Abstract
Lithium ion batteries have proven themselves the main choice of power sources for portable electronics. Besides consumer electronics, lithium ion batteries are also growing in popularity for military, electric vehicle, and aerospace applications. The present review attempts to summarize the knowledge about some selected membranes in lithium ion batteries. Based on the type of electrolyte used, literature concerning ceramic-glass and polymer solid ion conductors, microporous filter type separators and polymer gel based membranes is reviewed.
Keywords: lithium ion battery, Li ion conductor, separator, ceramic, polymer
1. Introduction
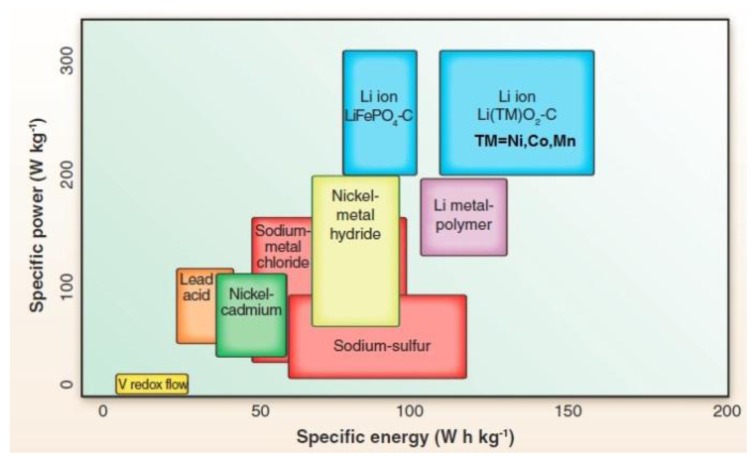
Since the first primary lithium ion batteries (LIBs) became commercially available in 1991, LIBs caught on quickly and have become the main power sources on the consumer electronics market [1,2]. LIBs are characterized by high specific energy and high specific power (Figure 1), which are the advantages that most other electrochemical energy storage technologies cannot offer [3]. In addition, some other advantages such as high efficiency, long life cycle and low self-discharge rate make lithium-ion batteries well suited for applications such as energy storage grid and electric transportation. Despite the overall advantages, scaling up LIB technology for these applications is still problematic due to safety, costs, operational temperature and materials availability [4].
Figure 1.
Ragone plots (power vs. energy density) for different rechargeable batteries [3].
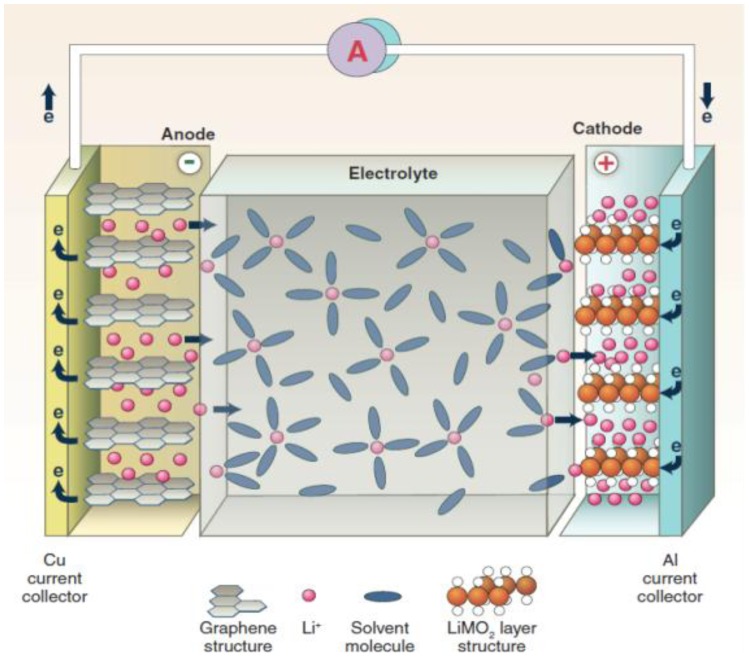
Figure 2 schematically shows a typical LIB [5], which consists of a cathode (LiMn1.5Ni0.5O4 spinel; layered structure LiMO2; LiMPO4 olivines, M = Mn, Fe and Co) and an anode (intercalated graphite; alloying materials Si and Sn), together with the electrolyte that allows Li ion transport but prevents electrodes from electronic contact [6]. During charging, Li deintercalates from the cathode and inserts into the anode. When discharging, Li intercalates into the cathode. In the processes of charge/discharge, Li ions transport between the anode and the cathode, which allows electrochemical energy storage within the battery and the conversion of chemical energy into electrical energy.
Figure 2.
Schematic of a lithium ion battery (LIB) consisting of the negative electrode (graphitic carbon) and positive electrode (Li-intercalation compound) [5].
The electrolyte usually functions as an electronic separator and ionic conductor between cathode and anode. It may consist of solvent, salt, separator, additive, and/or a solid ion-conducting membrane or a combination thereof. As in other electrochemical devices, the electrolyte should be durable in highly reductive and oxidative environments, highly ionic-conductive, and facilitate electrochemical reactions. Accordingly, if a liquid electrolyte, at least including solvent and salt, is used, an additional membrane is required to electronically separate two electrodes. At the same time, this membrane must be porous and allow the liquid electrolyte to flow through. In a commercial LIB, a porous plastic film as separator is soaked in LiPF6 which is dissolved in a mixture of organic solvents such as ethylene carbonate (EC), ethyl methyl carbonate (EMC), or diethyl carbonate (DEC). If the membrane itself is a Li ion conductor, the liquid electrolyte is not a necessity. Another case is to incorporate the liquid electrolyte into the polymer matrix to form a polymer gel electrolyte. These are the most common types of membranes used in a LIB. The main function of these membranes is to prevent the positive and negative electrodes electrically contacting each other, and allow rapid ionic transport to complete the circuit for the passage of current in lithium ion batteries. Therefore, they play very important roles in lithium ion batteries, and may affect the electrochemical energy efficiency.
2. Solid Li Ion Conductors
To simplify the cell design, and improve safety and durability, a solid electrolyte was used to eliminate the need of the liquid electrolyte. Two general classes of materials used for solid electrolytes in lithium-ion batteries include inorganic ceramics and organic polymers. The most obvious difference between these classes is the mechanical properties. Polymers are generally easier to process than ceramics, which reduce the fabrication costs. On the other hand, ceramics are more suitable for high temperature or other aggressive environments.
2.1. Ceramic-Glass
2.1.1. Na Super-ionic Conductor (NASICON) Structure
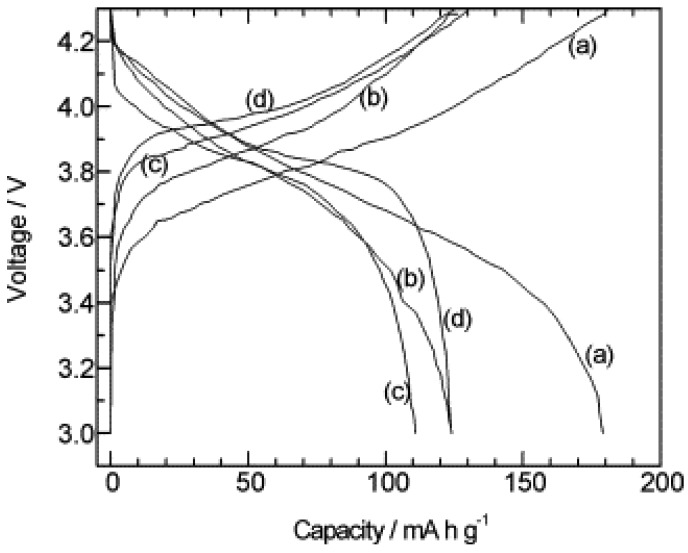
Among the LiM2(PO4)3 (M = Ti, Zr, Ge, Hf) NASICONs, the Ti-compound is supposed to exhibit high lithium ion conductivity at room temperature, due to its lower volume resistivity [7], which means the lowest cell volume of LiTi2(PO4)3 should show highest conductivity. Because the NASICON structure permits a wide range of ion substitution at the Ti and P sites making this structure a versatile family of solids, a lot of work focusing on substitution has been performed attempting to improve Li ion conductivity [8,9,10,11,12]. So far, the highest conductivity based on NASICON structure has been observed for Li1+xAlxGe2−x(PO4)3 (LAGP), which can reach 10−4 S cm−1 [9]. Another commonly used phosphate electrolyte is Li1+xTi2−xAlx(PO4)3 (LTAP) [11,13], and its modification at P sites (i.e., Li1+x+yTi2−xAlxSiy(PO4)3−y) [14]. Figure 3 shows the application of LTAP as the electrolyte in lithium-ion batteries [15].
Figure 3.
Charge and discharge curves of Li/PEO/LTAP/LiCoO2 cells at 50 °C at different annealing temperatures: (a) as-deposited; (b) 300 °C; (c) 400 °C and (d) 500 °C [15].
2.1.2. Garnet Structure
The nominal chemical compositions for this kind of structure are Li5La3M2O12 (M = Nb, Ta) and Li6ALa2M2O12 (A = Ca, Sr, Ba; M = Nb, Ta). Usually the Li ion conductivity based on the garnet structure is within 10−7 to 10−5 S cm−1 at room temperature [16,17,18]. The highest conductivity about 10−4 S cm−1 at room temperature has been obtained from Li5La3Ta2O12 with La sites substituted by Ba and/or Sr [19]. The unique feature of garnet-type materials may be that the total and bulk conductivities are nearly identical, which implies the grain boundary resistance should be very small. For example, Li6SrLa2Ta2O12 and Li6BaLa2Ta2O12 exhibit mainly bulk ionic conductivities of 8.9 × 10−6 and 5.4 × 10−5 S cm−1 at 22 °C, respectively [20]. According to bond valence models, the Li+ ion transport pathways in Li5La3M2O12 is directly related to the fully occupied octahedral sites [21], which indicates a vacancy-type ion transport is expected to be the dominant contribution. If this is the case, one should be careful when such membrane is used as the electrolyte in LIBs.
2.1.3. Perovskite Structure
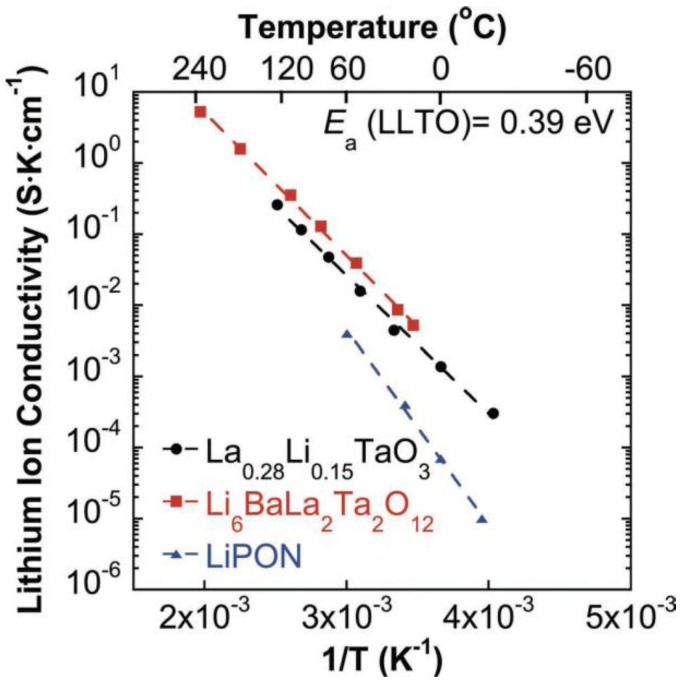
The perovskite (ABO3)-type lithium lanthanum titanate, like (Li, La)TiO3 (LLTO), shows the highest bulk lithium ion conductivity of 10−3 S cm−1 at room temperature, but the high grain boundary resistance makes total conductivity about 10−4 S cm−1 [22,23,24,25]. According to lithium ion transport properties of LLTO and structurally related materials [26], the high lithium-ion-conducting phase has an A-site deficient perovskite-type structure. Lithium ion conduction occurs due to the motion of lithium ions along A-site vacancies. The ionic conductivity is highly sensitive to the lithium content. Noteworthy is that the compound is not stable in direct contact with elemental lithium and rapidly undergoes Li-insertion with consequent reduction of Ti4+ to Ti3+, leading to high electronic conductivity. Due to this reason, Ti was substituted by Ta forming a new LLTO, namely La1/3−xLi3xTaO3. Recently, a crystalline, lithium-stable, fast lithium-ion conductor La1/3−xLi3xTaO3 directly with a thin copper foil current collector has been demonstrated for a lithium-free solid-state battery [27]. The conductivity can reach 1.5 × 10−5 S cm−1 at room temperature, which is 15 times higher than amorphous lithium phosphorus oxy-nitride (LiPON) compositions (see Figure 4).
Figure 4.
Arrhenius plot for the lithium-ion conductivity of La0.281Li0.155TaO3 compared with data for Li6BaLa2Ta2O12 and lithium phosphorus oxy-nitride (LiPON) [27].
2.1.4. Sulfide Glass
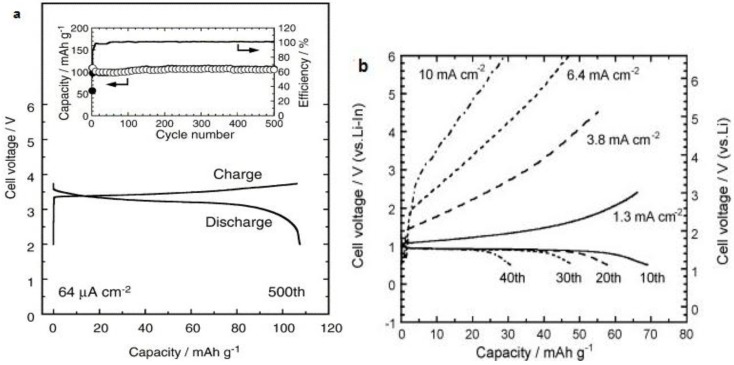
Effective ways to improve the Li ion conductivity in Li2S-based oxysulfide glasses and sulfide glass-ceramics have been discovered: (i) the combination of sulfide and oxide anions; (ii) the replacement of an oxide matrix by a sulfide one; and (iii) the precipitation of super-ionic metastable crystals [28]. Li2S-P2S5 systems have shown very good Li ion conductivity, higher than 10−3 S cm−1 [29,30,31]. The exploration of some other sulfide glasses has also been demonstrated, like Li3PO4-Li2S-SiS2 glass [32], LiI-Li2S-Ga2S3-GeS2 [33], and LiI-Li2S-Sb2S3-P2S5 [34], etc. The rate performances of all-solid-state cells by the utilization of highly conductive glass-ceramic electrolytes were investigated by scientists from Japan. Although Li2S-P2S5 electrolyte based LiCoO2-In battery showed very stable capacity within 500 cycles, the charge and discharge current density is very small (Figure 5a) [28]. Li-In/Li4Ti5O12 cell using 70Li2S-27P2S5-3P2O5 solid electrolyte exhibited very poor rate performance (Figure 5b) [35], which is probably caused by disconnected ionic pathway within the electrode.
Figure 5.
(a) Charge-discharge curves and cycling performance at 64μAcm−2 for the 500th cycle of In/LiCoO2 cells with 80Li2S-20P2S5 glass-ceramic [28]; (b) Charge-discharge curves of the all-solid-state Li-In/70Li2S-27P2S5-3P2O5/Li4Ti5O12 cell (discharge always at 64μAcm−2) [35].
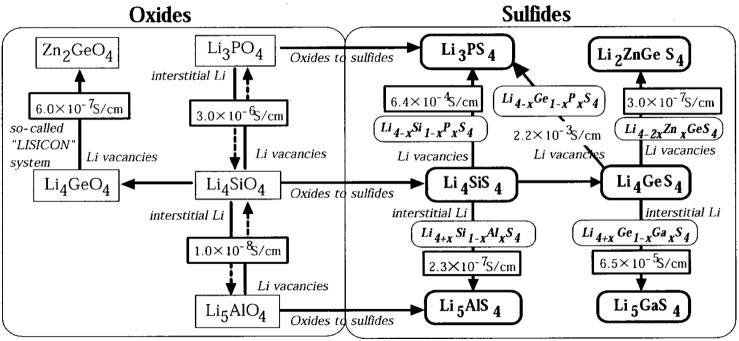
Lithium superionic conductor (LISICON) [Li14Zn(GeO4)4], an important Li ion conductor, is a general name for the glass-ceramic system. Based on some criteria of material design of crystalline ionic conductors (Figure 6): (1) mobile ions should have a suitable size for conduction pathways in the lattice; (2) there should be disorder in a mobile ion sublattice; and (3) highly polarizable mobile ions and anion sublattices are preferable, a new crystalline material family, thio-LISICON, was found in the Li2S-GeS2-P2S5 system, which showed the highest conductivity of 2.2 × 10−3 S cm−1 at 25 °C together with negligible electronic conductivity, high electrochemical stability, no reaction with lithium metal, and no phase transition up to 500 °C [36].
Figure 6.
The concept of material design for the lithium superionic conductor (LISICON) system, and materials belonging to the LISICON (oxides) and the thio-LISICON (sulfides) are summarized [36].
2.2. Solid Polymer Membranes
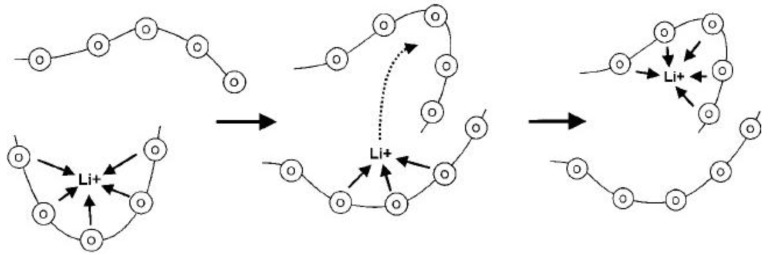
Containing no organic liquids, such polymer electrolyte is dry and solid. Poly(ethylene oxide) (PEO) is the earliest and most extensively studied system. The ionic conduction of PEO was discovered by Fenton in 1973 [37]. Since then, a lot of work has been done to increase the conductivity of dry polymer electrolytes and reduce the cost of manufacturing of devices incorporating such electrolytes. These complexes are formed by dissolving a lithium salt, LiX, in a PEO polymer matrix to form Li ion conductor for the application of lithium rechargeable polymer batteries [38,39,40]. Definitely the lithium salt properties are critical to optimizing these materials for electrolyte applications. The large soft anions are usually preferred to improve ionic conductivity of PEO-LiX polymer electrolytes, for example, the conductivities of PEO with LiClO4 are within 10−8 to 10−6 S cm−1 [41,42], while those of PEO with Li(CF3SO2)2N (LTFSI) [43] and Li(C2F5SO2)2N [44] are between 10−5 to 10−4 S cm−1. The mechanism of Li ion transport can be described as the motion of the Li ions between complex sites assisted by the segmental motion of the PEO matrix (see Figure 7) [45]. According to this model, the good conductivity can be ascribed to Li ion transport in the amorphous region in PEO. Therefore, the Li ion conductivity can be increased in two ways: (1) reducing crystallization of PEO and (2) weakening the interaction between Li ions and PEO chains. For the former, adding plasticizer [46] or inorganic oxides [47] may achieve that purpose while employing room temperature ionic liquid [48] can affect the interaction of Li ions with polymer.
Figure 7.
Schematic of the segmental motion assisted diffusion of Li ions in the poly(ethylene oxide) (PEO) matrix. The circles represent the ether oxygens of PEO [45].
3. Microporous Separators
This type of membranes usually works as the separator in liquid electrolyte batteries. The separator must physically keep anode and cathode from contacting with each other, while enable free ionic transport. Based on the morphology of the separator, there are generally two kinds of separators including microporous membranes and nonwoven films. Although separators are effective in preventing electrical shorts between anode and cathode, their presence in between the two electrodes decreases the effective conductivity of the electrolyte, raising cell impedance. This would be expected since the presence of the separator decreases the total cross sectional area of lithium ion conducting pathway, and the tortuosity of the open pores in the separator prolongs the ionic transport pathway. For this reason, one can imagine that the thinner the separator, the higher the ionic conductivity. However, it has been pointed out that there is a trade-off between the thickness of the separator and its mechanical properties. The detailed requirements for the separator in liquid lithium ion batteries are listed in Table 1 [49].
Table 1.
Separator requirements for liquid lithium ion batteries (LIBs).
| Parameter | Target | Note |
|---|---|---|
| Thickness (µm) | <25 | – |
| MacMullin number | <8 | The ratio of the resistance of the separator filled with electrolyte to the resistance of the electrolyte alone |
| Gurley (s) | ~25/mil | The time required for air to pass through the separator |
| Pore size (µm) | <1 | – |
| Porosity (%) | ~40 | – |
| Puncture strength (g/mil) | >300 | – |
| Melt integrity (°C) | >150 | – |
| Chemical stability | Long enough time | – |
| Thermal stability | <5% shrinkage | – |
| Tensile strength | <2% offset at 1,000 psi | – |
| Skew (mm/m) | <2 | – |
3.1. Microporous Membranes
The materials used for the microporous polymer membranes are semi-crystalline polyolefin materials, like polyethylene (PE), polypropylene (PP) and their blends PE-PP. The preparations of the microporous membranes can be classified into dry process and wet process. A thorough overview of these techniques has been provided previously [49,50]. In the dry process, the melted polymer is firstly extruded into uniaxially oriented tubular film. After a subsequent annealing at a temperature slightly lower than the melting point, the film was then stretched to form micropores through the orientation steps of cold stretch, hot stretch and relaxation. The wet process involves the following procedure: first, mixing polymer resins with paraffin oil, antioxidant etc. to form the homogeneous solution; second, extruding the solution to get the gel-like film; third, extracting the paraffin oil and additives to obtain the targeted microporous film.
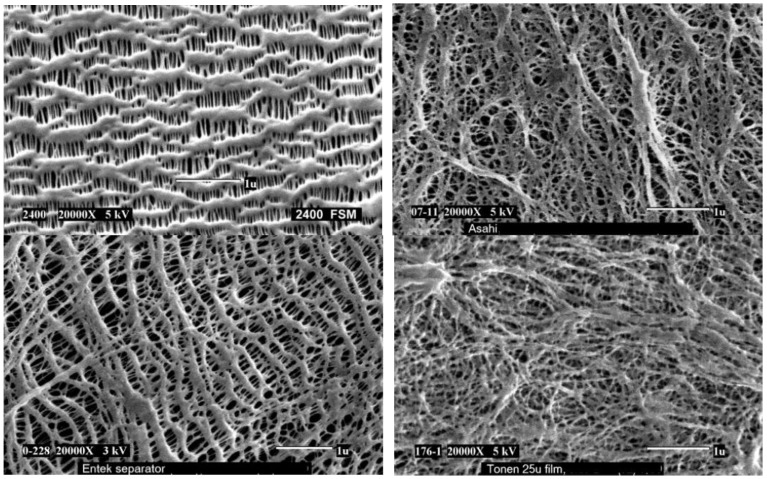
Table 2 lists some commercial separators based on microporous polyolefin membranes from 5 major manufacturers. Figure 8 shows scanning electron micrographs (SEMs) of some commercial separators [49]. The most attractive feature of the commercial separators is the fuse function, which is very important for protection from the external short-circuit and cell over-charge. Once the temperature reaches the melting point of the separator, the polymer can be flowable, and the pores will collapse forming less porous film or even a dense film, which decreases the ionic conductivity of the battery, stops the electrochemical and chemical reactions and thus protects the battery from thermal runaway. PP and PE separators have shutdown temperatures at about 165 °C and 135 °C respectively [51]. Another research topic based on commercial separator is concerned with the modification of polymer surfaces. To improve the compatibility of membranes/electrodes interfaces and electrolyte holding ability between the electrodes in the Li-ion cell, some functional groups have been attached on the polymer surfaces by electron beam irradiation and plasma [52,53].
Table 2.
Some commercial separators.
| Manufacturer | Material |
|---|---|
| Celgard LLC | PE, PP, PP/PE/PP |
| Asahi Kasei chemicals | PE, PP, and oxides-modified polyleffin |
| Entek membranes | PE, PP |
| SK energy | PE |
| Tonen | PE, PE-PP |
Figure 8.
Scanningelectron micrographs (SEMs) of (a) Celgard separator using dry process; (b) Asahi separator using wet process; (c) Entek separator using wet process; (d) Tonen separator using wet process [49].
3.2. Nonwoven Films
A nonwoven film is a textile product processed directly from fibers that are bonded together. The fibrous structure of nonwoven materials offers a high porosity, which is necessary for high electrolyte absorbance and low ionic resistance, and results in good charge/discharge cycles of the battery. In contrast to woven materials, the fibers in nonwovens are randomly distributed; an orientated microstructure does not occur. Compared with microporous membranes, a nonwoven film generally possesses much higher porosity and lower weight. The stochastic arrangement of the fibers is the main advantage of a nonwoven material compared with woven structures for the battery separator applications. Furthermore, it is convenient to prepare composite separators by using organic and inorganic materials simultaneously. However, the nonwoven separators have some drawbacks, such as large pore size and thicker nature. The tendency of particle penetration through the separator and the formation of dendrites during over-charging are very high in the Li-ion system. For this reason, membranes with small pores must be used. Table 3 gives the techniques of nonwoven film fabrication, related synthetic materials and film properties [50].
Table 3.
Fabrication of nonwoven films.
| Technique | Material | Film properties |
|---|---|---|
| Paper-making process | Polyolefin, PA, PTFE, PVDF, PVC, polyester, etc. | High porosity (60%–80%), large pore size (20–50 µm) |
| Melt-blowing method | ||
| Electrospinning |
Due to the disadvantages mentioned above, nonwoven films themselves are not suitable as the separator in liquid LIBs. There are two ways to employ the nonwoven films: (1) make gel polymer electrolyte as the supporting framework; (2) form composite separator by coating a layer of oxides particles on each side. Degussa commercialized a ceramic separator by coating oxides including alumina, zirconia, and silica on a thin poly(ethylene terephthalate) (PET) nonwoven film (see Figure 9) [54]. Oxide particles were obtained by hydrolyzing the precursors and suspended in an inorganic binder sol, and then the suspension was coated on a porous non-woven PET. After drying the coated PET at 200 °C a composite separator was obtained. Through this method, a separator having small pore-size, high air permeability as well as dimensional stability was developed.
Figure 9.
Schematic and SEMs of a Degussa composite separator [54].
4. Gel Polymer Electrolytes
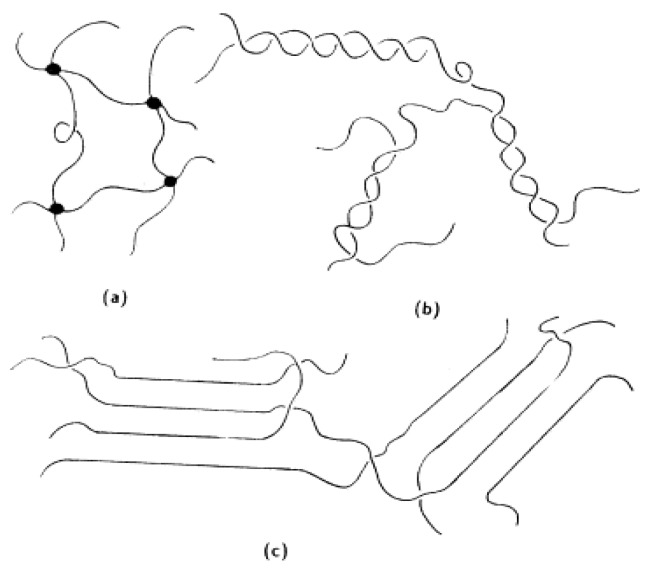
The polymer gel is formed by incorporating liquid electrolyte into the polymer matrix. The ionic conduction mechanism in polymer gels should be very similar to that in liquid electrolytes, and gels have better shape flexibility over liquids. Typically, liquid electrolyte is restrained by polymer chains within the polymer gel. Based on different structures, there are three gel networks (see Figure 10) [55]. The liquid electrolyte can also be restricted by nanofibers, as mentioned in the section of nonwoven films.
Figure 10.
Schematic representation of (a) a chemical gel network with junction points; (b) physical gel networks having junction zones and (c) fringed micelles, respectively [55].
Bellcore’s plastic Li-ion battery is a successful demonstration of the use of copolymer of vinylidene fluoride with hexafluoropropylene (PVDF-HFP) [56]. The addition of HFP introduces the amorphous domains into the polymer, which enables improved uptake of liquid electrolyte and thereby ionic conductivity. The crystalline regions provide enough mechanical integrity, and an overall plastic self-standing appearance, eliminating the need for cross-linking. A lot of work has been done to try to increase the conductivity of PVDF-HFP gels, such as changing the polymer matrix [57], adding oxide particles like Al2O3 and SiO2 [58], incorporating fully cyanoethylated cellulose derivative (DH-4-CN) [59], electronspinning BaTiO3 based PVDF-HFP [60], adding cross-linked dipoxy polyethylene glycol (DIEPEG) [61], and employing room temperature ionic liquids [62,63].
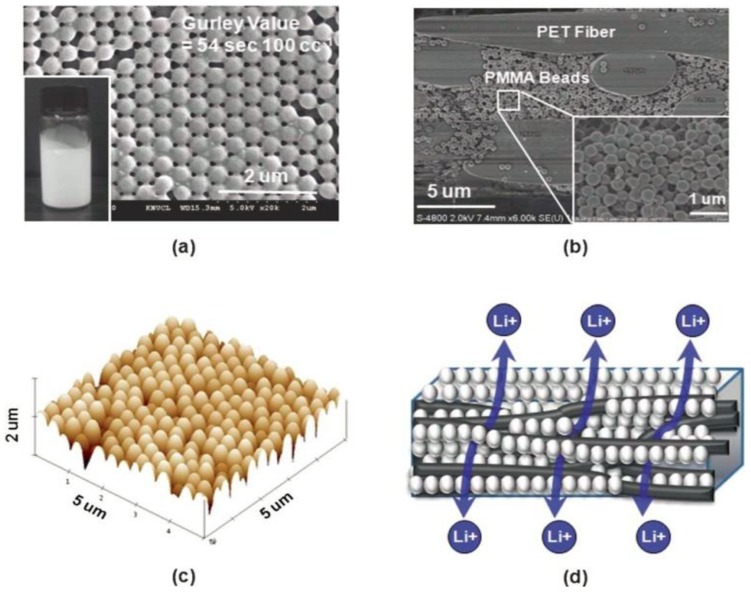
Poly(methyl methacrylate) (PMMA) is another important gel polymer, which has good compatibility with the liquid electrolytes, leading to good absorbing ability of the carbonate-based liquid electrolytes [64]. It is usually used to form blends [65], block copolymer [66], and layered structure to decrease the evaporation of the liquid electrolyte [67] or reduce leakage of the electrolyte [68]. A recent finding [69], in which close-packed PMMA particle arrays were introduced to a PET nonwoven separator, may inspire other research work on PMMA in LIBs. With nonwoven PET serving as a mechanical support, the well-connected interstitial voids formed between the close-packed PMMA colloidal particles in the PET nonwoven separator (see Figure 11). The highly-developed nanoporous structure and strong affinity for liquid electrolyte, the composite nonwoven separator allowed for more facile ion transport and superior electrolyte retention, which played a crucial role in improving the cell performance.
Figure 11.
(a) Surface SEM of the composite nonwoven separator;the inset is a photograph of Poly(methyl methacrylate) (PMMA) nanoparticles suspension; (b) Cross-section SEM; (c) AFM photograph of the composite nonwoven separator; (d) Schematic illustration of nanoporous structure [69].
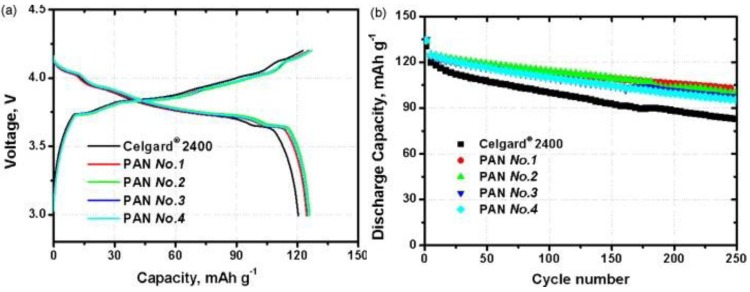
Poly(acrylonitrile) (PAN)-based gel electrolytes are considered of interest as separators in rechargeable LIBs. The expectation comes from the dimensional stability as well as the high conductivity at ambient (~10−3 S cm−1 at 25 °C) and subzero temperatures (~10−4 S cm−1 at −20 °C) of these electrolytes [70,71,72]. Later, the application of PAN-gel electrolyte was demonstrated in C-LiMn2O4 cell [73] and Li4Ti5O12-LiMn2O4 cells [74]. The drawback of polymer gel membranes is that liquid may eventually leak out from the membrane, which is deleterious both in terms of conductivity decay and, particularly, of battery reliability and safety. To solve this problem, oxide particles like Al2O3 were added in PAN-based, gel-type membranes [75]. The PAN nanofiber-based nonwoven separators for lithium-ion batteries have been developed by electrospinning technique. The cells using the PAN nonwovens showed better cycling performances than that of the Celgard due to smaller diffusion resistance of the separators [76,77,78] (see Figure 12).
Figure 12.
(a) Initial charge-discharge curves for the cells with the Celgard membrane and the Poly(acrylonitrile) (PAN) nonwoven membranes; (b) Discharge capacities vs. cycle numbers of the test cells at the 0.5C rate [78].
5. Conclusions
In this study, membranes used in lithium ion batteries have been reviewed. These membranes include solid state electrolytes which contains ceramic-glass and polymer Li ion conductors, microporous separators consisting of polyolefin-based microporous separators and nonwoven films, and gel polymer electrolytes. Each type of membrane can find its position in a particular battery application, which depends on specific requirements like rigid or flexible battery design, operating temperature and desired energy and power densities. For example, microporous polyolefin separators can satisfy most common demands in the batteries for mobile electronics. Ceramic Li ion conductors may be quite suitable for micro all-solid-state batteries by employing silicon technologies. For high energy and power density batteries, i.e., EV, HEV and grid energy storage LIBs, the safety requirement is a top priority, for example, present polyolefin separators cannot stand temperatures above the PP melting point (165 °C). Balancing performance, safety and cost should be the most important factors for the future research and development of LIB membranes.
Acknowledgments
Support from American Electric Power and the Virginia Tech Institute for Critical Technology and Applied Science is gratefully acknowledged.
References
- 1.Tarascon J.M., Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414:359–367. doi: 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- 2.Scrosati B., Garche J. Lithium batteries: Status, prospects and future. J. Power Sources. 2010;195:2419–2430. doi: 10.1016/j.jpowsour.2009.11.048. [DOI] [Google Scholar]
- 3.Dunn B., Kamath H., Tarascon J.M. Electrical energy storage for the grid: A battery of choices. Science. 2011;334:928–935. doi: 10.1126/science.1212741. [DOI] [PubMed] [Google Scholar]
- 4.Goodenough J.B., Kim Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009;22:587–603. doi: 10.1021/cm901452z. [DOI] [Google Scholar]
- 5.Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004;104:4303–4418. doi: 10.1021/cr030203g. [DOI] [PubMed] [Google Scholar]
- 6.Etacheri V., Marom R., Elazari R., Salitra G., Aurbach D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011;4:3243–3262. doi: 10.1039/c1ee01598b. [DOI] [Google Scholar]
- 7.Thangadurai V., Weppner W. Solid state lithium ion conductors: Design considerations by thermodynamic approach. Ionics. 2002;8:281–292. doi: 10.1007/BF02376081. [DOI] [Google Scholar]
- 8.Thokchom J.S., Kumar B. Composite effect in superionically conducting lithium aluminium germanium phosphate based glass-ceramic. J. Power Sources. 2008;185:480–485. doi: 10.1016/j.jpowsour.2008.07.009. [DOI] [Google Scholar]
- 9.Kumar B., Thomas D., Kumar J. Space-charge-mediated superionic transport in lithium ion conducting glass-ceramics. J. Electrochem. Soc. 2009;156:A506–A513. doi: 10.1149/1.3122903. [DOI] [Google Scholar]
- 10.Xu X., Wen Z., Yang X., Zhang J., Gu Z. High lithium ion conductivity glass-ceramics in Li2O-Al2O3-TiO2-P2O5 from nanoscaled glassy powders by mechanical milling. Solid State Ionics. 2006;177:2611–2615. doi: 10.1016/j.ssi.2006.04.010. [DOI] [Google Scholar]
- 11.Kosova N.V., Devyatkina E.T., Stepanov A.P., Buzlukov A.L. Lithium conductivity and lithium diffusion in NASICON-type Li1+xTi2−xAlx(PO4)3 (x = 0; 0.3) prepared by mechanical activation. Ionics. 2008;14:303–311. doi: 10.1007/s11581-007-0197-5. [DOI] [Google Scholar]
- 12.Hasegawa S., Imanishi N., Zhang T., Xie J., Hirano A., Takeda Y., Yamamoto O. Study on lithium/air secondary batteries—Stability of NASICON-type lithium ion conducting glass-ceramics with water. J. Power Sources. 2009;189:371–377. doi: 10.1016/j.jpowsour.2008.08.009. [DOI] [Google Scholar]
- 13.Takahashi K., Ohmura J., Im D., Lee D.J., Zhang T., Imanishi N., Hirano A., Phillipps M.B., Takeda Y., Yamamoto O. A super high lithium ion conducting solid electrolyte of grain boundary modified Li1.4Ti1.6Al0.4(PO4)3. J. Electrochem. Soc. 2012;159:A342–A348. [Google Scholar]
- 14.Zhang T., Imanishi N., Hasegawa S., Hirano A., Xie J., Takeda Y., Yamamoto O., Sammes N. Li/polymer electrolyte/water stable lithium-conducting glass ceramics composite for lithium—Air secondary batteries with an aqueous electrolyte. J. Electrochem. Soc. 2008;155:A965–A969. doi: 10.1149/1.2990717. [DOI] [Google Scholar]
- 15.Xie J., Imanishi N., Zhang T., Hirano A., Takeda Y., Yamamoto O. Li-ion transport in all-solid-state lithium batteries with LiCoO2 using NASICON-type glass ceramic electrolytes. J. Power Sources. 2009;189:365–370. doi: 10.1016/j.jpowsour.2008.08.015. [DOI] [Google Scholar]
- 16.Murugan R., Thangadurai V., Weppner W. Lithium ion conductivity of Li5+xBaxLa3−xTa2O12 (x=0-2) with garnet-related structure in dependence of the barium content. Ionics. 2007;13:195–203. doi: 10.1007/s11581-007-0097-8. [DOI] [Google Scholar]
- 17.Awaka J., Kijima N., Takahashi Y., Hayakawa H., Akimoto J. Synthesis and crystallographic studies of garnet-related lithium-ion conductors Li6CaLa2Ta2O12 and Li6BaLa2Ta2O12. Solid State Ionics. 2009;180:602–606. doi: 10.1016/j.ssi.2008.10.022. [DOI] [Google Scholar]
- 18.Murugan R., Thangadurai V., Weppner W. Lattice parameter and sintering temperature dependence of bulk and grain-boundary conduction of garnet-like solid Li-electrolytes. J. Electrochem. Soc. 2008;155:A90–A101. doi: 10.1149/1.2800764. [DOI] [Google Scholar]
- 19.Murugan R., Thangadurai V., Weppner W. Effect of lithium ion content on the lithium ion conductivity of the garnet-like structure Li5+xBaLa2Ta2O11.5+0.5x (x = 0-2) Appl. Phys. A. 2008;91:615–620. doi: 10.1007/s00339-008-4494-2. [DOI] [Google Scholar]
- 20.Thangadurai V., Weppner W. Li6ALa2Ta2O12 (A=Sr, Ba): Novel garnet-like oxides for fast lithium ion conduction. Adv. Func. Mater. 2005;15:107–112. doi: 10.1002/adfm.200400044. [DOI] [Google Scholar]
- 21.Thangadurai V., Adams S., Weppner W. Crystal structure revision and identification of Li+-ion migration pathways in the garnet-like Li5La3M2O12 (M = Nb, Ta) oxides. Chem. Mater. 2004;16:2998–3006. doi: 10.1021/cm031176d. [DOI] [Google Scholar]
- 22.Mei A., Wang X., Feng Y., Zhao S., Li G., Geng H., Lin Y., Nan C. Enhanced ionic transport in lithium lanthanum titanium oxide solid state electrolyte by introducing silica. Solid State Ionics. 2008;179:2255–2259. doi: 10.1016/j.ssi.2008.08.013. [DOI] [Google Scholar]
- 23.Jimenez R., Rivera A., Varez A., Sanz J. Li mobility in Li0.5−xNaxLa0.5TiO3 perovskites (0 ≤ x ≤ 0.5): Influence of structural and compositional parameters. Solid State Ionics. 2009;180:1362–1371. doi: 10.1016/j.ssi.2009.08.002. [DOI] [Google Scholar]
- 24.Bohnke O. The fast lithium-ion conducting oxides Li3xLa2/3−xTiO3 from fundamentals to application. Solid State Ionics. 2008;179:9–15. doi: 10.1016/j.ssi.2007.12.022. [DOI] [Google Scholar]
- 25.Zou Y., Inoue N. Structure and lithium ionic conduction mechanism in La4/3−yLi3yTi2O6. Ionics. 2005;11:333–342. doi: 10.1007/BF02430243. [DOI] [Google Scholar]
- 26.Stramare S., Thangadurai V., Weppner W. Lithium lanthanum titanates: A review. Chem. Mater. 2003;15:3974–3990. doi: 10.1021/cm0300516. [DOI] [Google Scholar]
- 27.Ihlefeld J.F., Clem P., Doyle B., Kotula P., Fenton K., Apblett C. Fast lithium-ion conducting thin-film electrolytes integrated directly on flexible substrates for high-power solid-state batteries. Adv. Mater. 2011;23:5663–5667. doi: 10.1002/adma.201102980. [DOI] [PubMed] [Google Scholar]
- 28.Minami T., Hayashi A., Tatsumisago M. Recent progress of glass and glass-ceramics as solid electrolytes for lithium secondary batteries. Solid State Ionics. 2006;177:2715–2720. doi: 10.1016/j.ssi.2006.07.017. [DOI] [Google Scholar]
- 29.Tatsumisago M., Mizuno F., Hayashi A. All-solid-state lithium secondary batteries using sulfide-based glass-ceramic electrolytes. J. Power Sources. 2006;159:193–199. doi: 10.1016/j.jpowsour.2006.04.037. [DOI] [Google Scholar]
- 30.Minami K., Hayashi A., Ujiie S., Tatsumisago M. Structure and properties of Li2S-P2S5-P2S3 glass and glass-ceramic electrolytes. J. Power Sources. 2009;189:651–654. doi: 10.1016/j.jpowsour.2008.09.044. [DOI] [Google Scholar]
- 31.Mizuno F., Ohtomo T., Hayashi A., Tadanaga K., Tatsumisago M. Lithium ion conducting solid electrolytes prepared from Li2S, elemental P and S. Solid State Ionics. 2006;177:2753–2757. doi: 10.1016/j.ssi.2006.04.024. [DOI] [Google Scholar]
- 32.Okamoto H., Hikazudani S., Inazumi C., Takeuchi T., Tabuchi M., Tatsumi K. Upper voltage and temperature dependencies for an all-solid-state In/LiCoO2 cell using sulfide glass electrolyte. Electrochem. Solid-State Lett. 2008;11:A97–A100. doi: 10.1149/1.2901600. [DOI] [Google Scholar]
- 33.Yao W., Martin S.W. Ionic conductivity of glasses in the MI + M2S + (0.1Ga2S3 + 0.9GeS2) system (M = Li, Na, K and Cs) Solid State Ionics. 2008;178:1777–1784. doi: 10.1016/j.ssi.2007.10.011. [DOI] [Google Scholar]
- 34.Nagamedianova Z., Hernández A., Sánchez E. Conductivity studies on LiX-Li2S-Sb2S3-P2S5 (X=LiI or Li3PO4) glassy system. Ionics. 2006;12:315–322. doi: 10.1007/s11581-006-0054-y. [DOI] [Google Scholar]
- 35.Kitaura H., Hayashi A., Tadanaga K., Tatsumisago M. High-rate performance of all-solid-state lithium secondary batteries using Li4Ti5O12 electrode. J. Power Sources. 2009;189:145–148. doi: 10.1016/j.jpowsour.2008.10.005. [DOI] [Google Scholar]
- 36.Kanno R., Murayama M. Lithium ionic conductor thio-LISICON: The Li2S-GeS2-P2S5 system. J. Electrochem. Soc. 2001;148:A742–A746. doi: 10.1149/1.1379028. [DOI] [Google Scholar]
- 37.Fenton D.E., Parker J.M., Wright P.V. Complexes of alkali metal ions with poly(ethylene oxide) Polymer. 1973;14:589. [Google Scholar]
- 38.Appetecchi G.B., Passerini S. Poly(ethylene oxide)-LiN(SO2CF2CF3)2 polymer electrolytes. J. Electrochem. Soc. 2002;149:A891–A897. doi: 10.1149/1.1483098. [DOI] [Google Scholar]
- 39.Villano P., Carewska M., Appetecchi G.B., Passerini S. PEO-LiN(SO2CF2CF3)2 polymer electrolytes. J. Electrochem. Soc. 2002;149:A1282–A1285. doi: 10.1149/1.1502688. [DOI] [Google Scholar]
- 40.Appetecchi G.B., Zane D., Scrosati B. PEO-based electrolyte membranes based on LiBC4O8 salt. J. Electrochem. Soc. 2004;151:A1369–A1374. doi: 10.1149/1.1774488. [DOI] [Google Scholar]
- 41.Shen C., Wang J., Tang Z., Wang H., Lian H., Zhang J., Cao C. Physicochemical properties of poly(ethylene oxide)-based composite polymer electrolytes with a silane-modified mesoporous silica SBA-15. Electrochimi. Acta. 2009;54:3490–3494. [Google Scholar]
- 42.An S., Jeong I., Won M., Jeong E., Shim Y. Effect of additives in PEO/PAA/PMAA composite solid polymer electrolytes on the ionic conductivity and Li ion battery performance. J. Appl. Electrochem. 2009;39:1573–1578. doi: 10.1007/s10800-009-9843-0. [DOI] [Google Scholar]
- 43.Marzantowicz M., Dygas J.R., Krok F., Tomaszewska A., Florjańczyk Z., Zygadło-Monikowska E., Lapienis G. Star-branched poly(ethylene oxide) LiN(CF3SO2)2: A promising polymer electrolyte. J. Power Sources. 2009;194:51–57. doi: 10.1016/j.jpowsour.2009.01.011. [DOI] [Google Scholar]
- 44.Fan L., Wang X., Long F., Wang X. Enhanced ionic conductivities in composite polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ionics. 2008;179:1772–1775. doi: 10.1016/j.ssi.2008.01.035. [DOI] [Google Scholar]
- 45.Meyer W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998;10:439–448. doi: 10.1002/(SICI)1521-4095(199804)10:6<439::AID-ADMA439>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 46.Ndeugueu J.L., Ikeda M., Aniya M. Correlation between the temperature range of cooperativity and the fragility index in ion conducting polymers. Solid State Ionics. 2010;181:16–19. doi: 10.1016/j.ssi.2009.11.011. [DOI] [Google Scholar]
- 47.Chen-Yang Y.W., Wang Y., Chen Y., Li Y., Chen H., Chiu H. Influence of silica aerogel on the properties of polyethylene oxide-based nanocomposite polymer electrolytes for lithium battery. J. Power Sources. 2008;182:340–348. doi: 10.1016/j.jpowsour.2008.04.001. [DOI] [Google Scholar]
- 48.Kim G.T., Appetecchi G., Carewska M., Joost M., Balducci A., Winter M., Passerini S. UV cross-linked, lithium-conducting ternary polymer electrolytes containing ionic liquids. J. Power Sources. 2010;195:6130–6137. doi: 10.1016/j.jpowsour.2009.10.079. [DOI] [Google Scholar]
- 49.Arora P., Zhang Z. Battery separators. Chem. Rev. 2004;104:4419–4462. doi: 10.1021/cr020738u. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources. 2007;164:351–364. doi: 10.1016/j.jpowsour.2006.10.065. [DOI] [Google Scholar]
- 51.Venugopal G., Moore J., Howard J., Pendalwar S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources. 1999;77:34–41. doi: 10.1016/S0378-7753(98)00168-2. [DOI] [Google Scholar]
- 52.Yao Z.P., Rånby B. Surface modification by continuous graft copolymerization. IV. Photoinitiated graft copolymerization onto polypropylene fiber surface. J. Appl. Poly. Sci. 1990;41:1469–1478. doi: 10.1002/app.1990.070410710. [DOI] [Google Scholar]
- 53.Ko J.M., Min B.G., Kim D.W., Ryu K.S., Kim K.M., Lee Y.G., Chang S.H. Thin-film type Li-ion battery, using a polyethylene separator grafted with glycidyl methacrylate. Electrochimi. Acta. 2004;50:367–370. doi: 10.1016/j.electacta.2004.01.127. [DOI] [Google Scholar]
- 54.Augustin S., Hennige V., Hörpel G., Hying C. Ceramic but flexible: New ceramic membrane foils for fuel cells and batteries. Desalination. 2002;146:23–28. doi: 10.1016/S0011-9164(02)00465-4. [DOI] [Google Scholar]
- 55.Song J.Y., Wang Y.Y., Wan C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources. 1999;77:183–197. doi: 10.1016/S0378-7753(98)00193-1. [DOI] [Google Scholar]
- 56.Tarascon J.M., Gozdz A.S., Schmutz C., Shokoohi F., Warren P.C. Performance of Bellcore’s plastic rechargeable Li-ion batteries. Solid State Ionics. 1996;86-88:49–54. doi: 10.1016/0167-2738(96)00330-X. [DOI] [Google Scholar]
- 57.Gentili V., Panero S., Reale P., Scrosati B. Role of the polymer matrix in determining the chemical-physical and electrochemical properties of gel polymer electrolytes for lithium batteries. Ionics. 2007;13:111–116. doi: 10.1007/s11581-007-0091-1. [DOI] [Google Scholar]
- 58.Gentili V., Panero S., Reale P., Scrosati B. Composite gel-type polymer electrolytes for advanced, rechargeable lithium batteries. J. Power Sources. 2007;170:185–190. doi: 10.1016/j.jpowsour.2007.04.008. [DOI] [Google Scholar]
- 59.Ren Z., Liu Y., Sun K., Zhou X., Zhang N. A microporous gel electrolyte based on poly(vinylidene fluoride-co-hexafluoropropylene)/fully cyanoethylated cellulose derivative blend for lithium-ion battery. Electrochimi. Acta. 2009;54:1888–1892. [Google Scholar]
- 60.Raghavan P., Zhao X., Kim J., Manuel J., Chauhan G., Ahn J., Nah C. Ionic conductivity and electrochemical properties of nanocomposite polymer electrolytes based on electrospun poly(vinylidene fluoride-co-hexafluoropropylene) with nano-sized ceramic fillers. Electrochimi. Acta. 2008;54:228–234. doi: 10.1016/j.electacta.2008.08.007. [DOI] [Google Scholar]
- 61.Ren Z., Sun K., Liu Y., Zhou X., Zhang N., Zhu X. Polymer electrolytes based on poly(vinylidene fluoride-co-hexafluoropropylene) with crosslinked poly(ethylene glycol) for lithium batteries. Solid State Ionics. 2009;180:693–697. doi: 10.1016/j.ssi.2009.02.033. [DOI] [Google Scholar]
- 62.Ye H., Huang J., Xu J., Khalfan A., Greenbaum S. Li ion conducting polymer gel electrolytes based on ionic liquid/PVDF-HFP blends. J. Electrochem. Soc. 2007;154:A1048–A1057. doi: 10.1149/1.2779962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrari S., Quartarone E., Mustarelli P., Magistris A., Fagnoni M., Protti S., Gerbaldi C., Spinella A. Lithium ion conducting PVDF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide ionic liquid. J. PowerSources. 2010;195:559–566. [Google Scholar]
- 64.Kim D.W., Oh B.K., Choi Y.M. Electrochemical performance of lithium-ion polymer cell using gel polymer electrolyte based on acrylonitrile-methyl methacrylate-styrene terpolymer. Solid State Ionics. 1999;123:243–249. doi: 10.1016/S0167-2738(99)00099-5. [DOI] [Google Scholar]
- 65.Rao M., Geng X., Liao Y., Hu S., Li W. Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J. Membr. Sci. 2012;399-400:37–42. doi: 10.1016/j.memsci.2012.01.021. [DOI] [Google Scholar]
- 66.Xiao Q., Wang X., Li W., Li Z., Zhang T., Zhang H. Macroporous polymer electrolytes based on PVDF/PEO-b-PMMA block copolymer blends for rechargeable lithium ion battery. J. Membr. Sci. 2009;334:117–122. doi: 10.1016/j.memsci.2009.02.018. [DOI] [Google Scholar]
- 67.Zhang H.P., Zhang P., Li Z.H., Sun M., Wu Y.P., Wu H.Q. A novel sandwiched membrane as polymer electrolyte for lithium ion battery. Electrochem. Comm. 2007;9:1700–1703. doi: 10.1016/j.elecom.2007.03.021. [DOI] [Google Scholar]
- 68.Xiao Q., Li Z., Gao D., Zhang H. A novel sandwiched membrane as polymer electrolyte for application in lithium-ion battery. J. Membr. Sci. 2009;326:260–264. doi: 10.1016/j.memsci.2008.10.019. [DOI] [Google Scholar]
- 69.Cho J.H., Park J., Kim J., Lee S. Facile fabrication of nanoporous composite separator membranes for lithium-ion batteries: Poly(methyl methacrylate) colloidal particles-embedded nonwoven poly(ethylene terephthalate) J. Mater. Chem. 2011;21:8192–8198. doi: 10.1039/c0jm04340k. [DOI] [Google Scholar]
- 70.Dautzenberg G., Croce F., Passerini S., Scrosati B. Characterization of PAN-based gel electrolytes. electrochemical stability and lithium cyclability. Chem. Mater. 1994;6:538–542. doi: 10.1021/cm00040a032. [DOI] [Google Scholar]
- 71.Alamgir M., Abraham K.M. Room temperature rechargeable polymer electrolyte batteries. J. Power Sources. 1995;54:40–45. doi: 10.1016/0378-7753(94)02037-4. [DOI] [Google Scholar]
- 72.Abraham K.M. Li+-conductive solid polymer electrolytes with liquid-like conductivity. J. Electrochem. Soc. 1990;137:1657–1658. doi: 10.1149/1.2086749. [DOI] [Google Scholar]
- 73.Abraham K.M., Choe H.S., Pasquariello D.M. Polyacrylonitrile electrolyte-based Li ion batteries. Electrochimi. Acta. 1998;43:2399–2412. doi: 10.1016/S0013-4686(97)10168-2. [DOI] [Google Scholar]
- 74.Peramunage D., Abraham K.M. The Li4Ti5O12/PAN electrolyte/LiMn2O4 rechargeable battery with passivation-free electrodes. J. Electrochem. Soc. 1998;145:2615–2622. doi: 10.1149/1.1838690. [DOI] [Google Scholar]
- 75.Appetecchi G.B., Romagnoli P., Scrosati B. Composite gel membranes: A new class of improved polymer electrolytes for lithium batteries. Electrochem. Comm. 2001;3:281–284. doi: 10.1016/S1388-2481(01)00137-0. [DOI] [Google Scholar]
- 76.Cho T.H., Sakai T., Tanase S., Kimura K., Kondo Y., Tarao T., Tanaka M. Electrochemical performances of polyacrylonitrile nanofiber-based nonwoven separator for lithium-ion battery. Electrochem. Solid-State Lett. 2007;10:A159–A162. doi: 10.1149/1.2730727. [DOI] [Google Scholar]
- 77.Liang Y., Ji L., Guo B., Lin Z., Yao Y., Li Y., Alcoutlabi M., Qiu Y., Zhang X. Preparation and electrochemical characterization of ionic-conducting lithium lanthanum titanate oxide/polyacrylonitrile submicron composite fiber-based lithium-ion battery separators. J. Power Sources. 2011;196:436–441. [Google Scholar]
- 78.Cho T.H., Tanaka M., Onishi H., Kondo Y., Nakamura T., Yamazaki H., Tanase S., Sakai T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources. 2008;181:155–160. doi: 10.1016/j.jpowsour.2008.03.010. [DOI] [Google Scholar]