Abstract
Mesenchymal stem cells (MSCs) have unique immunologic properties that may someday prove useful in cell-based therapy for various degenerative diseases. Its potential is limited, however, by several factors, including the rarity of these cells and difficulty in isolating them. To evaluate their potential as new sources for cell therapy, we isolated MSCs from human fetal tissue (hfMSC) derived from spontaneous abortus (8∼10 weeks) then studied their cell cycle and cell surface marker expression using a fluorescence-activated cell sorter (FACS), as well as the expression of differentiation markers using real-time polymerase chain reaction (RT-PCR). The hfMSCs were able to undergo PCR up to 20 times without displaying significant changes in morphology or expression of various stemness markers (Nanog and human telomerase reverse transcriptase [hAFP]), including germ layer markers (hNF68, alpha-cardiac actin, and hAFP). Also, teratomas were not seen in mice with severe combined immunodeficiency syndrome (SCID) that received a transplantation of hfMSCs with hTERT activity. The FACS analysis revealed that the majority of hfMSCs express mesenchymal markers CD13, CD44, CD71, CD90, CD105, CD253a, and HLA-ABC, but did not express CD31, CD34, CD38, CD45, and HLA-DR. Interestingly, hfMSCs derived from the cell membrane during early passages were negative for both HLA-ABC and HLA-DR, although HLA-ABC expression was detected during later passages (>20 passages). We found that hfMSCs could be differentiated into an osteogenic lineage; this was indicated by modulation of osteoblast markers specific for mRNA. We conclude that hfMSCs could be used as a new source of cells to treat patients with osteogenic diseases, as well as to understand the mechanisms of immunosuppression by MSCs.
Keywords: Mesenchymal stem cell, Fetal tissue, Osteogenic differentiation, HLA class
Introduction
Mesenchymal stem cells (MSCs) derived from various adult and fetal tissues have emerged as promising sources of cells with therapeutic effects achieved through tissue regeneration and repair (1, 2). MSCs derived from adult bone marrow (a representative source of stem cells) reportedly have therapeutic effects in several degenerative diseases, as well as a potential for multilineage differentiation (2, 3). They possess unique immunologic properties that may prove to be advantageous in immune-mediated disease (4, 5). Unfortunately, MSCs are relatively rare and are difficult to isolate from adult tissues (including bone marrow). If they are to be used to develop cell-based therapies in the future, several obstacles must be overcome, including the following: 1) the small number of MSCs available in bone marrow after it has been collected from donors; 2) age-dependent differences in differentiation capacity; 3) current trends in limiting MSCs to autologus use; and 4) a limited number of bone marrow donors (6). MSCs can be isolated from tissues other than adult bone marrow, including adipose tissue, umbilical cord blood, amnionic fluid, and fetal tissues. Differences in differentiation potential among these tissues can influence the specific lineage that can be induced in each type of cell (1, 7–9).
Researchers have been investigating the clinical potential of fetal tissue-derived MSCs for several years (2, 7). Until recently, little as known about the characteristics of fetal tissues-derived MSCs. Researchers have identified several potential advantages to these cells in immunological disorders by demonstrating their ability to consistently express human leukocyte antigens (HLA), including HLA-ABC (HLA class I), HLA-G, and HLA-E in long-term cultures. HLA-DR (HLA class II) is also expressed when these cells are exposed to interferon (IFN)-γ (10, 11). Additionally, Zhang et al recently reported that MSCs derived from fetal tissue is superior to that derived from adult tissues in terms of osteogenic capacity (12).
In this study, we attempted to isolate and characterize MSCs derived from tissue in the fetal membrane and fetal yolk sac to determine whether they can be established as new sources of therapeutic cells.
Materials and Methods
Isolation of human fetal tissues-derived mesenchymal stem cells (hfMSCs)
We collected human fetuses 8 to 10 weeks following therapeutic abortion. Each woman provided written, informed consent before the fetuses were collected. The fetal membrane and yolk sac were extracted, washed with culture medium (Bulbecco modified Eagle medium [DMEM] /F12, low glucose, 10% fetal bovine serum [FBS], 100 U/ml penicillin, and 100μg/ml streptomycin), and minced. The processed tissue was exposed to trypsin/EDTA (0.25% and 0.5 mM, respectively) for 20 minutes at 37°C to liberate individual cells. After centrifugation at 800× g for 20 minutes, isolated cells were collected and cultured at 37°C until significant growth was observed.
Reverse transcription polymerase chain reaction (RT-PCR) analysis
The total RNA was extracted using an RNeasy® Plus Mini-Kit (Qiagen Korea, Ltd., Seoul, South Korea). Reverse transcription was carried out using Superscript II (Invitrogen; Carlsbad, Calif, USA) and oligo-d (T)20 primers at 42°C for 1 h then incubated at 72°C for 15 minutes. The primer sequences are shown in Table 1. The synthesized template was amplified using h-Taq DNA polymerase (Solgent; Daejeon, South Korea) under the following conditions: denaturation at 95°C for 3 minutes; followed by 35 cycles of denaturation, each at 95°C for 30 seconds; annealing at 53 to 60°C for 45 seconds; and extension at 72°C for 45 seconds. Annealing temperatures were modified slightly depending on the primer sequences used. The products of the polymerase chain reaction (PCR) were separated on a 1% agarose gel and visualized by ethidium bromide staining.
Table 1.
Sequence of primers used for RT-PCR and length of fragments
| Genes | Sequences | Tm (°C) | Size (bp) |
|---|---|---|---|
| Nanog | F: 5′-TTC TTG ACT GGG ACC TTG TC-3′ | 54 | 300 |
| R: 5′-GCT TGC CTT GCT TTG AAG CA-3′ | |||
| NF-68 | F: 5′-GAG TGA AAT GGC ACG ATA CCT A-3′ | 58 | 500 |
| R: 5′-TTT CCT CTC CTT CTT CTT CAC CTT C-3′ | |||
| α-CA | F: 5′-GGA GTT ATG GTG GGT ATG GGT C-3′ | 58 | 500 |
| R: 5′-AGT GGT GAC AAA GGA GTA GCC A-3′ | |||
| hAFP | F: 5′-AGC TTG GTG GAT GAA AC-3′ | 50 | 200 |
| R: 5′-TCC AAC AGG CCT GAG AAA TC-3′ | |||
| TERT | F: 5′-GAG CTG ACG TGG AAG ATG AG-3′ | 55 | 300 |
| R: 5′-CTT CAA GTG CTG TCT GAT TCC AAT G-3′ | |||
| OC | F: 5′-ATG AGA GCC CTC ACA CTC CTC-3′ | 62 | 293 |
| R: 5′-GCC GTA GAA GCG CCG ATA GGC-3′ | |||
| Col I | F: 5′-CAT CTC AGA AGC AGA ATC TCC-3′ | 59 | 360 |
| R: 5′-CCA TAA ACC ACA CTA TCA CCT C-3′ | |||
| Run×2 | F: 5′-CCG CAC GAC AAC GCG ACC AT-3′ | 55 | 288 |
| R: 5′-CGC TCC GGC CCA CAA ATC TC-3′ | |||
| β-actin | F: 5′-TCC TTC TGC ATC CTG TCA GCA-3′ | 58 | 300 |
| R: 5′-CAG GAG ATG GCC ACT GCC GCA-3′ |
Cell cycle analysis
Fluorescence-activated cell sorter (FACS) analysis was carried out for human fetal tissue mesenchymal stem cells (hfMSCs; 106 cells) harvested from the culture surface using trypsin/EDTA. After being harvested, these cells were fixed in 70% ethanol at room temperature for 10 minutes and resuspended in 200μl of fixative. RNase (0.5μg/ml) and propidium iodide (5μg/ml) were added to the cells, and they were incubated for 30 minutes at 37°C. Treated cells were analyzed using a BD FACSVantageTM SE Cell Sorter equipped with Becton and Dickinson ModiFit LT software (BD Biosciences; San Diego, Calif, USA).
Cell-surface antigens analysis using FACS
To detect cell-surface antigens, we harvested hfMSCs using 2 mM EDTA/5% FBS in phosphate buffered saline (PBS). Harvested cells were rinsed and incubated in 2% FBS/PBS buffer containing either mouse immunoglobulin G as a reference or predetermined concentrations of fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies at 4°C for 30 minutes. Washed samples were analyzed using a BD FACSVantageTM SE Cell Sorter (Becton & Dickinson). Before the FACS was carried out, propidium idodide 5μg/ml was added to remove dead cells and debris. The results were analyzed using Cell-Quest software (Becton and Dickinson).
Osteogenic differentiation
Osteogenic differentiation of hfMSCs was performed as described with some modification (13). Once the hfMSC population reached a density of approximately 5×103 cells/cm2, we replaced the medium with DMEM containing 50μM L-ascorbic acid 2-phosphate, 10 mM glycerophosphate, and 1μM dexamethasone. The cells remained in this medium for 14 to 21 days, with the medium being changed every other day. The total RNA was extracted, and real-time polymerase chain reaction (RT-PCR) analysis was performed.
TRAP assay
Telomerase activity was measured using a PCR-based technique with a modified telomerase repeat amplification protocol (TRAP), which induces highly specific amplification of telomerase-mediated elongation products; these can be visualized with EtBr staining. This assay can be separated into 2 major steps: 1) telomerase adds telomeric repeats (TTAGGG) to the 3′ end of the synthetic P1-TS primer; and 2) the elongation products are amplified by PCR using the primers P1-TS and P2 to generate PCR products with the telomerase-specific 6-nucleotide segments. Unlike other TRAP assays, the telomerase-PCR assay contains all the compounds required for the telomerase reaction to take place and for PCR analysis in a ready-to-use reaction buffer, thereby combining both reactions to create a one-step/one-tube reaction. An additional advantage of this assay over the conventional assay is provided through the use of optimized primer sequences, which eliminates the need for “hot-start” PCR and avoids amplification artifacts (eg, primer dimmers).
Teratoma formation in NOD/SCID mice
We conducted all animal experimental procedures using protocols approved by the National Institutes of Health Guidelines. hfMSC cells (106 cells) were injected into the testicular capsules of 8-week-old mice with nonobese diabetes and severe combined immunodeficiency syndrome (NOD/SCID) (The Jackson Laboratory, Bar Harbor, Maine, USA). These mice were sacrificed 12 weeks after the injection. Their testicular tissue was fixed in 10% neutral buffered formalin then sectioned and examined histologically using hematoxylin and eosin (H&E) staining.
Results
Characterization of fetal tissues-derived MSCs (hfMSCs)
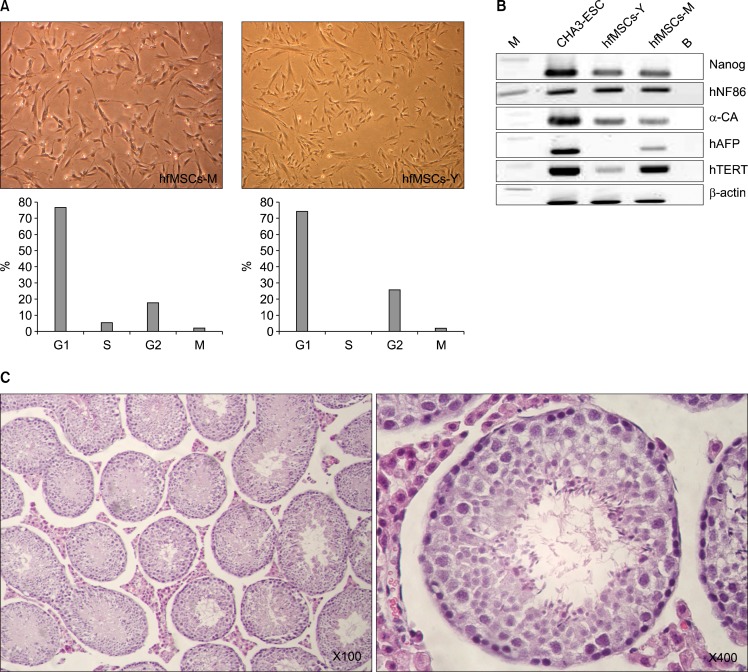
The morphology of hfMSCs isolated from the fetal membrane (hfMSCs-M) and yolk sac (hfMSCs-Y) were similar to those of typical mesenchymal stem cells. The S/G2 phase of the cell cycle (which indicates proliferative activity) was longer in hfMSCS-M compared with hfMSCs-Y (Fig. 1A). However, the differentiation marker nanog and the germ layer markers (including hNF68, a-CA, AFP, and NF68, and the human TERT gene) were similarly expressed in hfMSCs from both sources compared to CHA3-embryonic stem cells (Fig. 1B). These results indicate that hfMSCs have the potential for self-renewal and for differentiation into multiple lineages.
Fig. 1.
Morphology of hfMSCs derived from fetal membranes (hfMSCs-M) and the yolk sac (hfMSCs-Y). hfMSCs were assessed at passage number 8 to 10. (A) The morphologies of hfMSCs including hfMSCs-M and hfMSCs-Y were similar to the round-spindle shape of mesenchymal stem cells (Upper, ×100). Cell cycle analysis of hfMSCs showed increased S/G2 phase, indicating proliferative activity (Lower). (B) RT-PCR analysis for stem cells markers in embryonic stem cells (CHA3-ESC), hfMSCs-Y and hfMSCs-M. (C) Histological analysis for teratoma formation in the testicular capsules of 8-week-old NOD/SCID mice after hfMSCs transplantation.
To determine whether hfMSCs can induce teratomas, we transplanted all hfMSCs (including hfMSCs-M and hfMSCs-Y) into the testicular capsules of 8-week-old NOD/SCID mice and then examined the tissue after 12 weeks using H&E staining. No teratomas were observed (Fig. 1C). The fact that hfMSCs have the potential to differentiate into the 3 germ layers but do not have the potential to form teratomas suggests a potential for pluoripotency, which would be expected in an embryonic stem cell.
Expression of surface markers in hfMSCs
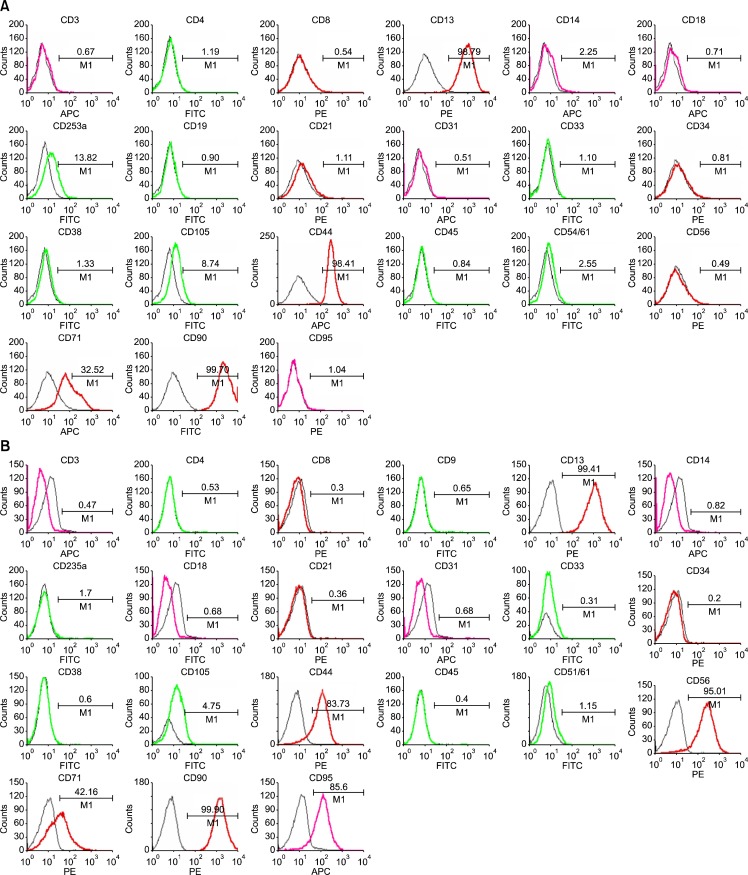
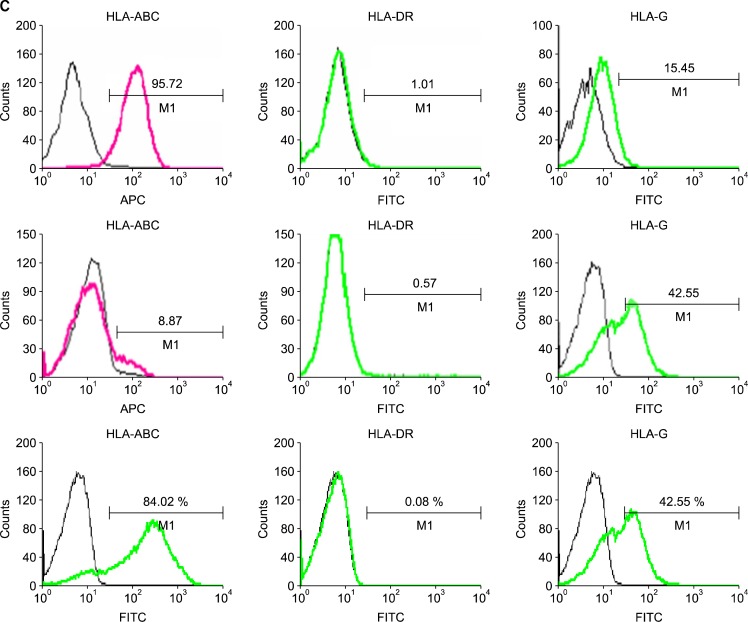
To confirm the surface phenotype of hfMSCs, we performed a FACS analysis using various mouse anti-human antibodies (Fig. 2A, B). The phenotypes of hfMSCs-M and hfMSCs-Y were similar to those of MSCs derived from adult bone marrow, ie, the analysis was negative for hematopoietic markers (eg, CD31, CD33, CD34, CD45, CD51/ 61, and HLA-DR) and positive for nonhematopoietic markers (eg, CD13, CD44, CD90, CD105, and HLA-G). There was no difference in the expression of any of these markers in hfMSCs (including hfMSCs-M and hfMSCs-Y) compared with bone-marrow MSCs. However, we did observe differences in the expression of CD56, CD95, and CD253a. Interestingly, HLA-ABC expression was not observed in hfMSCs-M; neither was the expression of HLA-ABC or HLA-DR. However, HLA-ABC expression was increased in hfMSCs-M in late passages (>20 passages) (Fig. 2C). This suggests that the expression of the HLA class could be affected by environmental factors, such as a long-term culture.
Fig. 2.
FACS analysis of expression of surface markers in hfMSCs. Expression of surface makers in hfMSCs-Y (A) and hfMSC-M (B) using FACS analysis. (C) Expression of HLA-class in hfMSCs-Y (Upper) and hfMSCs-M. HLA-ABC expression was not observed in hfMSCs-M in early passages (Middle), but HLA-ABC expression was increased in hfMSCs-M in late passages (>20 passages, Lower). The percentages are indicated together with the fluorescence intensity.
Osteogenic differentiation in hfMSCs
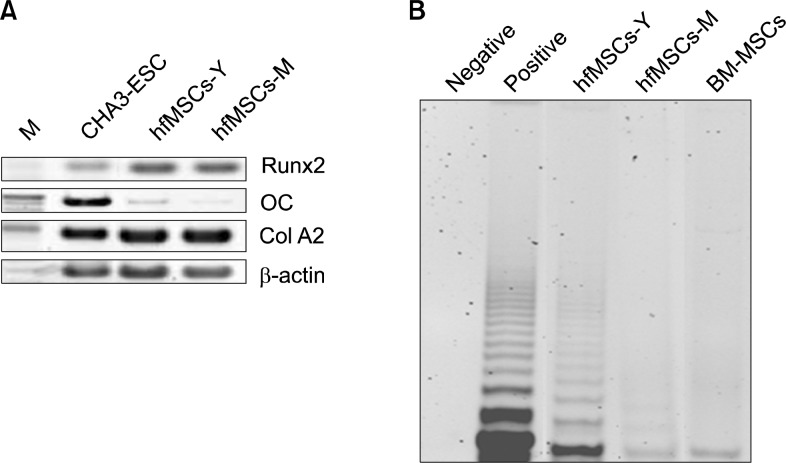
To determine the potential for MSCs to differentiate into osteogenic lineages, we induced osteogenic differentiation in hfMSCs then confirmed it by RT-PCR. Runx2 expression was strong in these hfMSCs after osteogenic differentiation, but osteocalcin (OC) (a marker of mature osteocytes) was only expressed weakly in the hfMSCs after osteogenic differentiation compared with CHA3-ESC (an embryonic stem cell). Coll A2 expression was similar to that of embryonic stem cells, even in the absence of osteogenic differentiation (Fig. 3A). These results suggest that hfMSCs have the potential to differentiate into osteogenic lineages.
Fig. 3.
Osteogenic differentiation and Telomerase activity of hfMSCs. (A) RT-PCR analysis of expression of osteogenic specific genes in hfMSCS after osteogenic differentiation. M: marker; lane 1: embryonic stem cells (CHA3, positive control); lane 2: after osteogenic differentiation of hfMSCs-Y; lane 3: after osteogenic differentiation of hfMSCs-M. (B) The Telomerase activity in hfMSCs and Bone marrow-derived MSCs (BM-MSCs).
Telomerase activity in hfMSCs
The Telomerase activity in embryonic stem cells is strong, otherwise, that of bone marrow-derived mesenchymal stem cells known to express weakly. To analyze telomerase activity in hfMSCs, we performed a TRAP assay in hfMSCs and bone marrow MSCs. The Telomerase activity observed in both hfMSCs-M and hfMSCs-Y was comparable to that seen in bone-marrow MSCs. However, the telomerase activity in hfMSCs-Y was strong than in the other stem cells (Fig. 3B). These results suggest that hfMSCs have a potential for proliferation more than bone marrow-derived mesenchymal stem cells.
Discussion
In the present study, we characterized hfMSCs derived from fetal membranes (hfMSCs-M) and the yolk sac (hfMSCs-Y). These cells are similar to those of MSCs isolated from other types of tissue (eg, bone marrow, adipose tissue, umbilical cord blood, amnionic fluid, and other fetal tissues) but, as reported in previous MSC studies, the hfMSCs demonstrated characteristics that are unique to their source tissue (7, 9, 13). Thus, although both types of tissue are fetal in origin, we found some differences between them, particularly in terms of the patterns of expression for surface markers and HLA-ABC.
In cell-based therapy, there is a risk that the stem cells transplanted into damaged tissue in the host will induce an immune response (14–16). For this reason, the rate for the expansion and the survival of grafted stem cells are limited. Therefore, it is important for hfMSCs to be able to provide advantages that can protect against immune rejection and the need for further engraftment while offering therapeutic advantages before pathology sets in (4). This immune advantage is associated with the patterns of human leukocyte antigens (HLA) expression patterns on the surface of transplanted cells. Undifferentiated MSCs express HLA class I (HLA-DR), but not HLA class II (HLA-ABC) (17). HLA-DR may function as an immunomodulator to promote tolerance of the transplanted cells in the recipient, given that it can attack natural killer (NK) cells exhibit in recipient (14, 15). Short-term co-culturing with MSCs reduces NK-cell cytotoxicity against HLA-DR-positive tumor cells but not against HLA-DR-negative cells. These observations suggest that MSCs have an inhibitory effect on NK-cell cytotoxicity against HLADR-positive cells, making them less susceptible to NK-mediated lysis than HLA-DR-negative cells (15–17). HLAABC expression is also induced in undifferentiated MSCs exposed to IFN-γ; however, IFN-γ-induced HLA-ABC cannot stimulate T cells (4, 11, 18), Still, all of these expression patterns may be involved in modulating the immune system in the tissue host.
We found that hfMSCs-Y express high levels of HLA class II (HLA-ABC) antigen; otherwise, extremely low levels of HLA class I (HLA-DR) antigen. We also found that hfMSCs express HLA-G antigen. These expression patterns could also be involved in modulating the immune system in the tissue recipient. However, hfMSCs-M expression of HLA-ABC was low during early passages but high with more than 20 passages (Fig. 1). This finding indicates that the characteristics of MSCs could vary with the age of the MSC. Therefore, it is important to obtain MSCs at their earliest stages of development.
The hfMSCs have been known to increase engraftment and have great potential for proliferation and osteogenesis (12, 19). hfMSCs proliferation involves the expansion and the function of transplanted stem cells (20). This proliferative capacity is associated with the expression of human telomerase reverse transcriptase (hTERT). Aggressive hTERT expression also induces teratoma formation (as seen with embryonic stem cells) and hfMSC proliferation (21). We found that hTERT activity in hfMSCs--including hfMSCs-M and hfMSCs-Y--is greater than in bone-marrow MSCs but does not induce the formation of teratomas (Fig. 3). These advantages suggest indirectly that hfMSCs could be used as a cell source for cell-based therapy.
We conclude that the various hfMSCs we characterized in this study could serve as models for understanding immunosuppressive mechanisms of MSCs. However, it is necessary to evaluate the characteristics of hfMSCs derived from each type of fetal tissue to be sure that they are highly efficient at immunosuppression.
Acknowledgments
We thank Min-Jung Baik, Su-Yeon Jeon in our laboratory for technicalassistance in these experiments. This work was supported by the Resettlement Funds for a newly appointed professor in CHA University and the Korea Research Foundation Grant funded by the Korean Government (MEST) (KRF-2008-313-E00247).
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interest.
References
- 1.In’t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–852. [PubMed] [Google Scholar]
- 2.Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X, Zhao RC. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J Lab Clin Med. 2003;141:342–349. doi: 10.1016/S0022-2143(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 5.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 6.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Fan CG, Tang FW, Zhang QJ, Lu SH, Liu HY, Zhao ZM, Liu B, Han ZB, Han ZC. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14:311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, Cho DJ, Kang SG, You J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251:116–123. doi: 10.1016/j.cellimm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Campagnoli C, Roberts IAG, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 11.Goherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZY, Teoh SH, Chong MS, Schantz JT, Fisk NM, Choolani MA, Chan J. Superior osteogenic capacity for bone tissue engineering of fetal compared to perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27:126–137. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 13.Mirmalek-Sani SH, Tare RS, Morgan SM, Roach HI, Wilson DI, Hanley NA, Oreffo RO. Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cells. 2006;24:1042–1053. doi: 10.1634/stemcells.2005-0368. [DOI] [PubMed] [Google Scholar]
- 14.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 15.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 16.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activatedNK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 18.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 19.Le Blanc K, Goherström C, Ringdén O, Hassan M, Mc-Mahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U, Nordén-Lindeberg S, Jansson M, Dalton A, Aström E, Westgren M. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 20.Ramasamy R, Tong CK, Seow HF, Vidyadaran S, Dazzi F. The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol. 2008;251:131–136. doi: 10.1016/j.cellimm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Zhao YM, Li JY, Lan JP, Lai XY, Luo Y, Sun J, Yu J, Zhu YY, Zeng FF, Zhou Q, Huang H. Cell cycle dependent telomere regulation by telomerase in human bone marrow mesenchymal stem cells. Biochem Bipphys Res Commun. 2008;269:1114–1119. doi: 10.1016/j.bbrc.2008.03.011. [DOI] [PubMed] [Google Scholar]