Abstract
Alzheimer’s Disease (AD) is a progressive, neurodegenerative disorder and the most prevalent senile dementia. The early symptom of memory dysfunction involves synaptic loss, thought to be mediated by soluble amyloid-beta (Aβ) oligomers. These aggregate species target excitatory synapses and their levels correlate with disease severity. Studies in cell culture and rodents have shown that oligomers increase intracellular calcium (Ca2+), impairing synaptic plasticity. Yet, the molecular mechanism mediating Aβ oligomers’ toxicity in the aged brain remains unclear. Here, we apply quantitative immunofluorescence in human brain tissue from clinically diagnosed mild cognitive impaired (MCI) and AD patients to investigate the distribution of phosphorylated (active) Ca2+/calmodulin-dependent protein kinase-α (p(Thr286)CaMKII), a critical enzyme for activity-dependent synaptic remodeling associated with cognitive function. We show that p(Thr286)CaMKII immunoreactivity is redistributed from dendritic arborizations to neural perikarya of both MCI and AD hippocampi. This finding correlates with cognitive assessment scores, suggesting that it may be a molecular read-out of the functional deficits in early AD. Treatment with oligomeric Aβ replicated the observed phenotype in mice and resulted in a loss of p(Thr286)CaMKII from synaptic spines of primary hippocampal neurons. Both outcomes were prevented by inhibiting the phosphatase calcineurin (CaN). Collectively, our results support a model in which the synaptotoxicity of Aβ oligomers in human brain involves the CaN-dependent subcellular redistribution of p(Thr286)CaMKII. Therapies designed to normalize the homeostatic imbalance of neuronal phosphatases and downstream dephosphorylation of synaptic p(Thr286)CaMKII should be considered to prevent and treat early AD.
Keywords: amyloid-beta oligomers, calcineurin (CaN), calcium/calmodulin dependent protein kinase II (CaMKII), Alzheimer’s disease, hippocampus, immunofluorescence
INTRODUCTION
Memory dysfunction is the first manifestation of amnestic mild cognitive impairment (MCI), a clinical entity that often progresses to Alzheimer’s Disease (AD) (Petersen et al., 1999). The early deficits of MCI and AD are thought to be due to synaptic loss (DeKosky & Scheff, 1990; Terry et al., 1991) mediated by soluble oligomeric Aβ species (Walsh et al., 2002; Selkoe 2002; Lacor et al. 2004; Koffie et al., 2009). In autopsy brain tissue, the level of soluble Aβ, but not plaque burden, is a robust predictor of synapse loss (Lue et al., 1999). Oligomers decrease synapse size and alter the protein composition of the post-synaptic density (PSD) (Lacor et al. 2007). The current explanations for Aβ oligomer synaptotoxicity postulate that disturbed Ca2+ dynamics in dendritic spines dysregulates downstream signaling, impairing long-term potentiation (LTP) (Arispe et al., 1993; Chen et al., 2002; Demuro et al., 2005; Kuchibhotla et al. 2008; Dineley et al., 2010), an activity-dependent form of synaptic plasticity thought to underlie memory formation. It is therefore likely that the environment of the aged brain, in which Ca2+ regulatory systems are already compromised (reviewed in Foster, 2007), perpetuates the negative effects of Aβ on Ca2+ homeostasis (Mattson et al., 1992; Arispe et al., 1993; Demuro et al., 2005). Nonetheless, the molecular mechanisms of Aβ oligomer synaptotoxicity in aging MCI and AD individuals are not fully understood.
Ca2+/calmodulin-dependent protein kinase II-α (CaMKII) is a highly expressed serine/threonine kinase that comprises 1–2% of total protein in brain and is the main component of the PSD (Kennedy et al. 1983, Lisman & Zhabotinsky, 2001). Upon LTP induction by high-frequency stimulation, Ca2+-activated calmodulin (CaM) binds to CaMKII, which autophosphorylates at threonine residue 286 (p(Thr286)CaMKII), enhancing kinase activity and association with the synapse (Shen & Meyer, 1999). Mutating Thr286 to alanine inhibits LTP and causes spatial learning deficits in rodents (Giese et al. 1998), suggesting a potential critical role of p(Thr286)CaMKII in mediating synaptic events underlying plasticity and cognition (Elgersma et al., 2004). Recent evidence suggests that CaMKII signaling is dysregulated in aged hippocampus (Nyffeler et al., 2007; Bodhinathan et al., 2010), and the presence of Aβ further intensifies conditions which impair synaptic plasticity; acute treatment with Aβ oligomers has been shown to perturb CaMKII autophosphorylation (Zhao et al. 2004; Zhao et al., 2010), while prolonged exposure to oligomers reduces the total pool of synaptic CaMKII expression, along with a reduction in the GluR1 subunit of postsynaptic AMPA receptors (Gu et al. 2009; Zeng et al., 2010). Most of these studies have examined total CaMKII (Wang et al. 2005, Tannenberg et al. 2006, Gu et al. 2009); few have probed the levels or localization of the phosphorylated active protein (Zhao et al. 2004; Srivareerat et al. 2009). Only one examined p(Thr286)CaMKII in the AD brain and reported significantly decreased levels in immunoblots of frontal cortex and hippocampus (Amada et al., 2005). Here we apply quantitative immunofluorescence to human brain tissue from clinically diagnosed MCI and AD patients to investigate the expression pattern of p(Thr286)CaMKII. The data demonstrate that p(Thr286)CaMKII immunoreactivity is redistributed from dendritic arborizations to neural perikarya of both MCI and AD hippocampi, and this significantly correlates with the patient cognitive assessment scores. Furthermore, this phenotype can be induced by Aβ oligomers, but not fibrils, in wild-type mice and primary hippocampal neurons. Both outcomes were prevented by inhibition of calcineurin (CaN). Collectively, these findings suggest that an Aβ oligomer-induced increase in CaN activity may mediate synaptic loss of p(Thr286)CaMKII in human hippocampal neurons, contributing to impairment in synaptic plasticity underlying cognitive dysfunction in AD patients.
MATERIALS AND METHODS
Human Tissue
Frozen mid-hippocampus was obtained from the Oregon Brain Bank at Oregon Health and Science University (OHSU) in Portland, OR, where brain tissue was donated by subjects enrolled in studies at the NIH-OHSU Layton Aging and Alzheimer Disease center. These cases have post-mortem intervals (PMI) of 24 hours or less. Each is examined by a neuropathologist for neurodegenerative pathology including neurofibrillary tangles (NFT) and neuritic plaques (NP). Using standardized CERAD criteria (Mirra et al. 1991), the cases are assigned an amyloid score based on the deposition of amyloid plaques in the brain (1=severe, 2=moderate, 3=mild, 4=none), and a Braak stage (1–6; with 6 being the most severe) indicative of the level and location of hyper-phosphorylated tau tangles (Braak and Braak, 1991). In addition to the pathological information detailed above, demographical data was received along with the frozen tissue. These can be found in Table 1 and include age, sex, duration of disease, and Mini Mental State Exam (MMSE) score (Folstein et al. 1975). Based on clinical and neuropathological observations, brains are classified as control, amnestic mild cognitive impairment (MCI), and Alzheimer (AD). Subjects were followed with annual neurologic and neuropsychologic evaluation, as well as a clinical dementia rating (CDR, Hughes et al., 1982) assigned by an experienced clinician with input from a collateral historian. Control subjects displayed no evidence of cognitive or functional impairment despite this rigorous assessment. MCI subjects were cognitively and functionally intact at the time of enrollment, and had progressed from CDR = 0 to a global CDR = 0.5 (with no sub-scores greater than 0.5) at the time of their last evaluation, within a year of brain autopsy. Subjects in the AD group were diagnosed by consensus at a clinical team conference, met NINDS-ADRDA criteria for a clinical diagnosis of AD, had CDR = 1.0 or greater, and had neuropathologic confirmation of diagnosis. Autopsies were performed after appropriate informed consent and tissue was used in accord with IRB-approved protocols. Neuropathologic AD assessment was performed according to NIA-Reagan consensus criteria (Ball et al. 1997). Subjects with moderate to frequent neuritic plaques and Braak stage VI neurofibrillary tangles, and who were clinically demented and had a diagnosis of probable AD were included in the AD group. Controls had no more than sparse neuritic plaques and no greater than Braak stage II neurofibrillary tangles. Individuals with Lewy body disease involving the brainstem (including substantia nigra), amygdala, or middle frontal gyrus were excluded from both AD and control groups, as were patients with vascular brain disease manifested by any grossly observed arterial territorial infarct, any grossly observed lacunar infarcts, or microvascular infarcts.
Table 1.
Demographical information of cases.
| Status | Case # | Age | Sex | PMI | Braak Stage |
Plaque Score |
MMSE | MMSE (years before death) |
|---|---|---|---|---|---|---|---|---|
| AD | 1673 | 81 | F | 5.5 | 6 | 2 | 17 | 0.5 |
| AD | 1678 | 76 | F | 25 | 6 | 2 | 1 | 0.8 |
| AD | 1688 | 75 | M | 17 | 6 | 1 | 0 | 2.2* |
| AD | 1746 | 61 | M | 4 | 6 | 1 | 2 | 1.4 |
| AD | 1756 | 68 | M | 11.5 | 6 | 1 | 7 | 2.2* |
| AD | 1766 | 63 | F | 3.5 | 6 | 1 | 18 | 5.2* |
| AD | 1770 | 82 | F | 6.5 | 6 | 1 | 15 | 1.2 |
| AD | 1774 | >89 | M | 3.25 | 6 | 1 | 2 | 0.1 |
| AD | 1776 | >89 | F | 6.25 | 6 | 2 | 6 | 1.2 |
| AD | 1777 | 67 | F | 20.5 | 6 | 1 | 9 | 1.0 |
| AD | 1811 | >89 | M | 18 | 6 | 2 | 21 | 0.6 |
| MCI | 781 | 89 | F | 20 | 3 | 2 | 22 | 0.1 |
| MCI | 811 | >89 | F | 12 | 5 | 1 | 20 | 0.3 |
| MCI | 975 | >89 | F | 4 | 2 | 3 | 25 | 0.8 |
| Control | 1104 | 86 | F | 16 | 2 | 3 | 29 | 0.6 |
| Control | 1229 | >89 | F | 12 | 2 | 3 | 30 | 0.3 |
| Control | 1563 | 80 | M | 2 | 1 | 3 | 30 | 1.0 |
| Control | 1731 | 74 | F | 7.5 | 2 | 3 | 29 | 2.8 |
Asterisk (*) denotes exclusion from MMSE correlation due to the lapse between cognitive testing and tissue collection. To preserve subject confidentiality, individuals over 89 years are listed only as >89.
Tissue preparation and antibody capture assay (ACA)
As previously described (Yang et al., 2007), hippocampal tissue was homogenized and sequentially extracted in buffer A (10 mM Tris, 1 mM ethylene glycol bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid, 1 mM dithiothreitol, and 10% sucrose, pH 7.5], and buffer B (buffer A + 1% Triton X-100). Phosphatase inhibitors (20 mM NaF and 1 mM sodium orthovanadate) and protease inhibitor cocktail (Sigma Cat #P2714; St. Louis, MO) were added to buffers immediately before use. Supernatant B was diluted 1:100 in a solution containing 100 mM Tris, 0.05% sodium azide, and 0.001% bromophenol blue and 100 µL of each sample was spotted onto 96-well plates that were incubated overnight at room temperature in a humidified chamber. Plates were washed twice with phosphate-buffered saline (PBS), blocked with 1% bovine serum albumin in PBS with 0.05% sodium azide, and washed again with PBS. ACAs were performed using tetramethylbenzidine with absorbances determined at 405 nm. Primary antibodies against CaMKII (mouse monoclonal) and p(Thr286)CaMKII (rabbit polyclonal), both from Santa Cruz Biotechnology; (Santa Cruz, CA), were used at 1:2000 dilution. Specificity was confirmed by Western blot of brain extracts before use in capture assays. These samples were prepared from hippocampal tissue from additional cases at the Oregon Brain Bank at OHSU, from the same clinicopathologic groups as the immunofluorescence studies described below.
Immunofluorescence
5mm sections of the mid hippocampus were brought out of storage at −80 °C and equilibrated to −20 °C before embedding in Tissue-Tek O.C.T. compound (Sakura Finetek USA; Torrance, CA). 10 µm sections were cut and affixed to Superfrost Plus slides (Thermo Fisher Scientific; Waltham, MA), for storage at −80°C. After briefly equilibrating to room temperature, they are rinsed in 0.1 M PBS and then fixed in ice-cold 4% paraformaldehyde for 15 minutes. After two brief washes in 0.1 M PBS, the sections were blocked and permeabilized for 1 hour in 0.1 M PBS containing 10% goat serum, 0.03% Triton-X, and 0.1% phosphatase inhibitor (Thermo Fisher Scientific; Waltham, MA). Incubation with primary antibodies in 0.1M PBS containing 10% serum and 0.1% phosphatase inhibitor was carried out overnight at room temperature. Antibodies used were CaMKII (mouse, 1:100) and p(Thr286)CaMKII (rabbit, 1:100) both from Santa Cruz Biotechnology (Santa Cruz, CA); PSD-95 (mouse, 1:500) from Abcam (Cambridge, MA); NeuN (mouse, 1:1000) from Chemicon/Millipore (Billerica, MA); and MAP2 (chicken, 1:7500) and 6E10 (mouse, 1:400) both from Covance (Princeton, NJ). Following two PBS washes, the slides were incubated with Alexa Fluor 488 and 594 secondaries (1:600; Invitrogen; Carlsbad, CA) in 0.1 M PBS containing 10% serum and 0.1% phosphatase inhibitor. Slides were again rinsed twice in PBS and once in distilled water before a 10 minute incubation with 0.3% Sudan Black B (EMD Chemicals; Gibbstown, NJ) in 70% ethanol to block lipofuscin autofluorescence (Romijn et al., 1999). After two more rinses in distilled deionized water, Vectashield containing 4',6-diamidino-2-phenylindole (DAPI) was applied (Vector Laboratories; Burlingame, CA), and coverslips were mounted. Nail polish was applied to seal the edges and signal. Treatment of primary hippocampal neurons was identical to the procedure described for immunohistochemistry, but Sudan Black B was omitted, and Vectashield mounting media did not contain DAPI. Experiments were performed in triplicate.
Preparation of Aβ
As previously described (Reese et al., 2008; Dineley et al., 2010) human amyloid beta 1–42 was purchased from the Dept. of Biophysics and Biochemistry, Yale University, New Haven, CT. To prepare oligomeric Aβ, lyophilized Aβ aliquots (0.3 mg) were dissolved in 0.2 ml of 1,1,1,3,3,3-Hexafluoro-2-propanol (HFP) and then added to 0.7 ml of distilled deionized H2O. Samples were loosely capped and stirred on a magnetic stirrer under a fume hood for 48 hr and then used within 36 hr. The quality of Aβ preparations was routinely checked by dot-blot with conformation-specific A-11 antibody (Kayed et al., 2003) and western blot. Fibrillar Aβ was prepared following the same procedure as for oligomeric Aβ, except that the samples were tightly capped and stirred for 7 days, then loosely capped for 48 hr. The final concentration of the oligomeric Aβ preparation was nominally calculated based on the concentration of the starting Aβ monomer.
Animals
C57BL/6 mice at 5 months of age were used. Animals were maintained at the UTMB vivarium under USDA standards (12 hr light/dark cycle, food and water ad libitum). At the end of each experiment, animals were sacrificed by exposure to halothane vapors followed by decapitation. For immunoblots, the brain was removed and dissected into cerebellum, hippocampus, and frontal and parietal cortices. For immunohistochemistry, the whole brain was removed and stored at −80 °C until sectioning, an approach chosen to mimic the conditions in which the human tissue was processed.
Intracerebroventricular (ICV) injection
Following an IACUC-approved protocol, ICV injections were performed via a modified free-hand method (Clark et al. 1968; Dineley et al., 2010). Briefly, mice were deeply anesthetized with a ketamine/xylazine mixture (65 and 7.5 mg/kg IP, respectively), the scalp was shaved and an incision was made through the midline to expose the top of the skull. A 28-gauge needle held with a haemostatic forceps to leave 3 mm of the needle tip exposed was lowered 1 mm posterior and 1 mm lateral of the bregma. The needle was connected through a 0.28 mm polyethylene tubing to a 25 µL syringe driven by an electronic programmable micro-infuser (Harvard Apparatus, Holliston, MA), which was used to deliver 3 µL/mouse at a rate of 6 µL/min. After injection, the needle was left in place for 1 min and the surgical wound stitched before placing the mouse to recover while lying on a heated pad under a warm light. Reliability and consistency of injections was routinely tested during actual experiments by injecting India ink in parallel blank animals and macroscopically observing proper coloration of the ventricles (Dineley et al., 2010). 18 hours after ICV, animals were given an intraperitoneal injection of either 1% DMSO in 0.9% saline or CaN inhibitor FK506 at 10 mg/kg (LC Laboratories; Woburn, MA). 5 animals were treated with saline, and 6 with oligomers. For fibrils and oligomers + FK506, 4 animals per group were injected. Half of the animals were used for biochemical analysis (Fig. 4) and half for immunofluorescence studies (Fig. 5).
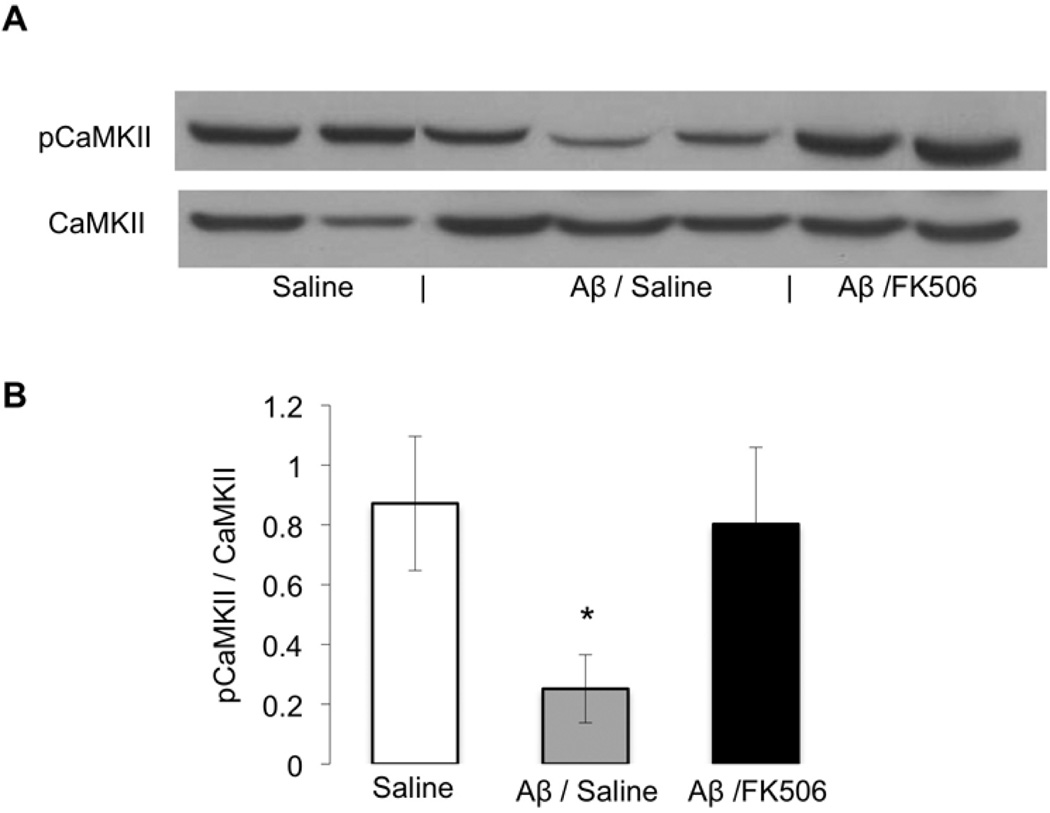
Figure 4. p(Thr286)CaMKII is reduced in a CaN-dependent manner in mice that received an ICV injection of oligomeric Aβ.
(A) Immunoblot of p(Thr286)CaMKII and total CaMKII; (B) ImageJ densitometric analysis of p(Thr286)CaMKII and total CaMKII in the ICV mouse hippocampus. Aβ/S (n=3) is significantly different (p = 0.011) from saline-treated animals (n=2) and Aβ/FK506 (n=2; p = 0.018).
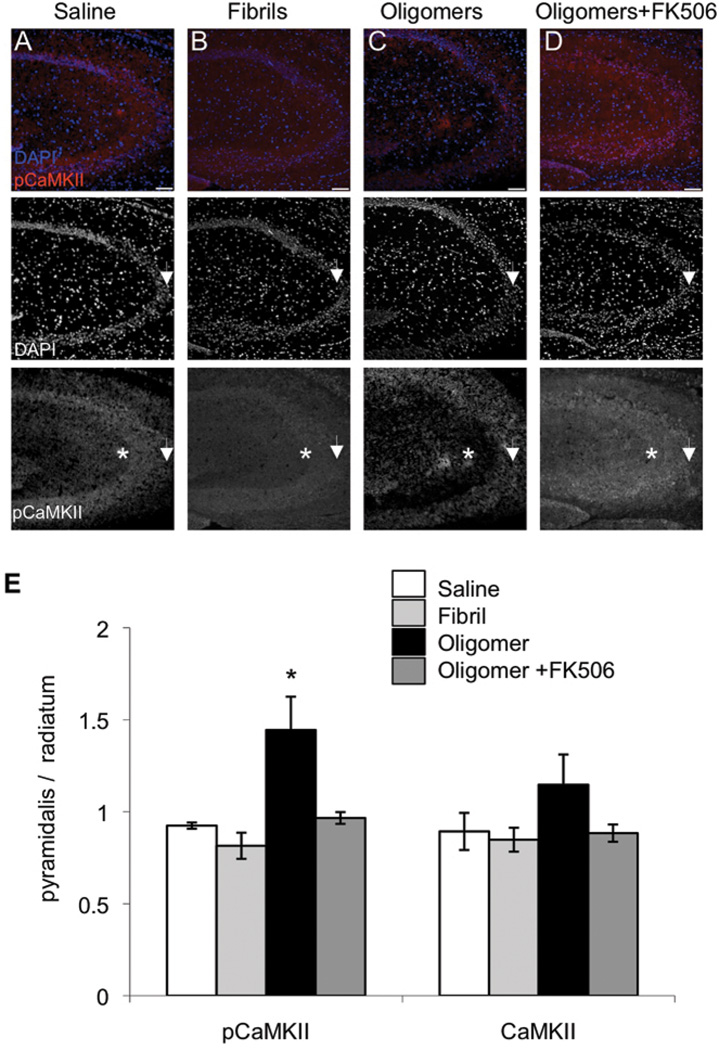
Figure 5. p(Thr286)CaMKII is decreased following ICV injection of Aβ oligomers, but not fibrils, in a CaN-dependent manner in wild-type mice.
Immunolabeling for DAPI (blue) and p(Thr286)CaMKII (red) shows the decreased immunoreactivity of p(Thr286)CaMKII in the stratum radiatum (merge A-D, scale bar 100µm; arrows denote CA3 pyramidal neurons and asterisks denote stratum radiatum); (E), Sections from mice treated with oligomers (1.44 ± 0.18; n = 4) showed a significantly higher p(Thr286)CaMKII ratio (F = 9.62; p = 0.002) as calculated in Fig.2A) compared to sections treated with saline (0.93 ± 0.17; n=3; p = 0.002), fibril (0.82 ± 0.07; n = 4; p = 0.0002), and oligomer+FK506 (0.97 ± 0.03; n = 4; p =0.002). This outcome was specific to the phosphorylation state: although there was a trend towards higher CaMKII ratio in oligomer treated sections (1.15 ± 0.16), it was not significantly different (F = 2.38; p = 0.125) from saline (0.89 ± 0.10), fibrils (0.85 ± 0.06), or oligomers ± FK506 (0.88 ± 0.05).
Western blot
Hippocampal tissue was homogenized in SDS lysis buffer containing 5 mM EDTA, 50 mM Tris, 2% SDS, 1 mM DTT, 1 mM PMSF and 1% protease cocktail inhibitors (Sigma, St. Louis, MO), centrifuged at 20,000×g for 5 minutes and the supernatants collected. Samples containing 30 µg of proteins, as determined by the BCA assay (Pierce, Rockford, IL), were subjected to SDS-PAGE using 12% gels, followed by electrophoretic transfer to nitrocellulose-backed membranes (Bio-Rad, Hercules, CA). Membranes were then blocked in 5% bovine serum albumin in 10 mM Tris-buffered saline containing 0.1% Tween 20, pH 7.5, (TBS-T) for one hour, then incubated with 1:1000 of the primary antibodies CaMKII (mouse) or (p(Thr286)CaMKII (rabbit), both from Santa Cruz Biotechnology (Santa Cruz, CA) overnight at 4°C. After two washes in TBS-T followed by a horseradish peroxidase (HRP) conjugated secondary antibody (Bio-Rad, Hercules, CA) against rabbit IgG (for polyclonal primaries) or mouse IgG (for monoclonal primaries). Detection was achieved using enhanced chemiluminescence (ECL; Pierce, Rockford, IL) and quantification of band intensity was performed with ImageJ (NIH) applied to TIFF files of scanned autoradiography images of membranes.
Primary Rat Hippocampal Cultures
Hippocampal cultures were prepared from embryonic day 18 rat embryos using previously described methods (Laezza et al., 2007a, 2007b, 2009). Briefly, hippocampi were dissected and dissociated using papain and trituration through a Pasteur pipette. Neurons were plated at low density (1–5×105 cells per dish) on poly-L-lysine-coated coverslips in 60 mm culture dishes in MEM supplemented with 10% horse serum. After 2–4 hr, coverslips (containing neurons) were inverted and placed over a glial feeder layer in serum-free MEM with 0.1% ovalbumin, 1 mM pyruvate (N2.1 medium; Invitrogen, Carlsbad, CA) and B-27 supplement (Invitrogen) separated by 1 mm wax dot spacers. The presence of the spacer prevented contact between the neurons on the coverslips and the glial feeder layer. Cultures were maintained in N2.1 medium for up to 30 days. To prevent glial overgrowth, cultures were treated with cytosine arabinoside (5µM; Calbiochem, La Jolla, CA) at 3 d in vitro (DIV). Immediately before experimental treatment, coverslips were flipped to directly expose the neuron to the culture medium. Following addition of the treatment solution, plates were placed back in the cell culture incubator to maintain pH, temperature, and humidity conditions. Experiments were performed in triplicate.
Microscopy
High-resolution TIFF images (1024x1024 pixels) were acquired using a confocal laser-scanning model (Bio-Rad Radiance 2000 with LaserSharp software; Hercules, CA) mounted on a Nikon E800 upright microscope. For histology slides 4x/0.20NA, 10x/0.45NA, 40x/0.95NA, and a 60x-oil/1.4NA objective were used (Nikon USA; Melville, NY), for primary hippocampal neurons only the 60x oil immersion objective was employed at the optimal iris setting (2.0 mm). Images were acquired with a blue diode and krypton lasers of 488 nm and 568 nm excitation. Images for comparisons were acquired with constant settings for laser power, detector gain, amplification gain, and offset.
Data Collection
Analysis was performed with ImageJ (NIH) and a set of plug-ins developed by Wright Cell Imaging Facility (available at: http://www.uhnres.utoronto.ca/facilities/wcif/imagej/). For human and mouse studies, equal areas (~50 mm2) were selected in the 10x TIFF files from the hippocampal regions described in results, and measured by a blinded operator for fluorescence intensity in ImageJ (see Fig.2B). We acknowledge that the quality of frozen tissue from humans is less than ideal, and tissue tearing can be problematic. For this reason, regions with large unstained patches without DAPI positive nuclei were omitted from analysis. For quantification of synaptic p(Thr286)CaMKII, masks of the synaptic regions were generated by thresholding the PSD95 signal in the Alexa 488 channel. We selected PSD95 positive puncta on proximal dendrites, and the resulting mask was transferred to the Alexa 594 channel. The pixel intensities for both channels (PSD95 and p(Thr286)CaMKII) were determined as average fluorescence intensity. In this way, synaptic p(Thr286)CaMKII (rather than dendritic) contributed to the obtained value. This also allowed us to measure the area of the PSD95 punctae,
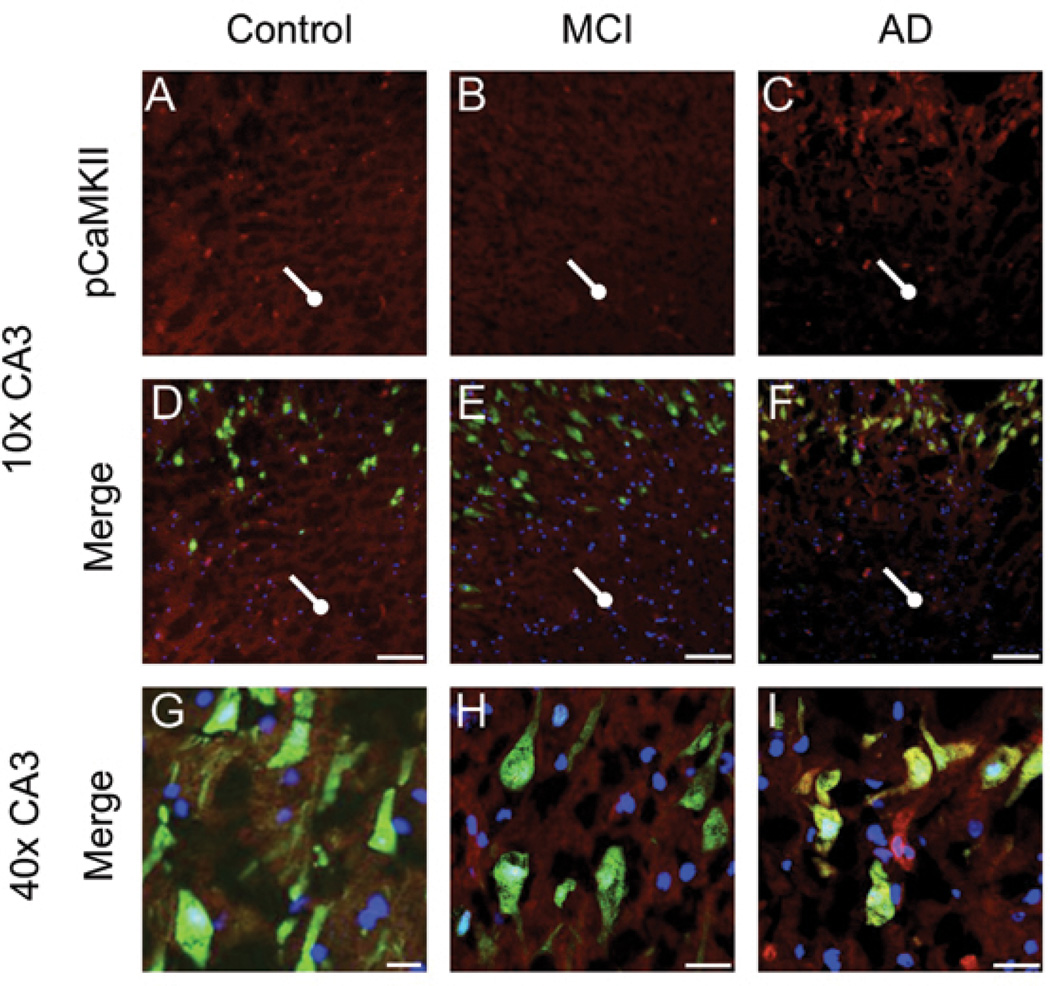
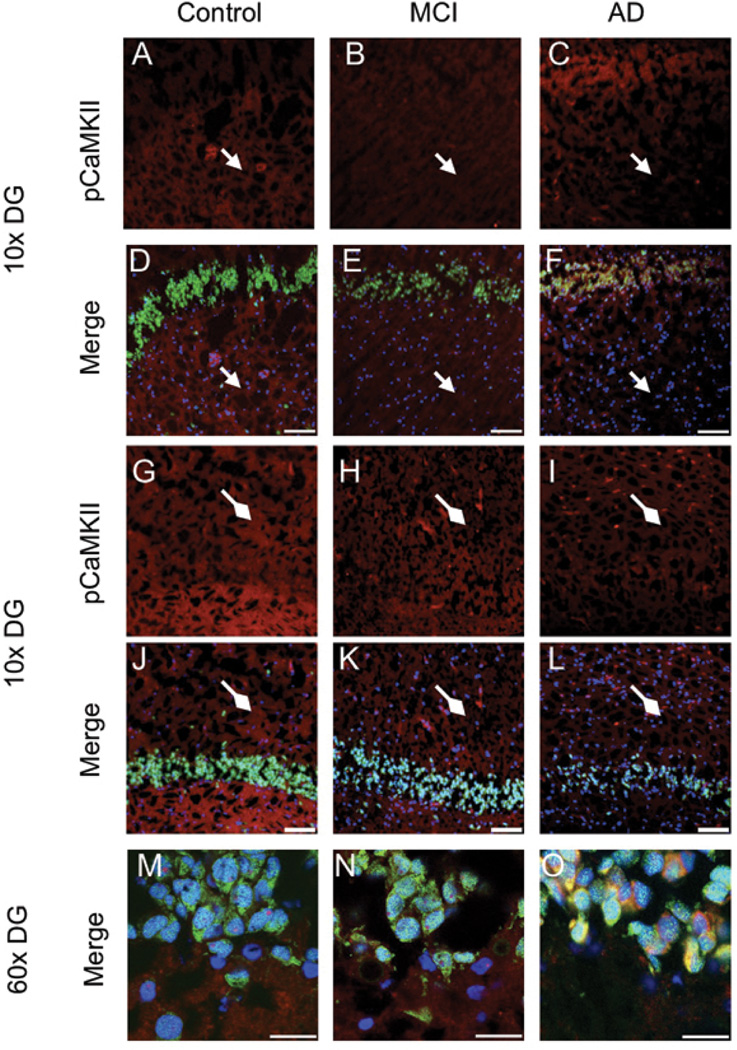
Figure 2. pCaMKII immunoreactivity is altered in MCI and AD CA3.
Expression of pCaMKII (red) and NeuN (green) was assessed in 10µm sections of hippocampus. DAPI mounting media was used to visualize nuclei (blue). pCaMKII immunoreactivity is decreased in the stratum radiatum (A-F, circleheads, scale bar 100µm, 10x), of MCI and AD CA3, relative to control. Conversely, immunoreactivity is increased around the soma of MCI and AD CA3 pyramidal neurons (G-I, scale bar 25µm, 40x).
Statistical Analysis
Data was tabulated and graphed with Microsoft Excel (Version 2008), and analyzed with StatPlus (AnalystSoft, StatPlus:mac Version 2009). Analyses with more than two groups were subject to one-way ANOVA and, if overall p < 0.05, was followed by Fisher least significant difference (LSD) (see Figs. 3B, 4B, 5E, 6E-F, 7E-F, S.Fig.3B, S.Fig.5E-F). For MMSE correlation, Pearson’s correlation coefficient (Figs. 3C-E) was calculated. Statistical significance of ACA data (S. Fig. 1) was determined by a Mann-Whitney U-test due to the different sample sizes. Error bars represent mean ± SEM in all figures except Fig.4B where two of the conditions only include two animals and error bars represent mean ± SD.
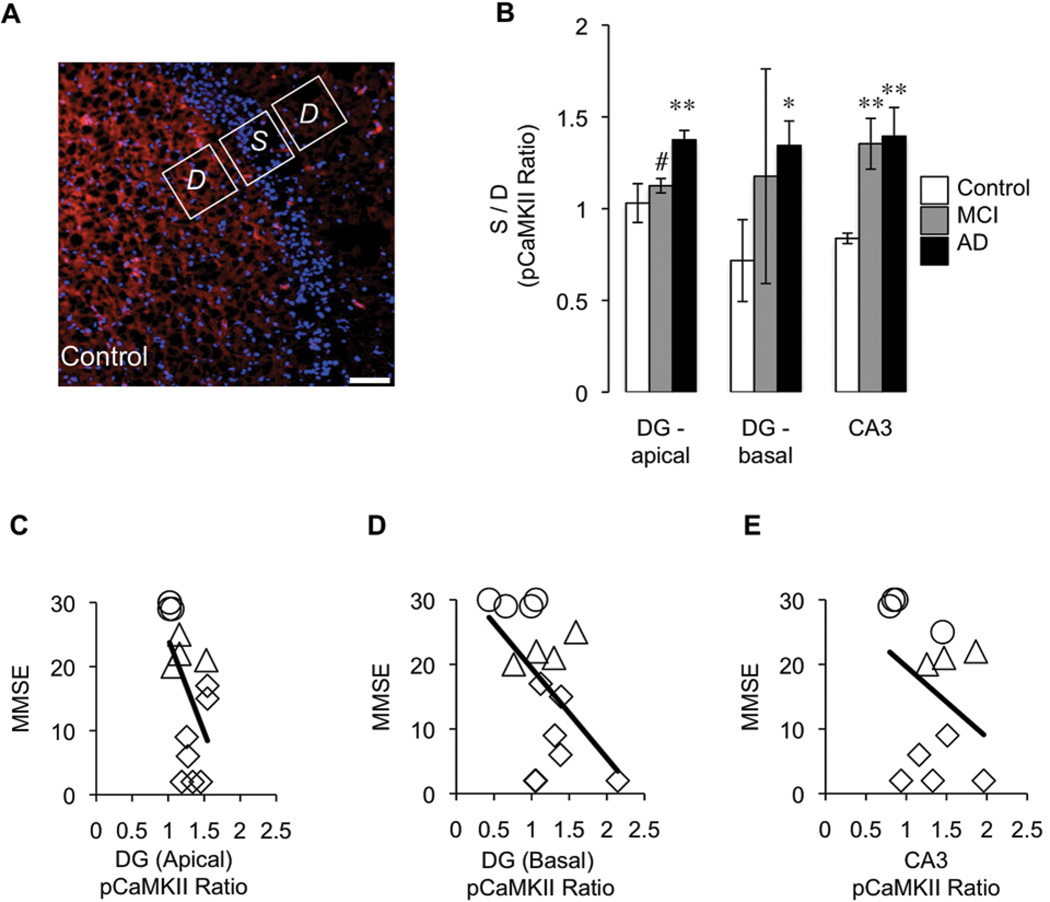
Figure 3. p(Thr286)CaMKII expression (red) shifts from dendritic arborizations to neuronal soma in MCI and AD hippocampus and this change correlates with decreased MMSE score.
(A) Representative micrograph of control DG illustrates how the pCaMKII ratio is calculated as the average fluorescence intensity of the neuronal soma (S) divided by the average intensity in the dendritic arborizations (D). For the latter, the hilus (DG basal dendrites), molecular layer (DG apical dendrites), and stratum radiatum (CA3 apical dendrites) were analyzed; (B) Graph showing the compiled data of 18 hippocampi. In the apical dendrites of the DG, p(Thr286)CaMKII distribution of AD is significantly different from control (p = 0.0004) and MCI (p = 0.010). The same is true of the basal dendrites in the DG, AD is significantly different from control (p = 0.0136). In CA3, MCI and AD are significantly different from control (p = 0.001 and p = 0.006, respectively). (C) Correlation of pCaMKII in DG (apical dendrites) with MMSE (n = 15, r = −0.540, p = 0.018); (D) Correlation of pCaMKII in DG (basal dendrites) with MMSE (n=15, r = −0.527, p = 0.021 ); (E) Correlation of pCaMKII in CA3 with MMSE (n = 12, r = −0.369, p = 0.12). No impairment – circle, mild impairment – triangle, severe impairment – diamond.
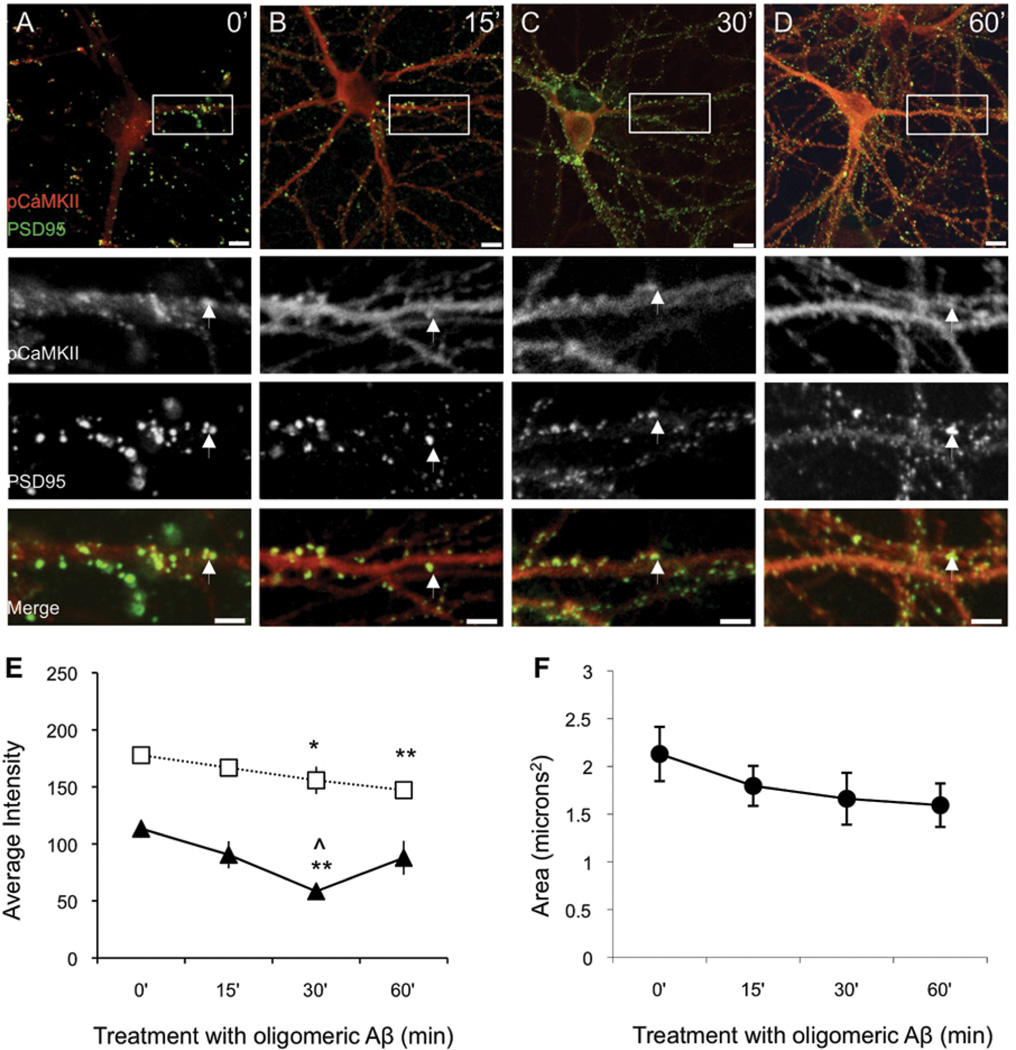
Figure 6. Oligomers decrease synaptic pCaMKII and synapse size in primary hippocampal neurons.
Primary hippocampal neurons (DIV21) were treated with 500nM oligomeric Aβ for 0, 15, 30, or 60 minutes (A-D, scale bar 10µm). Double immunolabeling for pCaMKII (red) and PSD95 (green), with merge shown below (scale bar 5µm). White arrowheads indicate representative synapses. (E) Oligomer-treated neurons had decreased pCaMKII (black triangles; F = 5.04; p = 0.005) and PSD95 fluorescence intensity (white squares; F = 3.57; p = 0.02) at the synapse; (F) Oligomers also decreased the average puncta size, although this trend was not significant (p = 0.377). ANOVA post-hoc test results are indicated by symbols. Significantly different from: 0’ (*), 15’ (^). Single symbol p < 0.05; double symbol p < .01. The values from (E) and (F) can be found in Supplementary Table 2.
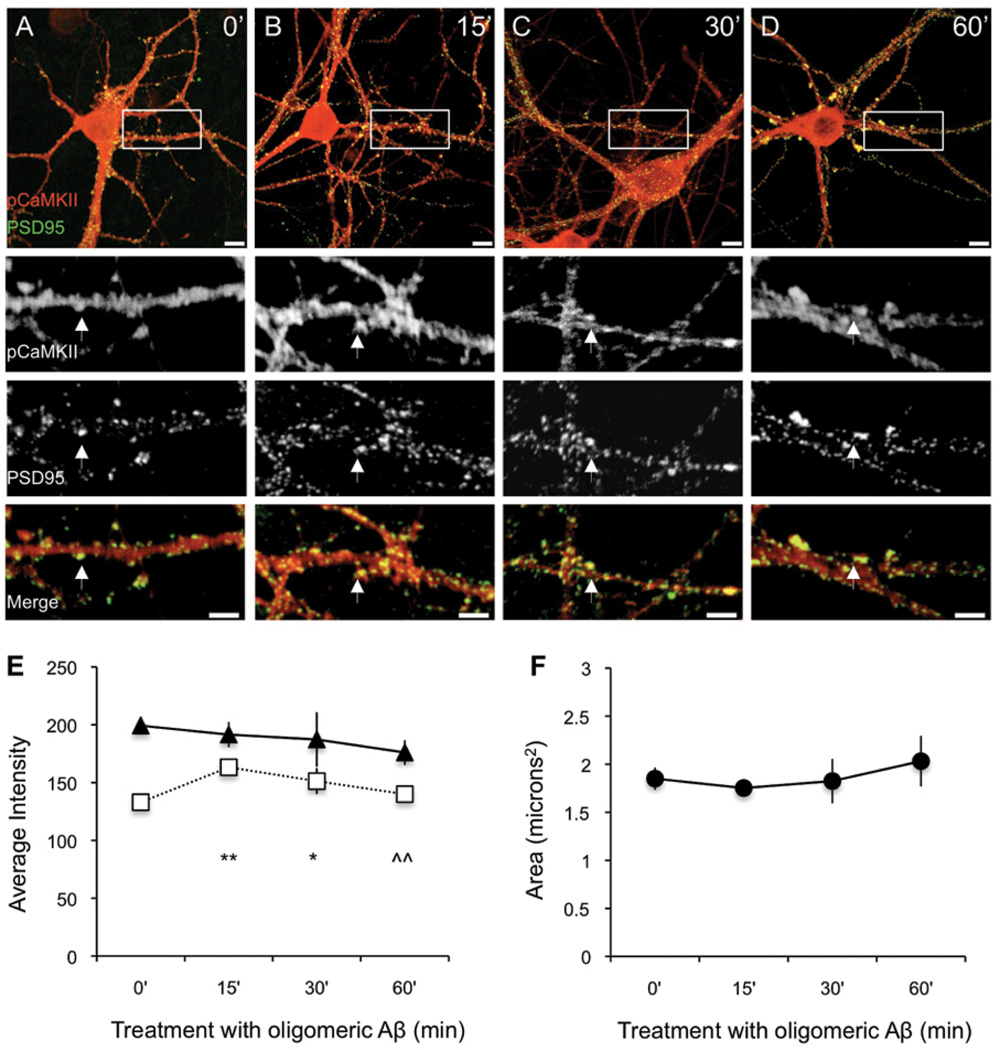
Figure 7. Inhibition of CaN prevents oligomer-induced decrease of synaptic pCaMKII.
After 30 minute exposure to 1µM FK506, primary hippocampal neurons (DIV20) were treated with 500nM oligomeric Aβ for 0, 15, 30, or 60 (A-D, scale bar 10µm). Double immunolabeling for pCaMKII (red) and PSD95 (green), with merge shown below (scale bar 5µm). White arrowheads indicate representative synapses. (E) Addition of FK506 prevented the oligomer-induced decrease of pCaMKII at the synapse (black triangles; F = 0.653; p = 0.594), and increased PSD95 intensity (white squares; F = 5.0325; p = 0.015); (F), The trending decrease in puncta size elicited by oligomers was also prevented (F = 0.498; p = 0.701). ANOVA post-hoc test results are indicated by symbols. Significantly different from: 0’ (*), 15’ (^). Single symbol p < 0.05; double symbol p < .01. The values from E and F can be found in Supplementary Table 2.
RESULTS
Phosphorylation of CaMKII at T286 is altered in MCI and AD hippocampi
Previous studies using western immunoblotting have reported a decrease in p(Thr286)CaMKII in AD frontal cortex and hippocampus (Amada et al., 2005) However, in preliminary experiments, an antibody capture assay (ACA) performed on hippocampal protein extracts from a panel of control and AD autopsy samples showed that p(Thr286)CaMKII was significantly increased in AD (S. Fig. 1). To further explore these opposing results, frozen hippocampal sections from 18 hippocampi (4 control, 3 MCI, and 11 AD; S. Table 1) were fixed and immunolabeled for p(Thr286)CaMKII and analyzed by confocal microscopy. Five anatomical regions were analyzed (S. Fig. 2): 1) the pyramidal neurons of Cornu Ammonis 3 (CA3); 2) the stratum radiatum, where the dendritic arborizations of CA3 neurons are located; 3) the dentate gyrus (DG) granule cells; 4) the molecular layer, which contains the apical dendrites of the DG; and 5) the hilus. This last region was included due to a striking shift of p(Thr286)CaMKII. While it does not contain the apical dendritic arborizations of the DG neurons, it is the location of the basal dendritic arborizations. In rodents, basal dendrites of granule cells are considered a transient phenomenon occurring during development (Jones et al., 2003) and also a pathological feature, formed after insults such as hypoxia-ischemia (Diaz-Cintra et al., 2009) and epileptic activity (Spigelman et al., 1998). However, in the higher primate brain, the basal dendrites are a normal feature of the adult hippocampus, (Seress and Mrzljak, 1987).
In control dentate gyrus, p(Thr286)CaMKII immunoreactivity is most prominent in the dendritic arborizations contained in the hilus and molecular layer. However, in MCI and AD tissue p(Thr286)CaMKII expression in these regions is noticeably decreased (Fig. 1, A-I). Instead, MCI and AD tissue show the greatest p(Thr286)CaMKII immunoreactivity in the perikarya of DG granule cells (Fig. 1, M-O). The same pattern is observable in the CA3, control stratum radiatum (Fig. 2A, D) shows higher immunoreactivity for p(Thr286)CaMKII compared to that of MCI and AD (Fig. 2, B-C, E-F). Again, the perikarya of CA3 pyramidal neurons exhibit enhanced p(Thr286)CaMKII signal (Fig. 2, G-I).
Figure 1. pCaMKII immunoreactivity is altered in MCI and AD dentate gyrus.
Expression of pCaMKII (red) and neuronal nuclei (NeuN, green) was assessed in 10µm sections of hippocampus. DAPI mounting media was used to visualize nuclei (blue). pCaMKII immunoreactivity is decreased in the hilus (A-F, arrowheads, scale bar 100µm, 10x), and molecular layer (G-L, diamond heads, scale bar 100µm, 10x), of MCI and AD DG, relative to control. However, immunoreactivity is increased around the soma of DG granule cells in MCI and AD (M-O, scale bar 25µm, 60x).
To quantify the observed redistribution of p(Thr286)CaMKII from the dendrites (D) to the soma (S), the average intensity of p(Thr286)CaMKII in the pyramidal layer of CA3 and the granule layer of the DG was compared to the levels in stratum radiatum or molecular layer (apical) and hilus (basal), respectively. These are expressed as ratio of [S / D] in both CA3 and DG (Fig. 3A). The resulting value, if greater than 1, indicates that a larger proportion of p(Thr286)CaMKII is in the perikarya of the neurons, rather than in the dendritic arborizations (Fig. 3B). Analysis of both apical and basal dendrites of the DG shows that in AD the ratio is significantly different from control. In the CA3, both MCI and AD are significantly different from control.
Subcellular distribution of p(Thr286)CaMKII correlates with cognitive dysfunction
Given the hypothesized role of CaMKII in plasticity and cognition, we performed within-subject correlational analyses to determine whether changes in the hippocampal distribution of p(Thr286)CaMKII corresponded to cognitive function as assessed by the Mini Mental State Examination (MMSE; Folstein et al., 1975). Cases were reclassified control, MCI, and AD subjects into three groups based on cognitive status: no impairment (26–30), mildly impaired (20–25), and severely impaired (0–19). The p(Thr286)CaMKII shift was then compared across these groups (Fig. 3C-E) to better determine when p(Thr286)CaMKII dysregulation occurs during AD pathogenesis. The correlation was highly significant in the DG (Fig. 3C; r = −0.540, p < 0.05; D; r = −0.527, p < 0.05), but was not significant in the CA3 (Fig. 3E; r = −0.369, p = 0.12). This may be because even in mild impairment, p(Thr286)CaMKII distribution in the CA3 is already perturbed. Also, due to limitations of the tissue, fewer cases were included in the CA3 analysis. Regardless, in the DG, p(Thr286)CaMKII redistribution is directly related to measurable cognitive decline, even in prodromal cases, suggesting that the degree of p(Thr286)CaMKII dysregulation can be a molecular read-out for patient symptoms. MMSE did not correlate with the total CaMKII ratio in the DG (n = 14; r = 0.141; p = 0.339), suggesting the observation is specific to the phosphorylation status of CaMKII. To rule out the possibility that the results are linked to ante mortem protein degradation, we determined that p(Thr286)CaMKII ratio did not correlate with post-mortem interval (n = 18; r = 0.149; p = 0.238).
Oligomeric Aβ induces p(Thr286)CaMKII changes in vivo
Oligomeric, but not fibrillar Aβ is known to alter Ca2+ dynamics and downstream Ca2+ signaling events (Demuro et al., 2005; Reese et al., 2008, Dineley et al., 2010). Although there are significant differences in p(Thr286)CaMKII localization between control, MCI, and AD hippocampi, with the methods used it was not possible to correlate these observations with a particular aggregated species of Aβ, given that a spectrum of soluble and insoluble forms of Aβ exist in the AD brain. We have previously used acute Intracerebroventricular (ICV) injections of homogenous Aβ preparations to show that only oligomers impair fear conditioning through aberrant activation of the phosphatase CaN (Dineley et al., 2010). Since CaN augments the activity of PP1, the major desphosphorylator of p(Thr286)CaMKII at the synapse (Strack et al. 1997 Lisman & Zhabotinsky, 2001), we predicted that oligomers would decrease p(Thr286)CaMKII levels in a CaN-dependent fashion. Autophosphorylation of CaMKII has also been shown to be an important event in single-trial learning (Irvine et al., 2005). Therefore, we performed ICV injection of either saline, Aβ oligomers or Aβ fibrils on wild-type mice. A subset of the mice treated with oligomers were additionally given a systemic injection of CaN inhibitor FK506, which we have used extensively in previous studies illustrating a central role for CaN in mediating cellular and behavioral effects of Aβ oligomers (Dineley et al., 2007; Reese et al., 2008; Taglialatela et al., 2009; Dineley et al., 2010). Twenty-four hours after ICV the animals were sacrificed and brains were collected. Western blots (Fig. 4A) showed that the treatment with oligomer Aβ significantly reduced the ratio of p(Thr286)CaMKII to total CaMKII in hippocampal homogenate (Fig. 4B; p < 0.05). Inhibition of CaN rescued this decrease, as animals treated with FK506 after oligomers ICV had significantly more p(Thr286)CaMKII than animals treated with oligomer alone (p < 0.05).
Parallel sets of whole brains were processed for immunofluorescence studies. Notably, similar to the results in human brain, oligomeric Aβ mice showed a shift of p(Thr286)CaMKII from the stratum radiatum to the CA3 pyramidal layer (Fig. 5A-D). This effect was reversed by treatment with the CaN inhibitor, raising the possibility that a similar mechanism of phosphatase hyperactivity may be at work in human MCI and AD hippocampi. On the other hand, animals treated with fibrillar Aβ did not exhibit a change in subcellular localization of p(Thr286)CaMKII in the hippocampus. The analysis of these experiments is shown in Figure 5E, where mice treated with oligomers showed a significant increase in p(Thr286)CaMKII ratio compared to PBS, fibril, and oligomer + FK506 treated mice (p < 0.01). The difference in CaMKII ratio was not significant and was attenuated by FK506 treatment, again suggesting that this shift in p(Thr286)CaMKII levels is due to a change in the phosphorylation state of the protein rather than localization of CaMKII itself. That CaN inhibition reverses this outcome implies that oligomeric Aβ may perturb p(Thr286)CaMKII signaling through increased phosphatase activity, and that similar mechanisms may be at work in MCI and AD hippocampus.
Oligomeric Aβ induces p(Thr286)CaMKII relocation in primary rat hippocampal neurons
Having established that oligomeric Aβ treatment is capable of affecting the level of p(Thr286)CaMKII in hippocampal dendritic arborizations of wild-type mice, we sought to further determine if this loss was specific to synaptic spines using primary rat hippocampal cultures. It has previously been demonstrated that synthetic Aβ oligomers target and bind to excitatory neurons synapses (Lacor et al., 2004). Similarly, we found that our preparation of Aβ oligomers localized to dendritic arbors at discrete puncta, while Aβ fibrils did not bind to primary hippocampal neurons (S. Fig. 4). To directly explore the effects of oligomers on synaptic p(Thr286)CaMKII parallel dishes of DIV 20–21 primary hippocampal neurons were treated with 500nM Aβ oligomer. Cells were fixed at 0 minutes, 15 minutes, 30 minutes, and 60 minutes then double labeled with a polyclonal anti-p(Thr286)CaMKII antibody and a monoclonal anti-PSD95 antibody. Confocal images were analyzed by selecting regions with robust PSD-95 puncta and measuring the area, as well as the fluorescence intensities of p(Thr286)CaMKII and PSD-95. Untreated neurons (Fig. 6A) showed the most intense p(Thr286)CaMKII staining in regions that also showed the strongest PSD-95 immunoreactivity. After 15 minutes of incubation with oligomeric Aβ p(Thr286)CaMKII immunoreactivity was decreased in PSD95 positive spines (Fig.6B), an observation that was more pronounced at 30 minutes (Fig.6C), but at 60 minutes synaptic p(Thr286)CaMKII levels had returned to levels similar to untreated (Fig.6D). These temporal observations are consistent with previous experiments we have performed using in vitro systems, where a rebound occurs following an acute challenge. In SY5Y human neuroblastoma cells, CaN-mediated dephosphorylation peaked at 15 minutes but normalized at 60 minutes (unpublished data). Interestingly, we observed that loss of synaptic p(Thr286)CaMKII is followed by a decrease in PSD95 puncta immunoreactivity (Fig. 6E) and size (Fig. 6F), although the latter is not statistically significant. In order to determine if inhibition of CaN would prevent the observed decreases in synaptic p(Thr286)CaMKII and PSD95, parallel sets of neurons were treated with 1µM of FK506 thirty minutes prior to addition of Aβ oligomer. We observed that CaN inhibition resulted in increased immunoreactivity for p(Thr286)CaMKII throughout the entire neuron (Fig.7A), likely through a PP1-mediated reduction of CaMKII phosphorylation (Mulkey et al., 2004), and prevented oligomer-induced synaptic loss of p(Thr286)CaMKII (Fig. 7E). In addition, FK506 increased PSD95 puncta intensity (Fig. 7E) and prevented the trend of decreased average PSD area (Fig. 6F) that was observed when neurons were treated with oligomers alone. In order to confirm that these outcomes were unique to oligomers, parallel dishes were treated with 500nM fibrillar Aβ (S. Fig. 5). These results show that the oligomer-induced decrease of synaptic p(Thr286)CaMKII is CaN-dependent and suggest that these pathways are attractive targets for future AD therapies.
DISCUSSION
Hippocampal synapse loss is a robust correlate of cognitive dysfunction in MCI and AD (Scheff et al., 2006), but the underlying mechanisms remain to be fully elucidated. The amnestic MCI cases present with atypical cognitive deficits, particularly in the memory domain, but are not yet classified as clinically demented. While not all MCI subjects progress to a diagnosis of AD, it is widely considered a transitional zone between normal aging and dementia (Petersen et al., 1999; 2001). The results presented here demonstrate that p(Thr286)CaMKII immunoreactivity is shifted in the hippocampi of MCI and AD cases, with a reduction in the dendritic arborizations and an increase in the neural perikarya. This outcome was more pronounced in the CA3, in that both MCI and AD hippocampi exhibited dysregulated localization of p(Thr286)CaMKII. In the DG, MCI was not significantly different from control. These results suggest that the CA3 is more vulnerable to changes in p(Thr286)CaMKII distribution, or that the DG is more resistant to the insults of AD (reviewed in Ohm, 2007). This may be in part due to neurogenesis that continues in the DG even in aged brain (Eriksson et al., 1998). Although the CA1, not the CA3, is hippocampal subregion affected earliest in AD pathology, we did not analyze the CA1 in these experiments. By end-stage AD, approximately half of CA1 neurons have been lost (West et al., 1994), making it difficult to assess the significance of the subcellular shift of p(Thr286)CaMKII in this region of the brain. In contrast, most neurons in CA3 are relatively well preserved in most cases of even established AD. The criticality of this region of the hippocampus for episodic memory processing (Kesner et al., 2008) suggested to us that a detailed investigation of more subtle changes such as those involved in CaMKII regulation might suggest important functional bases for the memory loss in AD. It is likely that there protective mechanisms for CA3 versus CA1 that are relevant to AD and that evaluation of CA1 might shed light on the nature of these. However, this was not the primary focus of our initial investigation.
Notably, the observation of p(Thr286)CaMKII redistribution in the DG correlates with cognitive test scores, suggesting that alterations of p(Thr286)CaMKII signaling may be involved in the cognitive decline that marks the progression of MCI and AD pathogenesis. The phenotype could be replicated in wild-type mice and primary hippocampal neurons by application of oligomeric Aβ and was prevented by inhibiting CaN. These results imply that oligomeric Aβ-mediated disruption of p(Thr286)CaMKII signaling is at least partially mediated through CaN. Similar studies in which oligomers reduced CaMKII autophosphorylation reported that co-treatment with neurotrophic factors may also attenuate p(Thr286)CaMKII (Zeng et al., 2010; Zheng et al., 2010).
Basal Ca2+ levels are increased in aged brain (Gibson et al., 1987); and AD has been hypothesized to be an exacerbation of this dysfunctional Ca2+ system (Khachaturian, 1989). The presence of soluble Aβ aggregates further disrupts Ca2+ homeostasis in several disease models. Synthetic oligomers increase intracellular Ca2+ in cultured cells (Demuro et al. 2005; Reese et al., 2008) and inhibit LTP and impair memory in mice through mechanisms involving the Ca2+-dependent phosphatase CaN (Dineley et al., 2010). Similarly, natural oligomers isolated from human brain inhibit LTP and reduce spine density in rodent brain (Shankar et al., 2008). Aged Tg2576 mice overexpressing mutated human amyloid precursor protein have increased resting Ca2+ levels (~20%) compared to aged wild-type mice (Kuchibhotla et al. 2008). It is believed that a modest increase of intracellular Ca2+ shifts the thresholds of LTP and long-term depression (LTD), increasing and lowering them respectively (Norris et al. 1996; Rosenzweig and Barnes, 2003; Jouvenceau & Dutar, 2006). LTD is induced following a protein phosphatase cascade, when activated CaN dephosphorylates and inactivates inhibitor-1, leading to increased PP1 activity (Lisman, 1989; Mulkey et al., 1994). PP1 in turn dephosphorylates p(Thr286)CaMKII (Lisman et al., 2002).
We found that the subcellular distribution of the active, phosphorylated form was dysregulated in MCI and AD hippocampus. Specifically, there was decreased immunoreactivity of p(Thr286)CaMKII in the dendritic arborizations and an increase near the cell soma. This paradoxical outcome may be explained by specific localization of phosphatase activity. Within the PSD, PP1 is the primary dephosphorylator of p(Thr286)CaMKII; outside of the PSD phosphatase 2A (PP2A) accounts for >70% of p(Thr286)CaMKII dephosphorylation (Strack et al. 1997). In AD hippocampi, PP2A mRNA levels are significantly reduced (Vogelsberg-Ragaglia et al., 2001) and PP2A inhibitors 1 and 2 are increased (Tanimukai et al., 2005). This would explain the net result of increased p(Thr286)CaMKII near the soma in MCI and AD brain. The subcellular localization and phosphorylation state of CaMKII are critical to the functionality of this important kinase (Kennedy, 2000). The decrease of p(Thr286)CaMKII in the dendritic arborizations implies that it would be unable to perform its traditional synaptic roles (Lisman et al., 2002; Hudmon et al., 2005), including the insertion and phosphorylation of postsynaptic AMPA receptors (Gu et al., 2009), and PSD scaffold proteins (Dosemeci and Jaffe, 2010). Indeed, we observed a decrease in PSD immunoreactivity following the shift of p(Thr286)CaMKII from the dendritic spines.
An alternative explanation of our results is that the pattern observed in human hippocampi is due to decreased CaMKII mRNA or protein in the dendrites. The localization, amplitude, duration, and frequency of Ca2+ transients are encoded by the phosphorylation status of CaMKII, effectively “tagging” active synapses (Colbran and Brown, 2004), and signaling for the transport of CaMKII mRNA to the dendrites is activity-dependent (Ouyan et al., 1999). Our findings are not likely to be a result of these pathways, as the immunoreactivity profile of the unphosphorylated protein is not significantly different between control, MCI, and AD (p = 0.39; S. Fig. 3).
It is challenging to link these observations to specific Aβ species in the MCI and AD brain because both soluble and insoluble forms are present; to overcome this limitation and determine whether aggregated Aβ induced a shift in p(Thr286)CaMKII we performed studies in wild-type mice and primary hippocampal neurons. Our data show that application of oligomers, but not fibrils, resulted in decreased p(Thr286)CaMKII in hippocampal homogenates and decreased immunoreactivity in mouse stratum radiatum. In agreement with previous evidence that oligomeric Aβ binds to primary hippocampal neurons and has deleterious effects on synapse composition, shape, and density (Lacor et al., 2004; 2007), our results showed that only oligomers elicited a decrease in synaptic p(Thr286)CaMKII.
We have previously shown that Aβ oligomers injected ICV in mice impair fear conditioned memory in a CaN-dependent fashion (Dineley et al., 2010). Here we show that this same paradigm also shifts the sub-hippocampal localization of p(Thr286)CaMKII in a manner similar to what is observed in AD brains, and this is prevented by CaN inhibition. Further supporting our hypothesis, pretreating primary neurons with a CaN inhibitor prior to the application of Aβ was sufficient to prevent the oligomer-mediated decrease in p(Thr286)CaMKII at the synapse.
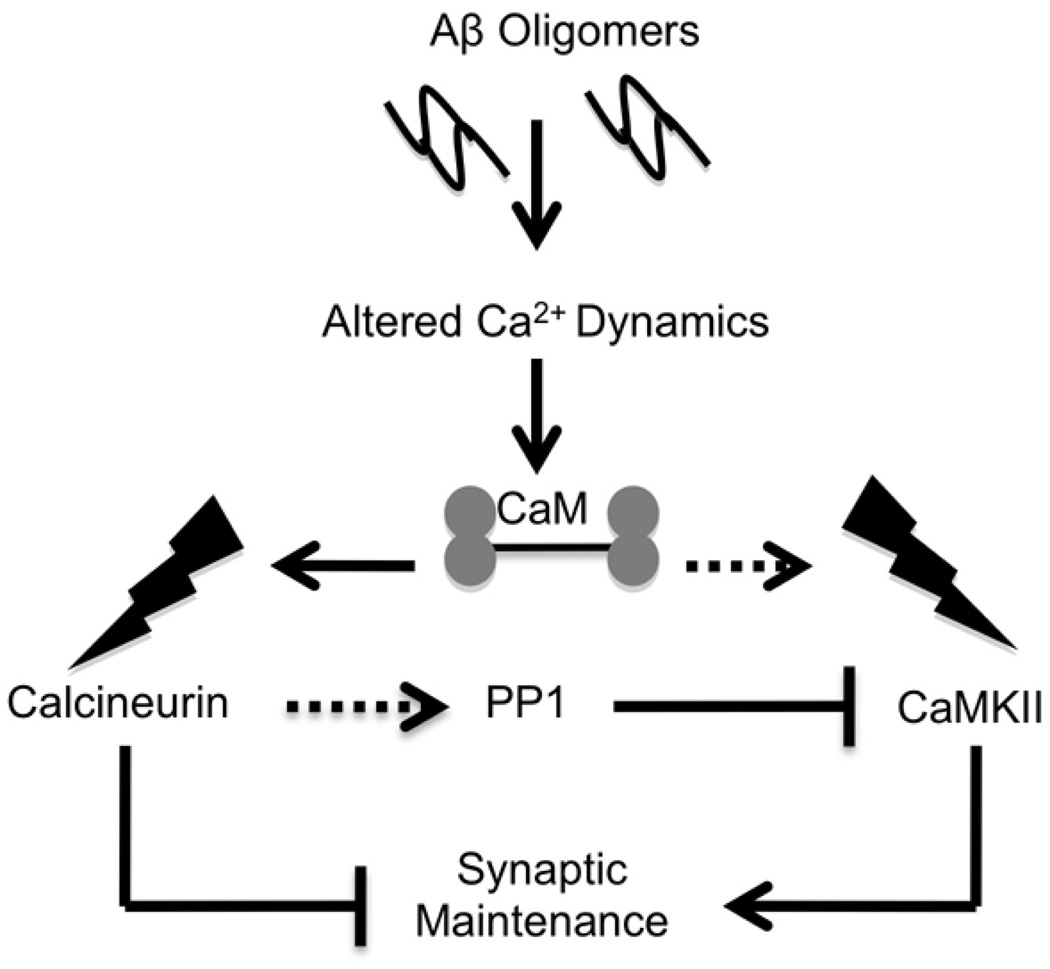
Due to the rapid oxidation of PP1 and CaN post-mortem, we do not explicitly address whether the decrease of CaMKII phosphorylation in dendritic arborizations is due to diminished phosphorylation or augmented dephosphorylation of the kinase in diseased human brain. However, the results obtained in disease models suggest that it is the latter. Within the dendritic spines, dephosphorylation of p(Thr286)CaMKII is mediated by PP1 (Hsieh-Wilson et al., 1999), which itself is indirectly controlled through CaN (Mulkey et al., 1994). The mature spine is an isolated, structured compartment where p(Thr286)CaMKII levels saturate the phosphatase activity of PP1 (Lisman and Zhabotinsky 2001). However, the spatio-temporal profile of Ca2+ dynamics is known to be dysregulated (increased) in the aged brain (Landfield et al., 1984; Gant et al., 2006). The phosphatase CaN, long known to be enriched in PSDs (Wood et al., 1980) is unique in that it is exquisitely responsive to Ca2+ fluctuation (Rusnak & Mertz, 2000), particularly within dendritic spines. Thus, given the derangement of Ca2+ dynamics in the aging brain, it is not surprising that CaN activity increases with age (Foster et al., 2001; Norris et al., 2002). Additional augmentation of intracellular Ca2+, similar to that elicited by Aβ oligomers (Demuro et al., 2005) further increases CaN activity and signaling (Agostinho et al., 2008; Reese et al., 2008; Abdul et al., 2009), while CaN inhibition prevents the loss of spines in the Aβ-treated brain slices (Shankar et al., 2007) as well as in transgenic AD mice (Rozkalne et al., 2010), and also precludes the loss of surface AMPA receptors (Zhao et al., 2010). In accordance with these findings in model systems, CaN is up-regulated in AD brain compared to age-matched control (Norris et al. 2005; Liu et al., 2005; Wu et al., 2010), an increase that correlates with low MMSE scores (Abdul et al., 2009). Taken together, these studies and the findings reported here suggest that Ca2+ dysregulation in MCI and AD hippocampi may be sufficient to initiate a PP1 dephosphorylation cascade resulting in a loss of p(Thr286)CaMKII at the synapse (Fig. 8).
Figure 8. Depiction of the hypothesized mechanism for Aβ oligomer induced loss of p(Thr286)CaMKII in dendritic spines.
The mature spine is an isolated, structured compartment where p(Thr286)CaMKII levels saturate the phosphatase activity of PP1. However, Ca2+ dynamics are known to be dysregulated in the aged brain, and Aβ oligomers have been shown to aberrantly increase CaN activity and signaling, including indirect activation of PP1 - the primary dephosphorylator of p(Thr286)CaMKII in spines. The ability of CaN inhibitor FK506 to attenuate p(Thr286)CaMKII decrease in mice and primary neurons further suggests that the normalization of synaptic Ca2+ signaling is a desirable target of early AD therapies.
CONCLUSIONS
Altered localization of p(Thr286)CaMKII in MCI and AD hippocampi correlates with MMSE, suggesting it may be directly related to cognitive dysfunction. That MCI tissue also shows the p(Thr286)CaMKII phenotype implies that these mechanisms are at work even in the initial stages of disease, a time when rationally designed therapies would be most effective in halting AD progression. Experiments in model systems further indicate that this phenomenon is provoked by Aβ oligomers, but not fibrils, and is at least partly mediated by CaN. Thus, the hypothesized mechanism likely involves dysregulation of Ca2+ signaling, and putatively explains why the aged brain is particularly vulnerable to the events described here. Collectively, these results imply that normalizing the balance of phosphatases and kinases at the synapse should be considered as a therapeutic target for cognitive dysfunction and synaptic loss in MCI and early AD pathogenesis.
Supplementary Material
Acknowledgements
This work was supported by a National Institute of Health Grant R01NS059901 (G.T.), Alzheimer’s Association grant IIRG-90755 (G.T.), a Mitchell Center Neurodegenerative Center Collaborative Grant (G.T.); NIA P30AG008017 (R.W.); L.C.R. is the recipient of a predoctoral fellowship by NINDS grant F31NS062558. The authors thank Zane Martin and Wen-Ru Zhang for technical assistance.
Abbreviations
- AD
Alzheimer Disease
- Aβ
amyloid-beta
- ACA
antibody capture assay
- CaN
calcineurin
- Ca2+
calcium
- CaMKII
Ca2+/calmodulin-dependent protein kinase II-α
- CDR
clinical dementia rating
- CA3
cornu ammonis 3
- DIV
days in vitro
- DAPI
4',6-diamidino-2-phenylindole
- DG
dentate gyrus
- ICV
intracerebroventricular
- LTP
long term potentiation
- MCI
mild cognitive impairment
- MMSE
mini-mental state exam
- NP
neuritic plaque
- PMI
post-mortem interval
- PSD
post-synaptic density
- PP1
protein phosphatase-1
Footnotes
The authors have no conflicts of interest.
Contributor Information
Lindsay C. Reese, Email: reeseli@ohsu.edu.
Fernanda Laezza, Email: felaezza@utmb.edu.
Randall Woltjer, Email: woltjerr@ohsu.edu.
REFERENCES
- Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, Levine H, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s Disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinho P, Lopes JP, Velez Z, Oliveira CR. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem. Intl. 2008;52:1226–1233. doi: 10.1016/j.neuint.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Amada N, Aihara K, Ravid R, Horie M. Reduction of NR1 and phosphorylated Ca2+/calmodulin-dependent protein kinase II levels in Alzheimer’s disease. Neuroreport. 2005;16:1809–1813. doi: 10.1097/01.wnr.0000185015.44563.5d. [DOI] [PubMed] [Google Scholar]
- Arispe N, Rojas R, Pollard HB. Alzheimer-disease and amyloid beta-protein forms calcium channels in bilayer membranes – blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball M, Braak H, Goethe J, Coleman P, Dickson D, Duyckaerts C, Gambetti P, Hansen L, Hyman B, Jellinger K, et al. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol. Aging. 1997;18(4 Suppl.):S1–S2. [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta. Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid β-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2002;77:354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat-brain postsynaptic density fraction contains a homolog of the drosophila disks-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Clark WG, Vivonia CA, Baxter CF. Accurate free-hand injection into the lateral brain ventricle of the conscious mouse. J. Appl. Physiol. 1968;25:318–321. doi: 10.1152/jappl.1968.25.3.319. [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr. Opin. Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. JBC. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Diaz-Cintra S, Xue BG, Spigelman I, Van K, Wong AM, Obenaus A, Ribak CE. Dentate granule cells form hilar basal dendrites in a rat model of hypoxia-ischemia. Brain Res. 2009;1285:182–187. doi: 10.1016/j.brainres.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Hogan D, Zhang W, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol. Learn. Mem. 2007;88:217–224. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Kayed R, Neugebauer V, Yu F, Zhang W, Reese LC, Taglialatela GT. Amyloid beta oligomers impair fear conditioned memory in a calcineurin dependent fashion. J. Neurosci. Res. 2010;13:2923–2932. doi: 10.1002/jnr.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemici A, Jaffe H. Regulation of phosphorylation at the postsynaptic density during different activity states of Ca2+/calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Comm. 2010;391:78–84. doi: 10.1016/j.bbrc.2009.10.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J. Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordberg C, Petersen DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state – practical method for grading cognitive state of patients for clinician. J. Psych. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J. Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J. Neurosci. 26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol. Aging. 1987;8:329–343. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at ThR286) of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Gu Z, Wenhua L, Yan Z. b-amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. JBC. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hsieh-Wilson LC, Allen PB, Watanabe T, Naim AC, Greengard P. Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1. Biochemistry. 1999;38:4365–4373. doi: 10.1021/bi982900m. [DOI] [PubMed] [Google Scholar]
- Hudmon A, LeBel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J. Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British J. Psych. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Irvine EE, Vernon J, Giese KP. ACaMKIIautophosphorylation contributes to rapid learning but is not necessary for memory. Nat. Neurosci. 2005;8:411–412. doi: 10.1038/nn1431. [DOI] [PubMed] [Google Scholar]
- Jones SP, Rahimi O, O’Boyle MP, Diaz DL, Claiborne BJ. Maturation of granule cell dendrites after mossy fiber arrival in hippocampal field CA3. Hippocampus. 2003;13:413–427. doi: 10.1002/hipo.10121. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Dutar P. A role for the protein phosphatase 2B in altered hippocampal synaptic plasticity in the aged rat. J. Physiol. Paris. 2006;99:154–161. doi: 10.1016/j.jphysparis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the post-synaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kennedy MB, Bennett MK, Erondu NE. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 1983;80:7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav. Neurosci. 2008;122:1217–1225. doi: 10.1037/a0013592. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. Calcium, membranes, aging, and Alzheimers Disease – Introduction and overview. Ann. NY. Acad. Sci. 1989;568:1–4. doi: 10.1111/j.1749-6632.1989.tb12485.x. [DOI] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid-beta associated with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA. 2009;10:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. A beta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong YS, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s- related amyloidβ oligomers. J. Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. A beta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Gerber BR, Lou JY, Kozel MA, Hartman H, Craig AM, Ornitz DM, Nerbonne JM. The FGF14(F145S) mutation disrupts the interaction of FGF14 with voltage-gated Na+ channels and impairs neuronal excitability. J. Neurosci. 2007a;27:12033–12044. doi: 10.1523/JNEUROSCI.2282-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Wilding TJ, Sequeira S, Coussen F, Zhang XZ, Hill-Robinson R, Mulle C, Huettner JE, Craig AM. KRIP6: a novel BTB/kelch protein regulating function of kainate receptors. Mol. Cell Neurosci. 2007b;34:539–550. doi: 10.1016/j.mcn.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Lampert A, Kozel MA, Gerber BR, Rush AM, Nerbonne JM, Waxman SG, Dib-Hajj SD, Ornitz DM. FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels. Mol. Cell Neurosci. 2009;42:90–101. doi: 10.1016/j.mcn.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+ dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Lisman JE. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc. Natl. Acad. Sci. USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA-receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. JBC. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Roger J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. Beta-amyloid peptides destabilize calcium homeostasis and render human cortical-neurons vulnerable to excitotoxicity. J. Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, Mckeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes HP, Vanbelle G, Berg L. The consortium to establish a registry for Alzheimers-Disease (CERAD): Standardization of the neuropathological assessment of Alzheimers-Disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J. Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, Landfield PW, Kraner SD. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J. Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyffeler M, Zhang WN, Feldon J, Knuesel I. Differential expression of PSD proteins in age-related spatial learning impairments. Neurobiol. Aging. 2007;28:143–155. doi: 10.1016/j.neurobiolaging.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ohm TG. The dentate gyrus in Alzheimer’s disease. Prog. Brain Res. 2007;163:723–740. doi: 10.1016/S0079-6123(07)63039-8. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB. Tetanic stimulation leads to increased accumulation of Ca2+/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J. Neurosci. 1999;19:7823–7833. doi: 10.1523/JNEUROSCI.19-18-07823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Reese LC, Zhang WR, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:825–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn HJ, van Uum JFM, Breedijk I, Emmering J, Radu I, Pool CW. Double immunolabeling of neuropeptides in the human hypothalamus as analyzed by confocal laser scanning fluorescence microscopy. J. Histochem. &Cytochem. 1999;47:229–235. doi: 10.1177/002215549904700211. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. In Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rozkalne A, Hyman BT, Spires-Jones TL. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol. Dis. 2010;41:650–654. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol. Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Seress L, Mrzljak L. Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and hippocampal formations. Brain. Res. 1987;405:169–174. doi: 10.1016/0006-8993(87)91003-1. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li SM, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Spiegelman I, Yan XX, Obenaus A, Lee EYS, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neuroscience. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Srivareerat M, Tran TT, Alzoubi KH, Alkadhi KA. Chronic psychosocial stress exacerbates impairment of cognition and long-term potentiation in beta-amyloid rat model of Alzheimer’s disease. Biological Psychiatry. 2009;65:918–926. doi: 10.1016/j.biopsych.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J. Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav. Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenberg RK, Scott HL, Tannenberg AEG, Dodd PR. Selective loss of synaptic proteins in Alzheimer’s disease: Evidence for an increased severity with APOE epsilon 4. Neurochem. Intl. 2006;49:631–639. doi: 10.1016/j.neuint.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Tanimukai H, Grundke-Iqbal I, Iqbal K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer’s disease. Am. J. Path. 2005;166:1761–1771. doi: 10.1016/S0002-9440(10)62486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VMY. PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp. Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Chen GH, Hu XY, Lu YP, Zhou JN, Liu RY. The expression of calcium/calmodulin-dependent protein kinase II-alpha in the hippocampus of patients with Alzheimer’s disease and its links with AD-related pathology. Brain Res. 2005;1031:101–108. doi: 10.1016/j.brainres.2004.10.061. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wood JG, Wallace RW, Whitaker JN, Cheung WY. Immunocytochemical localization of calmodulin and a heat-labile calmodulin-binding protein (CaM-BP80) in basal ganglia of mouse brain. J. Cell. Biol. 1980;84:66–76. doi: 10.1083/jcb.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J. Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Woltjer RL, Sokal I, Pan C, Wang Y, et al. Quantitative proteomics identifies surfactant-resistant alpha-synuclein in cerebral cortex of Parkinsonism- dementia complex of Guam but not Alzheimer’s disease or progressive supranuclear palsy. Am. J. Pathol. 2007;171:993–1002. doi: 10.2353/ajpath.2007.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Zhao DY, Xie CQ. Neurotrophins enhance CaMKII activity and rescue amyloid- beta induced deficits in hippocampal synaptic plasticity. J. Alz. Dis. 2010;21:823–831. doi: 10.3233/JAD-2010-100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DY, Watson JB, Xie CW. Amyloid beta prevents activation of calcium/calmodulin- dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J. Neurophys. 2004;92:2853–2858. doi: 10.1152/jn.00485.2004. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, Kinney GG, Shughrue PJ, Ray WJ. Inhibition of calcineurin-mediated endocytosis and AMPA receptors prevents amyloid β oligomer-induced synaptic disruption. JBC. 2010;285:7619–7632. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZQ, Sabirzhanov B, Keifer J. Oligomeric amyloid-beta inhibits the proteolytic conversion of brain-derived neurotrophic factor (BDNF), AMPA receptor trafficking, and classical conditioning. JBC. 2010;285:34708–34017. doi: 10.1074/jbc.M110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.