Abstract
The regeneration of damaged articular cartilage remains challenging due to its poor intrinsic capacity for repair. Tissue engineering of articular cartilage is believed to overcome the current limitations of surgical treatment by offering functional regeneration in the defect region. Selection of proper cell sources and ECM-based scaffolds, and incorporation of growth factors or mechanical stimuli are of primary importance to successfully produce artificial cartilage for tissue repair. When designing materials for cartilage tissue engineering, biodegradability and biocompatibility are the key factors in selecting material candidates, for either synthetic or natural polymers. The unique environment of cartilage makes it suitable to use a hydrogel with high water content in the cross-linked or thermosensitive (injectable) form. Moreover, design of composite scaffolds from two polymers with complementary physicochemical and biological properties has been explored to provide residing chondrocytes with a combination of the merits that each component contributes.
Keywords: Cartilage, Tissue engineering, Stem cells, Mechanical stimuli, Growth factors
Introduction
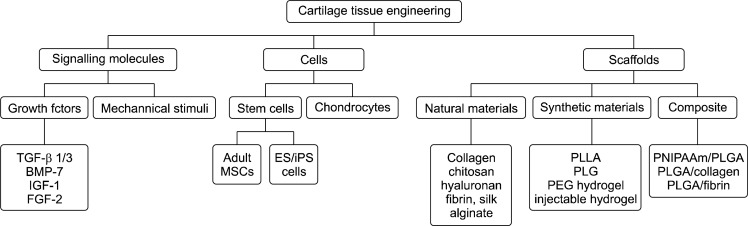
The connective tissue comprising articular cartilage of the knee is highly specialized for reducing joint frictions at the interface of two long bones, and merely contains chondrocytes in an avascular structure (1). The regeneration of damaged articular cartilage remains challenging due to its poor intrinsic capacity for repair. Thus, no surgical procedure has been able to reproduce the biological composition and biomechanical properties of the original cartilage (2). The use of specific treatment strategies has been decided by the nature or size of lesions, and the preference of the operating surgeon (2). However, the advent of tissue engineering has provided the revolutionary potential for treating cartilage-related diseases. It is believed that tissue engineering of articular cartilage will overcome the current limitations of surgical treatment by offering functional regeneration in the defect region. This technology involves ex vivo culturing of chondrocytes from autologous or allogeneic sources in extracellular matrix (ECM)-based constructs and subsequent implantation into the cartilage defect. Although recent progress has been made to engineer artificial cartilage via tissue engineering, the challenges remain significant. Selection of proper cell sources and ECM-based scaffolds, and incorporation of growth factors or mechanical stimuli are of primary importance to successfully produce artificial cartilage for tissue repair. In this portion of the review, suitable cell sources and ECM used in articular cartilage tissue engineering will be introduced, and growth factors and mechanical stimuli as cues for inducing the differentiation to chondrocytes (Fig. 1) will be discussed.
Fig. 1.
Components required for chondrocyte tissue engineering (modified from (2)).
Factors affecting cellular behavior of chondrocytes
Successful repair of cartilage defects by tissue engineering requires several factors including growth factors, cell sources, and mechanical stimuli other than scaffolds. These factors work together to generate artificially engineered cartilage. The ultimate goal of cartilage tissue engineering is to replace the cartilage defect with new tissue engineered from chondrocytes seeded into pre-formed scaffolds or hydrogels. Cell sources have been sought to provide functional characteristics of cartilage, and growth factors and mechanical stimuli were applied to guide the seeded cells for differentiation and maintenance of chondrocytes. Here, the representative factors affecting chondrogenic differentiation and maintenance are described.
Growth factors
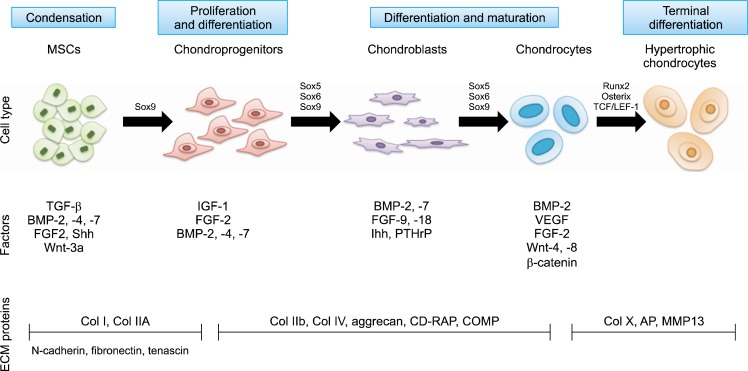
The proliferation and differentiation of both chondrocytes and MSC seeded in the appropriate scaffold are controlled primarily by nearby signally molecules such as hormones, cytokines, and growth factors which direct the specific signaling pathways and maintenance of the chondrocyte phenotype (2, 3). Several factors related to growth and differentiation of MSCs and chondrocytes for cartilage regeneration have been identified (Fig. 2). The successful regeneration of cartilage demands an improved understanding of the complex molecular events involved in the different pathways where each factors contribute. The key issue for chondrocyte tissue engineering is the spatio-temporal control of growth factor delivery to exert the maximal effect on the seeded cells. In most cases, growth factors were encapsulated in microspheres or hydrogel to achieve the controlled release toward surrounding cells.
Fig. 2.
MSC differentiation to chondrocytes and the related growth factors (modified from (3)). AP, alkaline phosphatase; CD-RAP, cartilage-derived retinoic acid-sensitive protein; Col, collagen; COMP, cartilage oligomeric protein; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor.
Transforming growth factor-β (TGF-β) has been used most frequently to induce MSC differentiation to chondrocytes, promote cell proliferation, and inhibit the activity of matrix matelloproteinases (MMP). In an in vitro study with TGF-β3 and MSC, TGF-β3 entrapped in gelatin microspheres were centrifuged with MSCs to form a pellet, and the viability and cartilage matrix production were evaluated with biochemical analysis, immunohistochemistry, and Western blot after four weeks of culture (4). The results showed higher DNA content and faster proliferation in pellets containing MSCs and TGF-β3-loaded microspheres. Heparin or sulfated polysaccharide can act as a platform to bind to and release growth factors like TGF-β3 (5). Rabbit MSCs were immobilized with heparin-bound TGF-β3 in a thermo-responsive p (NIPAAm-co-AAc) composite hydrogel for neo-cartilage formation (6). The data revealed that heparin-bound TGF-β3 facilitates the chondrogenic differentiation of rabbit MSCs. Furthermore, co-delivery of TGF-β3 and HA with MSCs synergistically enhanced the differentiation in the thermo-responsive p(NIPAAm-co-AAc) hydrogel after sub-cutaneous implantation in nude mice (7).
Bone morphogenetic proteins (BMPs) also belong to the TGF-β family. The BMP family consists of 20 subtypes, which have essential roles in chondrogenesis and osteogenesis. It is known that BMP-2 can stimulate the chondrogenic differentiation of MSCs and ESCs, and enhance the secretion of chondrocyte-specific markers, such as collagen type II and aggrecan (3). BMP can be delivered to MSCs or chondrocytes residing inside scaffolds in the form of peptide, protein, or plasmid. In a study in which BMP-2 and BMP-4 were transfected to a mouse mesenchymal stem cell line, C3H10T1/2, for differentiation into chondrocytes, culturing of the BMP-4-transfected cells in an alginate scaffold resulted in up-regulation of collagen type II and other hyaline cartilage proteins, suggesting differentiation into chondrocytes via a chondroprogenitor-like cells (8). As shown in Fig. 2, it is believed that sequential delivery of multiple growth factors enhanced cartilage tissue formation (9). However, the local concentration of each growth factor should be controlled tightly to minimize the side effects caused by over dose. After repeated injection of BMP-2 and TGF-β1 in a murine knee joint, fibrosis and osteophytosis were observed in the joint space (10).
Cell sources
Although several different sources have been sought for cartilage tissue engineering, chondrocytes, isolated from hyaline cartilage, are regarded as popular cells of choice. Chondrocytes, present predominantly in cartilage are the cells responsible for the production of ECM, such as collagens and proteoglycans, which supports and strengthens cartilage physically. Although chondrocytes are a popular source for cartilage tissue engineering, they suffer from several drawbacks such as instability in monolayer culture, additional surgery to procure and expand them, and limited potential for intrinsic repair. In contrast, adult mesenchymal stem cells are a reliable alternative cell source. Therefore, control in differentiation from MSCs to chondrocytes may be the key factor to produce high quality cartilages (Fig. 2). Although multiple cell sources are available, adult MSCs are highly preferred for cartilage tissue engineering due to the report that bone marrow-derived mesenchymal stem cells (BM-MSC) are capable of osteogenesis (11). Recently, adult adipose-derived stem cells (ASCs) were suggested as another cell source for cartilage repair, because of ease of isolation and multiple differentiation potentials. Chondrogenic differentiation was examined with subcutaneous implantation of an ASC-loaded PLGA scaffold in nude mice after in vitro culture in the presence of TGF-β1 for three weeks (12). Chondrocyte-specific markers, such as collagen type II and type Χ, cartilage oligomeric matrix protein (COMP), and aggrecan, were highly expressed even during in vitro culture, and ASC-seeded scaffolds exhibited a stable chondrogenic phenotype in a heterotopic model of cartilage transplantation.
Mechanical stimuli
Cell growth and ECM production can be enhanced with the use of bioreactors by creating a dynamically controlled environment, which promotes an active mass transfer of nutrients and waste between the inside and outside of the scaffolds. Dynamic culture also facilitates uniform distribution of seeded cells throughout the scaffold. Most seeded cells would be placed near the scaffold surface and provides the mechanical stimulation required for chondrocyte growth and differentiation (13). Articular cartilage is regularly subjected to various mechanical stimuli including hydrostatic pressure, compressive force, and shear strain. The composition and three-dimensional complex organization of ECM secreted from chondrocytes in articular cartilage are primarily responsible for its biomechanical and physical properties, supporting tissue homeostasis and remodeling. Generally, proliferation and biosynthesis in chondrocytes take place in response to external mechanical stimuli, and articular chondrocytes located at different depths from the tissue surface behave differently, responding to in vitro oscillatory tensile loading and tensile loading. This dynamic loading potently stimulates chondrocytes located at the superficial zone of artificial cartilage (14). However, chondrocytes at the deep zone remain unaffected by tensile loading with respect to the overall biosynthesis rate. It is thought that cartilage engineered with successful biochemical and mechanical properties could be created by loading scaffold under the mechanical stress similar to that of natural conditions. Intermittent hydrodynamic pressure applied to chondrocytes significantly enhanced collagen production, while preventing a significant decrease in total GAG levels, which is observed with control treatment (15). Furthermore, exposure of the chondrocytes to combined dynamic compression and shear strain for a week increased both collagen and proteoglycan synthesis up to approximately 80% more than that of the static control (16). Interestingly, IL-1-induced matrix degradation was inhibited in articular cartilage under mechanical loading of 0.5 MPa stress (17). It was reported that dynamic loading on cultured hydrogels enhanced anabolic and catabolic activities, but continuous loading inhibited catabolic activity (18, 19). Mechanical stimuli also exert great influence on the differentiation of MSCs, resulting in enhanced secretion of proteoglycan and collagen content, up-regulation of cartilage-specific markers (e.g. aggrecan, Sox-9, and collagen type II), and greater compressive strength (20). Crosslinking density in a PEG hydrogel influenced ECM production under both static and dynamic conditions (21). Indeed, based on the previous reports, it is suggested that mechanical stimuli are essential factors for maintaining cartilage integrity (22).
ECM-based scaffolds
ECM scaffolds should ideally furnish chondrocytes with the optimal physical conditions mimicking the natural ECM microenvironment of cartilages. Construction of these scaffolds involves fabrication of a three-dimentional network of ECM similar to that of the original structure, and provision for structural support and internal space for the residing cells to adhere, proliferate, and differentiate. Therefore, general requirements of three-dimensional ECM scaffolds includes high porosity, controlled degradation, mechanical stiffness and strength, and biocompatibility, and They also should exhibit adequate surface properties for proper tissue formation of chondrocytes (23). Higher porosity allows for the migration and proliferation of adhering cells, and the exchange of nutrients and waste products. Controlled biodegradability also is important so as not to hinder the formation of newly regenerating tissue within the scaffold. Biocompatibility may facilitate cellular attachment and differentiation.
In most cases, biodegradable polymers including synthetic or natural polymers have been used in cartilage tissue engineering. These polymers are formed usually in sponges, hydrogels, or nanofibers. Recently, composite scaffolds composed of mixture of different polymers were designed to provide chondrocytes residing in the matrix with the combination of merits that each component contributes.
Natural materials have been preferred because of their intrinsic advantages of biocompatibility and biodegradability over synthetic materials and because they have demonstrated an improved capacity for cell attachment. They can be divided in two groups: protein-based scaffolds (e.g. collagen, fibrin and silk) and carbohydrate-based scaffolds (e.g. hyaluronan, alginate, agarose, and chitosan).
Collagen is a natural component of cartilage and is known to play an important role in cellular adhesion and differentiation through specific interaction between ligands on collagen chain and adhering cells (2). Therefore, it is widely used for engineering artificial tissue in a broad spectrum of organs. Type II collagen also plays an essential role in the maintenance of chondrocyte function. Bovine BMSCs seeded on type II collagen represent the most prominent phenotype of chondrocyte differentiation with the addition of transforming growth factor (TGF)-β1 in a time dependent manner (24). It was also found that chondrogenic differentiation only was detected in three-dimensional hydrogels, not in monolayer cultures.
Hyaluronan is a primary physiological component of ECMs in articular cartilage, which can be chondrogenic to mesenchymal stem cells. In the transplantation study with autologous BMSCs embedded in the hyaluronic acid sponge, BMSC-loaded scaffold were implanted in full-thickness osteochondral defects of the rabbit knee (25). Histological findings revealed that newly formed cartilage tissue at the implantation site were very similar to the surrounding normal tissue, which is much better than that of the untreated group. It is usually chemically modified for easy fabrication into solid scaffolds because of its highly hydroscopic nature (26). The common form of the modification is the esterification of a carboxylic acid present at the C6 position of hyaluronic acid. The benzyl ester of hyaluronic acid has been commercialized as Hayff-11 and tested clinically.
Alginate is an anionic polysaccharide which forms a hydrogel instantly in the presence of divalent cations such as calcium ion. Alginate beads encapsulating cells are commonly produced when cells dispersed in alginate solution are dropped into a calcium chloride solution. In an in vitro study using rabbit BMSCs, it was reported that alginate and agarose gels containing BMSCs induced the greatest increase in the expression of cartilage-specific markers such as aggrecan and type II collagen, in comparison to type I collagen gels (27). Furthermore, when rabbit BMSCs encapsulated in alginate beads were deployed in cartilage defects of rabbit, the beads remained satisfactorily within the defect regions, which were progressively replaced by the regenerating tissue. Histological findings showed that viable chondrogenic cells were filled in the defects. In a clinical study on 17 patients enrolled with the inclusion criteria of isolated lesion of the femoral condyle (grades III and IV), significant improvement was observed in patients with lesions larger than 3 cm2 when autologous chondrocytes were isolated and suspended in an alginate-agarose mixture solution and subsequently implanted in lesions of patients (28). However, alginate gel suffers from instability in physiological solution due to a loss in mechanical strength and intergrity induced by the replacement of divalent calcium ions with monovalent sodium or potassium ions.
Synthetic materials including poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and their copolymer, poly(lactic acid-co-glycolic acid) (PLGA) have been tested for cartilage tissue engineering potential since the American Food and Drug Administration approved its use in the human body. They are thought to have advantages including their ease in molding and the ability to design their degradation rate to match tissue growth into the scaffold. However, these synthetic materials are not as preferred for cartilage tissue engineering as materials of natural origin, because the acidic byproducts generated during the degradation process cause an inflammation reaction, giant cell reaction, and acute chondrocyte death due to the abrupt drop in pH in the local microenvironment inside the engineered cartilage. The closed cartilage environment deters the rapid clearance of acidic byproducts from the degrading synthetic scaffolds, eventually inducing undesirable side reactions in cartilage. Another disadvantage of these materials is their poor cell attachment. Commonly, these materials were modified to possess an anchorage site for cell adhesion or mixed with other naturally-derived materials (29).
Recently, hydrogel-based scaffolds have gained greater attention for cartilage tissue engineering applications because of their similarity to the natural cartilage environment. Hydrogels contain high water content similar to that of natural cartilage, which serves as a suitable environment for chondrocytes. These materials are composed of synthetic or natural-based hydrophilic biomaterials cross-linked by physical, ionic, or chemical interactions. They also can be injected transcutaneously into the defect region of the joint, which avoids the invasive surgery required for the implantation of a prefabricated scaffold. PEG is a popular biocompatible hydrophilic polymer approved by FDA, and it has been extensively explored for formulating hydrogels to encapsulate bioactive drugs or cells. It is thought that crosslinked PEG hydrogel may provide a better environment for culturing chondrocytes in terms of high water content and mechanical strength, because chondrocytes are surrounded by hydrophilic ECM components in high abundance. When BMSC and embryonic stem cell-derived cells (ESC) were encapsulated in this hydrogel with a mechanical stimulus, gene expression of cartilage-related markers such as Sox-9, type II collagen, and aggrecan, was noticed (20). However, it was determined that highly crosslinked PEG hydrogel might hinder proliferation and proteoglycan synthesis of encapsulated chondrocytes (21, 30). As the seeded cells grow and form new tissue inside the hydrogel, the scaffold should degrade accordingly.
In situ injectable hydrogel systems have generated increased interest for cartilage repair applications (31). They can be injected with encapsulated cells and/or bioactive materials of interest into the cartilage defect in a minimally invasive manner and easily fill the three-dimensional shape of the defect for facilitated integration with the additional mechanical property of temperature-dependent sol-gel transition. Poly (N-isopropylacrylamide) (PNIPAAm) exhibits reversible phase separation with a lower critical solution temperature (LCST) of approximately 32°C. Thus, chondrocytes cells can be dispersed in PNIPAAm solution at room temperature (RT) lower than LCST and injected into the cartilage defect for in situ gelation.
A few specific characteristics of single ECM component might be insufficient to create the optimal environment to mimic the natural proliferation and differentiation of chondrocytes in cartilage. Therefore, in most cases a combination of multiple components to address various features required for culturing chondrocytes is desired. Most synthetic material-based scaffolds suffer from poor anchorage of chondrocytes or stem cells, and incorporation of natural ECMs including collagen, fibrin and hyaluronic acid in synthetic scaffolds for cell attachment has been a popular approach studied extensively. Synthetic materials provide relatively high mechanical strength with a tunable degradation rate, whereas their hydrophobicity and lack of cellular anchorage site are drawbacks for their application in tissue engineering. Conversely, naturally-derived ECM polymers support excellent cellular adhesion and growth due to their specific cell interaction peptides and hydrophilicity, even though their weak mechanical properties make it difficult to use them in load-bearing region such as cartilage. Thus, these characteristics of naturally or synthetically originated materials drive us to attempt a combination of the two materials in order to afford higher mechanical strength, tunable degradation, and cellular attachment (32). Another purpose of hybrid scaffolds is to incorporate thermally or chemically responsive components in natural or synthetic material-based scaffolds to increase the bioavailability of seeded cells while minimizing their leakage out of the scaffolds. Fibrin possesses chemically active gelling property, which typically occurs during blood coagulation in addition to the advantages described previously. Several studies investigating fibrin-based hybrid scaffold demonstrated its potential for the promotion of homogeneous cell distribution and cartilaginous tissue formation (33–35). PNIPAAm was also used for this purpose, and it possessed the merit of a synthetic thermoresponsive polymer for tissue engineering with versatile manipulation of its chemical structure and molecular weight as well as easy conjugation with other components.
Conclusion
Great scientific advances have been achieved for cartilage tissue engineering in the last two decades. Rational design of the scaffolds having greater biological affinity to host cartilage environment as well as sophisticated manipulation of diverse cell sources including stem cells would bring us the exciting prospect for the realization of clinically usable, engineered cartilage.
Acknowledgments
This work is partially supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare &?Family Affairs, Republic of Korea (A084869), the BioImaging Research Center at GIST, Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0002940) and Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (Grant RT104-01-01). The author also acknowledges the support from a grant of Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 08082-1).
Footnotes
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Tuzlakoglu K, Reis RL. Biodegradable polymeric fiber structures in tissue engineering. Tissue Eng Part B Rev. 2009;15:17–27. doi: 10.1089/ten.teb.2008.0016. [DOI] [PubMed] [Google Scholar]
- 2.Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg Br. 2009;91:565–576. doi: 10.1302/0301-620X.91B5.21832. [DOI] [PubMed] [Google Scholar]
- 3.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D. Cartilage engineering: a crucial combination of cells, bio-materials and biofactors. Trends Biotechnol. 2009;27:307–314. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Fan H, Zhang C, Li J, Bi L, Qin L, Wu H, Hu Y. Gelatin microspheres containing TGF-beta3 enhance the chondrogenesis of mesenchymal stem cells in modified pellet culture. Biomacromolecules. 2008;9:927–934. doi: 10.1021/bm7013203. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Woo DG, Yang HN, Na K, Park KH. Transforming growth factor beta-3 bound with sulfate polysaccharide in synthetic extracellular matrix enhanced the biological activities for neocartilage formation in vivo. J Biomed Mater Res A. 2009;91:408–415. doi: 10.1002/jbm.a.32271. [DOI] [PubMed] [Google Scholar]
- 6.Park JS, Woo DG, Yang HN, Lim HJ, Chung HM, Park KH. Heparin-bound transforming growth factor-beta3 enhances neocartilage formation by rabbit mesenchymal stem cells. Transplantation. 2008;85:589–596. doi: 10.1097/TP.0b013e3181639b3a. [DOI] [PubMed] [Google Scholar]
- 7.Na K, Kim S, Woo DG, Sun BK, Yang HN, Chung HM, Park KH. Synergistic effect of TGFbeta-3 on chondrogenic differentiation of rabbit chondrocytes in thermo-reversible hydrogel constructs blended with hyaluronic acid by in vivo test. J Biotechnol. 2007;128:412–422. doi: 10.1016/j.jbiotec.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Weber M, Steinert A, Jork A, Dimmler A, Thürmer F, Schütze N, Hendrich C, Zimmerman U. Formation of cartilage matrix proteins by BMP-transfected murine mesenchymal stem cells encapsulated in a novel class of alginates. Biomaterials. 2002;23:2003–2013. doi: 10.1016/s0142-9612(01)00329-5. [DOI] [PubMed] [Google Scholar]
- 9.Martin I, Suetterlin R, Baschong W, Heberer M, Vunjak-Novakovic G, Freed LE. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J Cell Biochem. 2001;83:121–128. doi: 10.1002/jcb.1203. [DOI] [PubMed] [Google Scholar]
- 10.van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. Differential effects of local application of BMP-2 or TGF-beta 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–317. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- 11.Friedenstein AJ. Osteogenetic activity of transplanted transitional epithelium. Acta Anat (Basel) 1961;45:31–59. doi: 10.1159/000141739. [DOI] [PubMed] [Google Scholar]
- 12.Mehlhorn AT, Zwingmann J, Finkenzeller G, Niemeyer P, Dauner M, Stark B, Südkamp NP, Schmal H. Chondrogenesis of adipose-derived adult stem cells in a poly-lactide-co-glycolide scaffold. Tissue Eng Part A. 2009;15:1159–1167. doi: 10.1089/ten.tea.2008.0069. [DOI] [PubMed] [Google Scholar]
- 13.Nikolaev NI, Obradovic B, Versteeg HK, Lemon G, Williams DJ. A validated model of GAG deposition, cell distribution, and growth of tissue engineered cartilage cultured in a rotating bioreactor. Biotechnol Bioeng. 2010;105:842–853. doi: 10.1002/bit.22581. [DOI] [PubMed] [Google Scholar]
- 14.Vanderploeg EJ, Wilson CG, Levenston ME. Articular chondrocytes derived from distinct tissue zones differentially respond to in vitro oscillatory tensile loading. Osteoarthritis Cartilage. 2008;16:1228–1236. doi: 10.1016/j.joca.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12:1337–1344. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 16.Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. Multi-axial mechanical stimulation of tissue engineered cartilage: review. Eur Cell Mater. 2007;13:66–73. doi: 10.22203/ecm.v013a07. [DOI] [PubMed] [Google Scholar]
- 17.Torzilli PA, Bhargava M, Park S, Chen CT. Mechanical load inhibits IL-1 induced matrix degradation in articular cartilage. Osteoarthritis Cartilage. 2010;18:97–105. doi: 10.1016/j.joca.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryant SJ, Nicodemus GD, Villanueva I. Designing 3D photopolymer hydrogels to regulate biomechanical cues and tissue growth for cartilage tissue engineering. Pharm Res. 2008;25:2379–2386. doi: 10.1007/s11095-008-9619-y. [DOI] [PubMed] [Google Scholar]
- 19.Nicodemus GD, Bryant SJ. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage. 2010;18:126–137. doi: 10.1016/j.joca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 21.Nicodemus GD, Bryant SJ. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. J Biomech. 2008;41:1528–1536. doi: 10.1016/j.jbiomech.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Detamore MS. Tissue engineering the mandibular condyle. Tissue Eng. 2007;13:1955–1971. doi: 10.1089/ten.2006.0152. [DOI] [PubMed] [Google Scholar]
- 24.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 25.Kayakabe M, Tsutsumi S, Watanabe H, Kato Y, Takagishi K. Transplantation of autologous rabbit BM-derived mesenchymal stromal cells embedded in hyaluronic acid gel sponge into osteochondral defects of the knee. Cytotherapy. 2006;8:343–353. doi: 10.1080/14653240600845070. [DOI] [PubMed] [Google Scholar]
- 26.Tortelli F, Cancedda R. Three-dimensional cultures of osteogenic and chondrogenic cells: a tissue engineering approach to mimic bone and cartilage in vitro. Eur Cell Mater. 2009;17:1–14. doi: 10.22203/ecm.v017a01. [DOI] [PubMed] [Google Scholar]
- 27.Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000;16:571–577. doi: 10.1053/jars.2000.4827. [DOI] [PubMed] [Google Scholar]
- 28.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 29.Munirah S, Kim SH, Ruszymah BH, Khang G. The use of fibrin and poly(lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: an in vivo analysis. Eur Cell Mater. 2008;15:41–52. doi: 10.22203/ecm.v015a04. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt O, Mizrahi J, Elisseeff J, Seliktar D. Immobilized fibrinogen in PEG hydrogels does not improve chondrocyte-mediated matrix deposition in response to mechanical stimulation. Biotechnol Bioeng. 2006;95:1061–1069. doi: 10.1002/bit.21072. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira JT, Santos TC, Martins L, Picciochi R, Marques AP, Castro AG, Neves NM, Mano JF, Reis RL. Gellan gum injectable hydrogels for cartilage tissue engineering applications: in vitro studies and preliminary in vivo evaluation. Tissue Eng Part A. 2010;16:343–353. doi: 10.1089/ten.TEA.2009.0117. [DOI] [PubMed] [Google Scholar]
- 32.Dai W, Kawazoe N, Lin X, Dong J, Chen G. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials. 2010;31:2141–2152. doi: 10.1016/j.biomaterials.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 33.Munirah S, Kim SH, Ruszymah BH, Khang G. The use of fibrin and poly (lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: an in vivo analysis. Eur Cell Mater. 2008;15:41–52. doi: 10.22203/ecm.v015a04. [DOI] [PubMed] [Google Scholar]
- 34.Lind M, Larsen A, Clausen C, Osther K, Everland H. Cartilage repair with chondrocytes in fibrin hydrogel and MPEG polylactide scaffold: an in vivo study in goats. Knee Surg Sports Traumatol Arthrosc. 2008;16:690–698. doi: 10.1007/s00167-008-0522-1. [DOI] [PubMed] [Google Scholar]
- 35.Sha’ban M, Yoon SJ, Ko YK, Ha HJ, Kim SH, So JW, Idrus RB, Khang G. Fibrin promotes proliferation and matrix production of intervertebral disc cells cultured in three-dimensional poly(lactic-co-glycolic acid) scaffold. J Biomater Sci Polym Ed. 2008;19:1219–1237. doi: 10.1163/156856208785540163. [DOI] [PubMed] [Google Scholar]