associated with alcohol consumption. AHB is a by-product in the methionine-to-glutamyltathione pathway (Figure 3), and its production is directly related to the rate of hepatic glutathione synthesis as oxidative stress increases (27). AHB has been reported to be a novel biomarker of insulin resistance perhaps via oxidative stress (28, 29). Other metabolites in this AHB-related pathway are those in the branched-chain amino acid pathway (Figure 3), and they may also be predictors of insulin resistance and glucose intolerance (30). As expected, the MetScore_AHB showed a significant and positive association with prevalent biomarker of inflammation and oxidative stress as well as positive associations with incident hypertension and incident CHD, which supported the hypothesis that AHB and its related metabolites serve as an oxidative mediator of associations of alcohol with disease.
In the metabolomic data reported in the current study, alcohol consumption was positively associated with most lysophosphatidylcholines (ie, 1-palmitoleoyl-glycerophosphocholine, 1-stearoyl-glycerophosphoethanolamine, and 2-arachidonoyl-glycerophosphoethanolamine) except 1-pentadecanoyl-glycerophosphocholine, which was inversely associated. Lysophosphatidylcholines are products of phospholipase A2 enzyme activity and have a direct role in toxic inflammatory responses in a variety of organ systems (31). Lysophosphatidylcholines are one of the major byproducts of phospholipid oxidation (32), which may play a role in atherosclerotic plaque characteristics (33). The MetScore_LysoPC showed a positive relation with the biomarker of inflammation as well as incident CHD after an average 17 y of follow-up.
All of the identified and replicated metabolites in the amino acid pathway showed positive associations with alcohol consumption, except for 5-oxoproline. 5-Oxoproline is a product of γ-glutamyl dipeptides via the enzyme γ-glutamyl cyclotransferase. Therefore, it follows that alcohol consumption is most likely associated with lower levels of 5-oxoproline because alcohol consumption lowers the levels of its precursors (ie, γ-glutamyl dipeptides).
Established metabolomic biomarkers of alcohol consumption emerged as a positive control in our study. Concentrations of the long-chain unsaturated fatty acids were uniformly and positively associated with alcohol consumption in our study, which was consistent with previous reports with the hypothesis that the increased concentration of unsaturated fatty acids mediates the protective effect of moderate alcohol drinking on cardiovascular diseases (34, 35).
The 2 sex steroids 4-androsten-3β,17β-diol disulfate 1 [which is a testosterone precursor (36)] and 5α-androstan-3β,17β-diol disulfate [which is a metabolite from dehydroepiandrosterone and dihydrotestosterone (37)] were identified and replicated to be positively associated with alcohol consumption. Sex-stratified results showed consistent associations with alcohol consumption for these 2 sex steroids between men and women (Figure 2). Although similar results were reported in European women (38, 39), to our knowledge, this is the first study to report associations of alcohol consumption with blood sex-hormone concentrations in a large sample of African Americans. Alcohol might influence sex-steroid concentrations through several mechanisms (38) such as the hypothalamic-pituitary-adrenal axis (40). Alcohol use may increase breast cancer risk in women (41) and prostate cancer risk in men (42) at least partially through an effect on sex-steroid concentrations (43, 44). Therefore, an examination of the relation between alcohol consumption and sex-steroid concentrations might be of interest for future studies of breast and prostate cancer risk.
It has been reported that moderate alcohol consumption may have a causal protective effect on cardiovascular disease. Protective associations of alcohol consumption have been reported primarily in studies of whites (45–47) but rarely in studies of African Americans. The mechanism of any such protective effects is contentious, and a multitude of risk-raising (eg, via AHB-related and lysophosphatidylcholines pathways) and risk-lowering (eg, γ-glutamyl dipeptides) pathways are likely implicated. A possible J-shaped relation between alcohol consumption and disease has been widely discussed (48, 49). In the current study, associations of alcohol consumption volume with incident hypertension and incident HF showed a suggestive J shape (see Supplementary Figure 1 under “Supplemental data” in the online issue). The relation between alcohol consumption and metabolite levels (tested by a quadratic term of the alcohol variable; data not shown) or the relation between MetScores and incident cardiovascular diseases (see Supplementary Figure 3 under “Supplemental data” in the online issue) did not support a J-shaped relation. We identified one targeted metabolomic study of alcohol consumption from a European white population (50) that had limited data on amino acids, sphingolipids, and lysolipids. In that study and the data reported in the current study, amino acid and sphingolipid levels were not related to alcohol consumption, and a subset of lysolipids (eg, 1-palmitoleoyl-glycerophosphocholine) was related to the alcohol consumption volume.
We used an untargeted bottom-up metabolomic approach with no previous assumptions to maximize the novelty of our findings, and the well-defined ARIC Study enabled us to verify our hypothesis of an intermediate effect of alcohol-related metabolites on disease by using longitudinal data. However, there are several aspects of this work that should be explored further, such as whether these results would be similar in other race groups and how the pattern of consumption (eg, binge drinking or regular frequency) would influence the results presented in this study. Because of the ARIC Study design, we were unable to separate effects of ethanol itself from effects of the nonalcoholic content of alcoholic beverages or address the question about whether the type of alcoholic beverage consumed influences changes in the intestinal microbiota. A potential measurement bias in alcohol consumption from the questionnaire was also of note. Nevertheless, our study was valuable as one of the first population-based research on metabolomic measures of usual alcohol consumption, providing a solid starting point for future research in this area.
In conclusion, this study identified and replicated metabolomic biomarkers of alcohol consumption in well-defined samples of African Americans from the ARIC Study. In addition, we showed that metabolomic biomarkers may provide insight into the underlying pathophysiologic mechanisms relating alcohol consumption to disease, especially inflammation and oxidative stress. The 3 subpathways identified in our analysis (ie, AHB-related metabolites, γ-glutamyl dipeptides, and lysophosphatidylcholines) emphasized the important role of inflammation in associations of alcohol with cardiovascular disease, although additional population and experimental studies are warranted. These data support the application of metabolomic technologies to better understand the complex relation between usual alcohol consumption habits and health and disease.
Supplementary Material
Acknowledgments
We thank the staff and participants in the ARIC Study for their important contributions.
The authors’ responsibilities were as follows—YZ: data analyses, data interpretation, and manuscript writing; BY: data analyses and critical revision of the manuscript; DA: technical support and critical revision of the manuscript; LMS: critical revision of the manuscript; JAN: research design and critical revision of the manuscript; and EB: research design, acquisition of data, and critical revision of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AHB, 2-hydroxybutyrate; ARIC, Atherosclerosis Risk in Communities; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HF, heart failure; m/bdl, missing or below the detection limit; MetScore, a composite metabolomic score; MetScore_AHB, a composite metabolomic score for AHB and metabolites in an AHB-related subpathway; MetScore_GLU, a composite metabolomic score for metabolites in γ-glutamyl dipeptide pathway; MetScore_LysoPC, a composite metabolomic score for phospholipids; MI, myocardial infarction.
REFERENCES
- 1.Friedmann PD. Clinical practice. Alcohol use in adults. N Engl J Med 2013;368:365–73. [DOI] [PubMed] [Google Scholar]
- 2.Poli A, Marangoni F, Avogaro A, Barba G, Bellentani S, Bucci M, Cambieri R, Catapano AL, Costanzo S, Cricelli C, et al. Moderate alcohol use and health: a consensus document. Nutr Metab Cardiovasc Dis 2013;23:487–504. [DOI] [PubMed] [Google Scholar]
- 3.Anderson P, Cremona A, Paton A, Turner C, Wallace P. The risk of alcohol. Addiction 1993;88:1493–508. [DOI] [PubMed] [Google Scholar]
- 4.Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol Clin Exp Res 2005;29:902–8. [DOI] [PubMed] [Google Scholar]
- 5.Harrigan GG, Maguire G, Boros L. Metabolomics in alcohol research and drug development. Alcohol Res Health 2008;31:26–35. [PubMed] [Google Scholar]
- 6.Sempos CT, Rehm J, Wu T, Crespo CJ, Trevisan M. Average volume of alcohol consumption and all-cause mortality in African Americans: the NHEFS cohort. Alcohol Clin Exp Res 2003;27:88–92. [DOI] [PubMed] [Google Scholar]
- 7.Volcik KA, Ballantyne CM, Fuchs FD, Sharrett AR, Boerwinkle E. Relationship of alcohol consumption and type of alcoholic beverage consumed with plasma lipid levels: differences between whites and African Americans of the ARIC study. Ann Epidemiol 2008;18:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen SL, McCarthy DM. Differences in acute response to alcohol between African Americans and European Americans. Alcohol Clin Exp Res 2013;37:1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 10.Demirovic J, Nabulsi A, Folsom AR, Carpenter MA, Szklo M, Sorlie PD, Barnes RW. Alcohol consumption and ultrasonographically assessed carotid artery wall thickness and distensibility. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation 1993;88:2787–93. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs FD, Chambless LE, Folsom AR, Eigenbrodt ML, Duncan BB, Gilbert A, Szklo M. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2004;160:466–74. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Yu B, Alexander D, Mosley TH, Heiss G, Nettleton JA, Boerwinkle E. Metabolomics and incident hypertension among blacks: the atherosclerosis risk in communities study. Hypertension 2013;62:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Yu B, Alexander D, Manolio TA, Aguilar D, Coresh J, Heiss G, Boerwinkle E, Nettleton JA. Associations between metabolomic compounds and incident heart failure among African Americans: the ARIC Study. Am J Epidemiol 2013;178:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosamond WD, Folsom AR, Chambless LE, Wang CH. Coronary heart disease trends in four United States communities. The Atherosclerosis Risk in Communities (ARIC) study 1987-1996. Int J Epidemiol 2001;30(suppl 1):S17–22. [DOI] [PubMed] [Google Scholar]
- 16.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009;81:6656–67. [DOI] [PubMed] [Google Scholar]
- 17.Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol 2009;37:521–35. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: The Atherosclerosis Risk in Communities Study. Hypertension 2001;37:1242–50. [DOI] [PubMed] [Google Scholar]
- 19.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 1997;16:2529–42. [DOI] [PubMed] [Google Scholar]
- 20.Blakesley RE, Mazumdar S, Dew MA, Houck PR, Tang G, Reynolds CF, 3rd, Butters MA. Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology 2009;23:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meghwal M, Goswami TK. Piper nigrum and piperine: an update. Phytother Res 2013;27:1121–30. [DOI] [PubMed] [Google Scholar]
- 22.Orwar O, Li X, Andine P, Bergstrom CM, Hagberg H, Folestad S, Sandberg M. Increased intra- and extracellular concentrations of gamma-glutamylglutamate and related dipeptides in the ischemic rat striatum: involvement of glutamyl transpeptidase. J Neurochem 1994;63:1371–6. [DOI] [PubMed] [Google Scholar]
- 23.Soga T, Sugimoto M, Honma M, Mori M, Igarashi K, Kashikura K, Ikeda S, Hirayama A, Yamamoto T, Yoshida H, et al. Serum metabolomics reveals gamma-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol 2011;55:896–905. [DOI] [PubMed] [Google Scholar]
- 24.Loguercio C, Piscopo P, Guerriero C, De Girolamo V, Disalvo D, Del Vecchio Blanco C. Effect of alcohol abuse and glutathione administration on the circulating levels of glutathione and on antipyrine metabolism in patients with alcoholic liver cirrhosis. Scand J Clin Lab Invest 1996;56:441–7. [DOI] [PubMed] [Google Scholar]
- 25.Lang CA, Mills BJ, Mastropaolo W, Liu MC. Blood glutathione decreases in chronic diseases. J Lab Clin Med 2000;135:402–5. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu H, Kiyohara Y, Kato I, Kitazono T, Tanizaki Y, Kubo M, Ueno H, Ibayashi S, Fujishima M, Iida M. Relationship between plasma glutathione levels and cardiovascular disease in a defined population: the Hisayama study. Stroke 2004;35:2072–7. [DOI] [PubMed] [Google Scholar]
- 27.Lord RS, Bralley JA. Clinical applications of urinary organic acids. Part I: detoxification markers. Altern Med Rev 2008;13:205–15. [PubMed] [Google Scholar]
- 28.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010;5:e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmuller G, Adamski J, Tuomi T, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013;62:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham TJ, Yao L, Lucena A. Product inhibition of secreted phospholipase A2 may explain lysophosphatidylcholines’ unexpected therapeutic properties. J Inflamm (Lond) 2008;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keaney JF. ed. Oxidative stress and vascular disease. Norwell, MA:. Kluwer Academic Publishers,2000. [Google Scholar]
- 33.Gonçalves I, Edsfeldt A, Ko NY, Grufman H, Berg K, Bjorkbacka H, Nitulescu M, Persson A, Nilsson M, Prehn C, et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol 2012;32:1505–12. [DOI] [PubMed] [Google Scholar]
- 34.de Lorgeril M, Salen P, Martin JL, Boucher F, de Leiris J. Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking. Am Heart J 2008;155:175–81. [DOI] [PubMed] [Google Scholar]
- 35.Pawlosky RJ, Salem N., Jr Perspectives on alcohol consumption: liver polyunsaturated fatty acids and essential fatty acid metabolism. Alcohol 2004;34:27–33. [DOI] [PubMed] [Google Scholar]
- 36.Chen F, Knecht K, Leu C, Rutledge SJ, Scafonas A, Gambone C, Vogel R, Zhang H, Kasparcova V, Bai C, et al. Partial agonist/antagonist properties of androstenedione and 4-androsten-3beta,17beta-diol. J Steroid Biochem Mol Biol 2004;91:247–57. [DOI] [PubMed] [Google Scholar]
- 37.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci 2006;26:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onland-Moret NC, Peeters PH, van der Schouw YT, Grobbee DE, van Gils CH. Alcohol and endogenous sex steroid levels in postmenopausal women: a cross-sectional study. J Clin Endocrinol Metab 2005;90:1414–9. [DOI] [PubMed] [Google Scholar]
- 39.Rinaldi S, Peeters PH, Bezemer ID, Dossus L, Biessy C, Sacerdote C, Berrino F, Panico S, Palli D, Tumino R, et al. Relationship of alcohol intake and sex steroid concentrations in blood in pre- and post-menopausal women: the European Prospective Investigation into Cancer and Nutrition. Cancer Causes Control 2006;17:1033–43. [DOI] [PubMed] [Google Scholar]
- 40.Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res 1996;20:240–54. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuccolo L, Lewis SJ, Donovan JL, Hamdy FC, Neal DE, Smith GD. Alcohol consumption and PSA-detected prostate cancer risk–a case-control nested in the ProtecT study. Int J Cancer 2013;132:2176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin X, Zhang H, Ye D, Yao X, Zhang S, Dai B. Variations in circulating sex steroid levels in metastatic prostate cancer patients with combined androgen blockade: observation and implication. Andrology 2013;1:512–6. [DOI] [PubMed] [Google Scholar]
- 44.Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE. Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses’ Health Study II. Breast cancer research. Breast Cancer Res 2013;15:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djoussé L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians’ Health Study I. Circulation 2007;115:34–9. [DOI] [PubMed] [Google Scholar]
- 46.Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension 2008;51:1080–7. [DOI] [PubMed] [Google Scholar]
- 47.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson PL. J-curve revisited: cardiovascular benefits of moderate alcohol use cannot be dismissed. Med J Aust 2013;198:419–22. [DOI] [PubMed] [Google Scholar]
- 49.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation 2007;116:1306–17. [DOI] [PubMed] [Google Scholar]
- 50.Jaremek M, Yu Z, Mangino M, Mittelstrass K, Prehn C, Singmann P, Xu T, Dahmen N, Weinberger KM, Suhre K, et al. Alcohol-induced metabolomic differences in humans. Transl Psychiatry 2013;3:e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.