Abstract
Peripheral arterial disease (PAD) may ultimately cause to the loss of the affected limb due to gangrene or infection. Some patients with PAD may have severe coexisting diseases and diffuse involvement of their distal arteries, and so they are poor candidates for revascularization procedures. Angiogenesis has recently been suggested to be a new emerging treatment strategy for patients with PAD. Angiogenesis is defined as the sprouting of new capillaries from pre-existing vascular structures; this process plays a major role in the development of collateral vessels in an ischemic limb. Yet, the exact mechanism of angiogenesis is currently poorly understood. It has been established that angiogenesis is initiated by hypoxia and it requires various pro-angiogenic factors such as vascular endothelial growth factor. Therapeutic angiogenesis is aimed at enhancing natural angiogenesis by the administration of the cells or genes that can trigger angiogenesis and this can lead to pain relief and wound healing by the development of collateral vessels. Most of the recent clinical trials have reported that stem cell therapy for promoting angiogenesis in patients with PAD improves the ischemic symptoms and enhances wound healing. However, there are several limitations to approve a standard treatment for PAD such as small sample size in several prevous studies, their diverse inclusion criteria and the lack of standard assessment methods for the safety and outcome. Therefore, multicenter, large-scale and randomized controlled studies are needed to prove the safety and efficacy of the clinically applying stem cells for therapeutic angiogenesis in patients with PAD.
Keywords: Stem cells, Angiogenesis, Peripheral arterial disease
Peripheral arterial disease
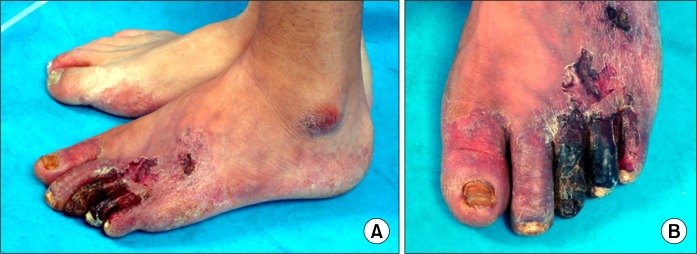
Peripheral arterial disease (PAD) is defined as a disorder caused by stenosis or occlusion in the aorta or the larger arteries of the arms and legs, and it is the result of atherosclerosis, inflammatory processes, embolism or thrombus. It is a progressive disease that leads to hemodynamic compromise of the affected limb and it may finally result in limb amputation due to gangrene or infection (Fig. 1).
Fig. 1.
Gangrenous change in patient with peripheral arterial disease.
The prevalence of PAD has been estimated to be 3∼12% in the general population and its incidence rises with age (1–7). For people more than 65 years old, the annual incidence is 10-fold higher as compared to 61 cased per 10,000 person-years for males and 54 cases per 10,000 person-years for females (8). According to the third National Health and Nutrition Examination Survey (9), the adjusted odds ratio for the prevalence of PAD was significantly greater with tobacco use and the risk of progression for critical limb ischemia was increased by the coexistence with diabetes mellitus, tobacco use, a ankle-brachial index less than 0.5, an age greater than 65 years and hypercholesterolemia (10, 11).
A number of risk factors for PAD have been established: atherosclerosis involved in the lower extremity is the most common and important risk factor for asymptomatic or symptomatic PAD. In addition, the presence of PAD strongly suggests that coronary artery disease and/or cerebrovascular disease coexist with PAD because they may share the same risk factors and pathogenesis.
The patients with PAD complaints a variety of clinical manifestations such as intermittent claudication, critical limb ischemia that is defined as ischemic rest pain, ulcer or gangrene, although some asymptomatic patients do exist. PAD is objectively diagnosed by measuring the ankle-brachial index (ABI) and PAD defined as less than a 0.9 of ABI value. However, patients with diabetes mellitus or severe chronic kidney disease show calcification in the same arteries that have PAD involvement, so ABI measurement doesn’t reflect the presence or severity of PAD. In that case, the toe-brachial pressure index should be measured and this is more predictive of substantial arterial disease (12). Once PAD is diagnosed, further evaluation must be done for determining the presence of coexisting atherosclerotic diseases such as coronary artery disease or cerebrovascular disease.
Fist of all, the management for PAD must include risk factor modification and medical therapy, and then the PAD patients can be considered for surgical or endovascular revascularization based on the degree of ischemic symptoms and the extent of PAD. The revascularization procedures as a treatment for PAD may be indicated for 1) life-style limiting claudication despite that risk factor modification, medical treatment and an appropriate exercise program have been done, 2) pain at rest due to ischemia and 3) non-healing ischemic ulceration or gangrene (13). However, in spite of optimal treatment, a third of PAD patients continue to suffer typical intermittent claudication that profoundly impairs their quality of life (14). Approximately 40% of the patients with critical limb ischemia will lose their legs within 6 months and 20% will die (10), and this mortality is mostly caused by coexisting coronary artery or cerebrovascular disease. In addition, some PAD patients often have severe coexisting morbidities such as old age, coronary artery disease, cerebrovascular disease, complicated diabetes mellitus or severe pulmonary disease, and so they are poor candidates for surgical or endovascular revascularization.
In addition to atherosclerotic PAD, another cause of PAD is thromboangiitis obliterans (or Buerger’s disease), which is a non-atherosclerotic, inflammatory disease involving the small and medium-sized arteries and veins in the limbs. The underlying pathogenesis of Buerger’s disease is unknown, but it is thought to be strongly associated with tobacco use. It typically occurs in young male smokers and 75 to 80% of these patients present with ischemic rest pain and/or ulceration (15). In most patients with critical limb ischemia caused by Buerger’s disease, surgical or endovascular revascularizations may not be indicated because of the diffuse disease involvement of the distal nature and there are no available distal target vessels for revascularization (16).
For these reasons reasons, angiogenesis has recently been suggested as a new emerging treatment strategy for patients with PAD.
Therapeutic angiogenesis
Under normal circumstance, the circulating endothelial progenitor cells (EPCs) are quiescent, yet under ischemic situation such as limb ischemia, the EPCs may be mobilized and they proliferate and angiogenesis is then initiated.
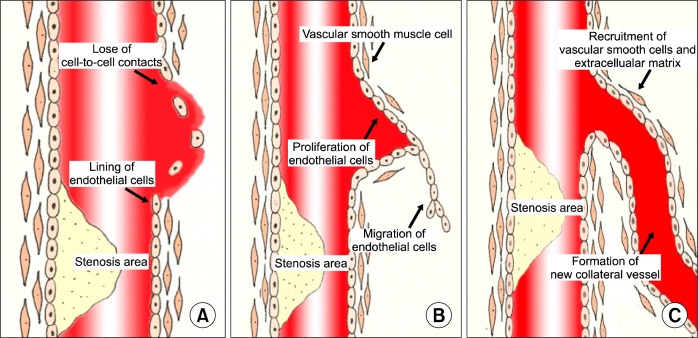
Angiogenesis, arteriogenesis and vasculogenesis contribute to new vessel formation and each term shares or has separate mechanisms (Fig. 2). This review summarized the definition and mechanism of angiogenesis because it mainly serves to form new vessels under various ischemic conditions.
Fig. 2.
Stages in angiogenesis.
Definitions
Angiogenesis is defined as the sprouting of new capillaries from pre-existing vascular structures and this process is initiated by the migration and proliferation of endothelial cells. As a result, new capillary networks are formed and these consist of tubes made of endothelial cells that lack additional wall structures, including smooth muscle cells or adventitial stabilizing structures and cells (17). So, remodeling of the extracellular matrix and adjacent tissue plays an important role in angiogenesis. Angiogenesis has recently becomes a term to indicate the growth and remodeling of primitive vessels into a complex network (18).
Arteriogenesis refers to the growth of pre-existing arteriolar collateral vessels and their caliber and wall thickness being increased during arteriogenesis. In comparison with angiogenesis, sheer stress rather than hypoxia is likely to be the important factor that stimulates arteriogenesis (19).
In contrast, vasculogenesis describes in situ differentiation from bone marrow-derived EPCs that differentiate into endothelial cells and they fuse into luminal structures (20). Vasculogenesis leads to new vessel formation in the embryo. In adult, new vessel formation occurs mainly through angiogenesis rather than vasculogenesis.
Mechanisms of angiogenesis
Hypoxia is the most important triggering factor that affects the initiation of angiogenesis. Oxygen tension plays a major role in the expression of numerous genes such as the vascular endothelial growth factor (VEGF) family and pro-angiogenic growth factor (20). In addition, hypoxia stimulates the transcription of promoter genes and this is mediated by the activation of hypoxia-inducible farctor-1 (HIF-1) and it leads to increased VEGF.
The key steps during angiogenesis are summarized as endothelial activation, migration, proliferation and reorganization (20). These steps are strictly regulated by pro- and anti-angiogenic factors.
VEGF is derived from platelets and it is a specific mitogen for endothelial cells and it is induced by hypoxia via HIF-1. It binds with tryrosine-kinase receptors, and then it stimulates not only endothelial proliferation and differentiation, but also increased endothelial permeability. Other growth factors such as TGF-B, FGF or PDGF also increase the expression of VEGF, and inhibitory factors such as angiostatin decrease the VEGF expression (Table 1).
Table 1.
Factors/cytokines affecting angiogenesis
| Stimulation | Inhibition |
|---|---|
| Hypoxia | Angiostatin |
| Nitric oxide | Endostatin |
| Hypoxia inducible factor-1 (HIF-1) | Troponin-I |
| Vascular endothelial growth factor (VEGF) | Thromboplastin |
| Acid and basic fibroblast growth factor (FGF) | Tissue inhibitors of matrix metalloproteinase (TIMPs) |
| Angiopoietin 1 (ANG-1) | Suramin |
| Platelet derived growth factor (PDGF) | |
| Hepatocyte growth factor (HGF) | |
| Granulocyte macrophage-colony stimulating factor (GM-CSF) | |
| Monocyte chemotactic protein-1 (MCP-1) | |
| Transforming growth factor (TGF)α&β | |
| Tumor necrosis factor (TNF)α |
Although the initiation of angiogenesis is caused by hypoxia and several growth factors, vascular endothelial cells may also be activated. This triggers cell-to-cell contacts to break and adjacent supporting cells to loosen and it leads to the migration of endothelial cells into distant sites. Angiopoietin 2 is an inhibitor of Tie2 signaling, and it may detach smooth muscle cells and loosen the extracellular matrix (21, 22). The release of matrix metalloproteinase, plasminogen, chymase or heparanase degrades the extracellular matrix and leads to the induction of growth factors (b-FGF, VEGF and IGF-1) (23). The fusion of endothelial cells into the pre-existing vessels forms the lumen of a collateral vessel. The molecules associated with lumen formation are integrins (αv, β3 and α5) and myocyte enhancer binding factor 2C (MEF2C) transcription factor; in contrast, thrombospondin (TSP)-1 inhibits lumen formation (18).
The migration of endothelial cells makes up the loops in the distal aspect of the occluded or stenotic site and this consequently forms the endothelial lining of the new vessel’s lumen. In this manner, a new collateral vessel is created by the connection of endothelial cells, and then this becomes a mature network by the recruitment of vascular smooth muscle cells and the extracellular matrix (Fig. 2).
Clinically, it has been reported that some conditions such as old age, diabetes mellitus and hypercholesterolemia reduce the activity of angiogenesis (24); in contrast, a well-designed program of exercise training stimulates angiogenesis and improves the ischemic symptoms in patients with PAD.
Therapeutic angiogenesis aims to enhance natural angiogenesis by administering the cells or genes that can trigger the initiation of angiogenesis and the release of pro-angiogenic growth factors. Thus, therapeutic angiogenesis has become an alternative therapeutic strategy for various kinds of ischemic disease.
Stem cells as a source of angiogenesis
Stem cells are defined as undifferentiated cells that are capable of self-renewal and differentiation into the specialized cells through their replication (25). In order for any of the cells that are isolated from peripheral blood, bone marrow etc. to be defined as stem cells, it must be proved that the cells differentiate or transform into specific cells or tissue under specific conditions.
There are three recent revolutionary discoveries for stem cells that have demonstrates stem cells’ clinical potential in a variety of diseases (26). First, stem cells have been discovered in organs that were previously thought to lack regenerative potential. Second, organ-specific adult stem cells exhibit much more plasticity than we originally thought. This fact means that stem cells derived from one type of cell or tissue can differentiate into unrelated types of tissue. So, it had been proposed that the use of adult stem cells should be considered as a treatment strategy for diseases that require vascular regeneration. Third, embryonic stem cells can be isolated and differentiated into a variety of cell types in vitro. So, their differentiation ability may hold promise for the treatment of many types of disease that require tissue repair, such as stroke, neuro-degenerative disease and myocardial infarction.
Stem cells can be divided into two types, that is, embryonic and adult stem cells. Embryonic stem cells are derived from the fetus that is in the blastocyst stage and these cells have various abilities to differentiate into multiple cellular lineages. However, there are some problems with using embryonic stem cells in the clinical field because these cells tend to develop into tumors such as teratoma, and most of all, the ethical controversies for their procurement and use remain to be solved. In contrast, adult stem cells are distributed throughout the body and they have the potential to differentiate into specific tissue under certain circumstances, but this potential is quiescent under normal physiologic conditions.
Although adult stem cells are distributed throughout the body, there are tissue-specific locations for each type of stem cell. For example, hematopoietic and mesenchymal stem cells are primarily located in bone marrow and peripheral blood (27), neural stem cells are located in ependymal cells and astrocytes (28), and skeletal-muscle stem cells are located in muscle fiber (29).
In most clinical trials, peripheral blood, bone marrow, cord blood or placenta have been used as the sources of stem cells because these tissues have a rich content of stem cells and the cells are easily and safely harvested for isolation (30).
Several experimental studies have reported that purified stem cells are able to generate cardiomyocytes and vascular structures (31, 32).
The exact mechanisms of angiogenesis by stem cells in various ischemic vascular diseases are poorly understood. It is thought that stem cells may cause the release of a variety of angiogenic factors such as VEGF and bFGF, and these factors mediate a paracrine effect into the ischemic lesion, and as a result, angiogenesis is triggered.
For PAD, most trials have reported that peripheral blood or bone marrow-derived stem cell transplantation improved the ischemic symptoms, such as claudication, ischemic rest pain and enhance wound healing in patients with ulceration (Table 2).
Table 2.
Critical trials of stem cell therapy in peripheral arterial disease (≥10 patients)
| Authors | Disease | Stem cell source | Route of cell application | Results |
|---|---|---|---|---|
| Tateishi-Yuyama E, et al. 2002 | ASO* | Bone marrow-derived mononuclear cells and peripheral blood mononuclear cells (as a control) | Intramuscular | Improvement: Pain free walking time, rest pain, ABI‡, TcPO2§ |
| Miyamoto K, et al. (37) 2004 | ASO*+TAO† | Bone marrow-derived mononuclear cells | Intramuscular | Improvement: Pain free walking time, VAS‖, and ABI‡ |
| Kawamura A, et al. (38) 2005 | ASO* | Peripheral blood stem cells (CD34 positive cells) | Intramuscular | Prevention of limb amputation: 22 limbs of 30 limbs (73%) |
| Huang P, et al. 2005 (39) | ASO* | Peripheral blood mononuclear cells | Intramuscular | Improvement: Pain, wound healing, ABI‡ |
| Kawamura A, et al. (40) 2006 | ASO* | Peripheral blood stem cells (CD34 positive cells) | Intramuscular | Improvement: subjective symptoms, temperature Visualization of arteries on CT |
| Bartsch T, et al. (41) 2006 | ASO* | Bone marrow-derived mononuclear cells | Combined intramuscular and intraarterial | Improvement: walking distance, ABI‡, perfusion |
| Kim DI, et al. (42) 2006 | TAO† | Whole bone marrow | Fenestration through tibia bone | Improvement: Ischemic symptoms and wound healing Development of collateral vessels on angiography |
| Durdu S, et al. (43) 2006 | TAO | Bone marrow-derived mononuclear cells | Intramuscular | Improvement: Rest pain, peak walking time, QOL¶, ABI‡ Development of collateral vessels (angiography) |
ASO*: Atherosclerosis obliterans,
TAO†: Thromboangiitis obliterans,
ABI‡: Ankle-brachial index,
TcPO2§: Transcutaneous oxygen pressure,
VAS‖: Visual analog scale,
QOL¶: Quality of life.
However, most of the clinical trials and treatments have shown several limitations so that they cannot yet be approved as a standard treatment for PAD. These limitations are 1) too small a sample size, 2) the lack of data for atherosclerotic PAD, 3) diverse inclusion criteria, 4) the absence of standard methods for assessing the safety and outcome and 5) the lack of the reports with long-term follow-up after using stem cells for treating PAD. Most of all, a great portion of clinical trials for PAD have not had a control group, and this is a weakness to prove the effectiveness of stem cells therapy.
In addition, some studies have reported serous adverse events, including myocardial infarctions, in-stent stenosis (33, 34) and arteriovenous fistula (35). There are several possible mechanisms for these adverse events (36). First, controlling the migration of stem cells into lesion sites may modify the risk and benefit of stem cell therapy. Second, the differentiation of stem cells may not be as directed as we have desired. As a result, acceleration of new arterial plaque or plaque instability has been demonstrated.
Conclusions
In conclusion, most of the previous clinical trials have reported that stem cell therapy could serve as a novel therapeutic modality for patients with PAD. However, there are several limitations to directly apply stem cell therapy in the clinical field. First, the mechanisms of angiogenesis by administration of stem cells have not been yet proven. Second, most of the trials have had a small number of subjects, a variety of inclusion criteria and they were non-randomized trials. Third, there has been the lack of standard reporting for the outcomes, adverse events and safety. Fourth, the most effective type of stem cells, the timing of administration, the amount and the route of administration that causes angiogenesis in patients with PAD have not been established.
Therefore, multicenter, large-scale and randomized controlled clinical trials may be fundamental and mandatory to prove the safety and efficacy of promoting angiogenesis by the administration of stem cells and for this therapy to become a standard treatment strategy for the patients suffering with PAD.
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interests.
References
- 1.Criqui MH. Peripheral vascular disease-epidemiological aspects. Vasc Med. 2001;3(Suppl 6):3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Godman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FGR, Housley E, Cawood EH, Macintyre CA, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 4.Bowlin SJ, Medalie JH, Flocke SA, Zyzanski SJ, Goldbourt U. Epidemiology of intermittent claudication in middle-aged men. Am J Epidemiol. 1994;140:418–430. doi: 10.1093/oxfordjournals.aje.a117264. [DOI] [PubMed] [Google Scholar]
- 5.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication: a risk profile from the Framing-ham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.cir.96.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192. doi: 10.1161/01.atv.18.2.185. [DOI] [PubMed] [Google Scholar]
- 7.He Y, Jiang Y, Wang J, Fan L, Li X, Hu FB. Prevalence of peripheral arterial disease and its association with smoking in a population-based study in Beijing, China. J Vasc Surg. 2006;44:333–338. doi: 10.1016/j.jvs.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33:13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 10.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Oline JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/ Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 12.Ubbink DT, Tulevski II, den Hartog D, Koelemay MJ, Legemate DA, Jacobs MJ. The value of non-invasive techniques for the assessment of critical limb ischaemia. Eur J Vasc Endovasc Surg. 1997;13:296–300. doi: 10.1016/s1078-5884(97)80101-3. [DOI] [PubMed] [Google Scholar]
- 13.Arain FA, Leslie T, Copper JR. Peripheral Arterial Disease: Diagnosis and Management. Mayo Clin Proc. 2008;83:944–950. doi: 10.4065/83.8.944. [DOI] [PubMed] [Google Scholar]
- 14.Al Mheid I, Quyyumi AA. Cell therapy in peripheral arterial disease. Angiology. 2009;59:705–716. doi: 10.1177/0003319708321584. [DOI] [PubMed] [Google Scholar]
- 15.Olin JW. Cecel Medicine. 23th ed. Philadelphia: Saunders ELSEVIER; 2008. Thromboangiitis obliterans (Buerger’s disease) p. 570. [Google Scholar]
- 16.Olin JW. Thromboangiitis obliterans (Buerger’s disease) N Engl J Med. 2000;343:864–869. doi: 10.1056/NEJM200009213431207. [DOI] [PubMed] [Google Scholar]
- 17.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 19.Schaper W, Ito WD. Molecular mechanism of coronary vessel growth. Circ Res. 1996;79:911–919. doi: 10.1161/01.res.79.5.911. [DOI] [PubMed] [Google Scholar]
- 20.Collinson DJ, Donnelly R. Therapeutic anigogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation? Eur J Vasc Endovasc Surg. 2004;25:9–23. doi: 10.1016/j.ejvs.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 22.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 23.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MJ, Kim MH, Kim SA, Chang JS. Age-related deterioration of hematopoietic stem cells. International J Stem Cells. 2008;1:55–63. doi: 10.15283/ijsc.2008.1.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and morrow-derived stem cells for nonmalignant disease. JAMA. 2008;299:925–936. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 26.Kaji EH, Leiden JM. Gene and stem cell therapies. JAMA. 2001;285:545–550. doi: 10.1001/jama.285.5.545. [DOI] [PubMed] [Google Scholar]
- 27.Ko IK, Kim BS. Mesenchymal stem cells for treatment of myocardial infarction. International J Stem Cells. 2008;1:49–54. doi: 10.15283/ijsc.2008.1.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SU, Lee HJ, Park IH, Chu K, Lee ST, Kim NH, Roh JK, Kim SK, Wang KC. Human neural stem cells for brain repair. International J Stem Cells. 2008;1:27–35. doi: 10.15283/ijsc.2008.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kørbling M, Estrov Z. Adult stem cells for tissue repair-a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 30.Lee KB, Kim AK, Kim MJ, Do YS, Shin SW, Kim JS, Park CJ, Kang KS, Kim BS, Joh JH, Oh WI, Hong HK, Kim DI. Angiogenesis induced by autologous whole bone marrow stem cells transplantation. International J Stem Cells. 2008;1:64–69. doi: 10.15283/ijsc.2008.1.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 32.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entam ML, Michael LH, Hirschi KK, Goo-dell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischemia by autologous transplantation of bone marrow cells: a pilot study and a randomized controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 34.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Lee DS, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem cells mobilized with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomized clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto K, Nishigami K, Nagaya N, Akutsu K, Chiku M, Soma T, Miyata Sm, Higashi M, Tanaka R, Nakatani T, Nonogi H, Takeshita S. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–2684. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch AT. Critical limb ischemia and stem cell research: informed adverse event reporting anchoring hope with informed adverse event reporting. Circulation. 2006;114:2581–2583. doi: 10.1161/CIRCULATIONAHA.106.666719. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto M, Yasutake M, Takano H, Takagi H, Takagi G, Mizuno H, Kumita S, Takano T. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetroformin (TF) perfusion scintigraphy. Cell Transplant. 2004;13:429–437. doi: 10.3727/000000004783983837. [DOI] [PubMed] [Google Scholar]
- 38.Kawamura A, Horie T, Tsuda I, Ikeda A, Egawa H, Imamura E, Iida J, Sakata H, Tamaki T, Kukita K, Meguro J, Yonekawa M, Kasai M. Prevention of limb amputation in patients with limbs ulcers by autologous peripheral blood mononuclear cell implantation. Ther Apher Dial. 2005;9:59–63. doi: 10.1111/j.1774-9987.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–2160. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura A, Horie T, Tsuda I, Abe Y, Yamada M, Egawa H, Iida J, Sakata H, Onodera K, Tamaki T, Furui H, Kukita K, Meguro J, Yonekawa M, Tanaka S. Clinical study of therapeutic angiogenesis by autologous peripheral blood stem cell (PBSC) transplantation in 92 patients with critically ischemic limbs. J Artif Organs. 2006;9:226–233. doi: 10.1007/s10047-006-0351-2. [DOI] [PubMed] [Google Scholar]
- 41.Bartsch T, Falke T, Brehm M, Zeus T, Kögler G, Wernet P, Strauer BE. Transplantation of autologous adult bone marrow stem cells in patients with severe peripheral arterial occlusion disease. Med Klin (Munich) 2006;101(Suppl 1):195–197. [PubMed] [Google Scholar]
- 42.Kim DI, Kim MJ, Joh JH, Shin SW, Do YS, Moon JY, Kim NR, Lim JE, Kim AK, Eo HS, Kim BS, Cho SW, Yang SH, Park CJ, Shim JS. Angiogenesis facilitated by autologous whole bone marrow stem cell transplantation for Buerger’s disease. Stem Cells. 2006;24:1194–1200. doi: 10.1634/stemcells.2005-0349. [DOI] [PubMed] [Google Scholar]
- 43.Durdu S, Akar AR, Arat M, Sancak T, Eren NT, Ozyurda U. Autologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II–III thromboangiitis obliterans. J Vasc Surg. 2006;44:732–739. doi: 10.1016/j.jvs.2006.06.023. [DOI] [PubMed] [Google Scholar]