Abstract
A 5-year-old mare was treated for recurrent colic and weight loss by surgical removal of an intraluminal cecal mass. Microscopic examination revealed vascular hamartoma. A 6-month follow-up showed an improvement in the general condition of the mare. Vascular hamartoma should be one of the differential diagnoses for weight loss and colic.
Résumé
Coliques récurrentes causées par un hamartome vasculaire caecal chez une jument Arabe. Une jument de 5 ans a été traitée pour coliques récurrentes et perte de poids par exérèse chirurgicale d’une masse caecale intraluminale. L’examen microscopique a révélé un hamartome vasculaire. Un suivi de 6 mois a montré une amélioration de l’état général de la jument. L’hamartome vasculaire doit faire partie du diagnostic différentiel de l’amaigrissement et des coliques.
(Traduit par les auteurs)
Intestinal tumors in horses are rare. Polyp (1,2), granuloma (3), neurofibroma (4), myxosarcoma (5), gastrointestinal stromal tumors (GIST) (6), adenoma and adenocarcinoma tumors (7,8–11) have been described. Vascular hamartoma, also termed angiomatous hamartoma, is a benign vascular proliferation that is rarely described in horses. The name may be confused with hemangioma or angiomatosis/hemangiomatosis. To the authors’ knowledge, intraluminal intestinal vascular hamartoma has not been reported in horses. This report describes a case of vascular hamartoma developing in the cecum and causing recurrent colic in a mare.
Case description
A 5-year-old Arabian mare was referred for recurrent colic and weight loss over a 3-week period. The mare was routinely vaccinated and was last dewormed 3 mo earlier. She was smaller than other foals of the same age and breed, but had grown without noticeable diseases. In the past 3 wk the mare had several bouts of colic, each one resolved by the referring veterinarian by administration of paraffin oil by nasogastric intubation and intravenous injections of butylscopolamine and dipyrone. The mare’s fecal production was markedly decreased during these 3 wk. The mare also received antibiotics (penicillin once, followed 2 wk later by ceftiofur for 8 d), but despite these treatments she remained uncomfortable and anorectic.
Upon admission to the hospital, the mare had a poor body condition, weighed 265 kg, and was severely depressed. There was moderate ventral edema and the left neck was indurated due to multiple intramuscular injections. The mare was normothermic at 36.8°C; her heart rate (HR) was high at 60 beats/min (bpm). Pink tacky mucous membranes and capillary refill time (CRT) between 2 and 3 s indicated a slightly dehydrated state (estimated dehydration 6% to 7%). Gastrointestinal sounds were decreased.
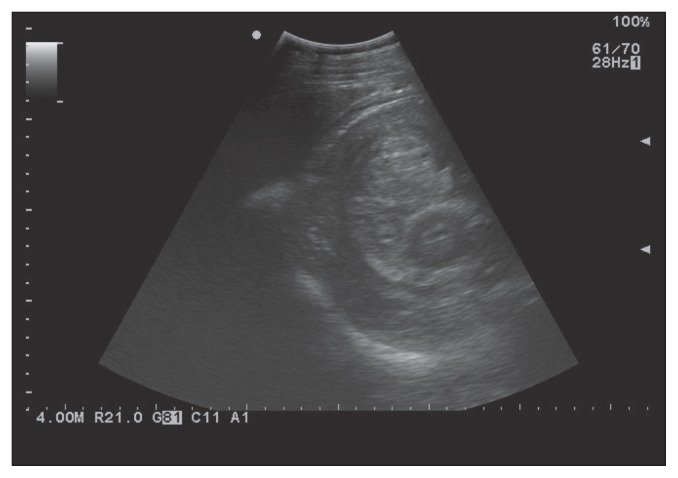
On rectal examination small firm masses (approximately 1-cm diameter) were palpated on the right side, along the cecal band, and were suspected to be reactive lymph nodes. Peritoneal fluid obtained by abdominocentesis was contaminated with blood but total protein concentration was within normal limits. Analysis of blood showed a decrease in total protein concentration (46 g/L) caused by a hypoalbuminemia of 17 g/L [reference range (RR): 58 to 75 g/L and > 25 g/L, respectively). Packed cell volume (PCV) was within normal limits at 42% (RR: 32% to 53%). Plasma fibrinogen was slightly increased at 4 g/L, but serum amyloid A inflammatory protein was severely increased at 2085 mg/L (RR: 2 to 4 g/L and < 7 mg/L, respectively). White blood cell count and biochemistry were within normal values. Coproscopic examination showed presence of strongyle eggs (between 10 and 100 eggs/5 g feces). An abdominal ultrasound examination revealed a heterogenous vascularized mass (14 cm wide × 20 cm long) in the cecum and an intussusception or an intraluminal mass was suspected (Figure 1). Based on the chronicity of the clinical signs and the suspicion of an abdominal mass or intussusception, an exploratory laparotomy was recommended and elected.
Figure 1.
Echographic examination of the right flank showed a heterogenous vascularized mass in the cecum.
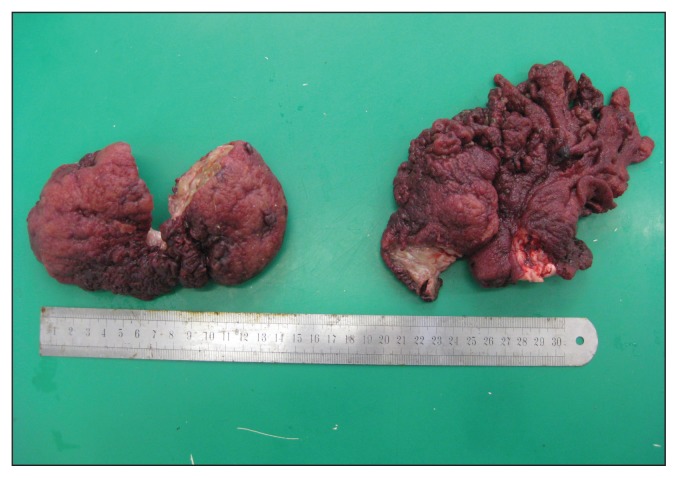
The mare was premedicated with xylazine (Rompun® 2%; Bayer Sante, Puteaux, France), 0.8 mg/kg body weight (BW), IV, and morphine (Morphine® 10 mg/mL; Cooper, Melun, France) 0.1 mg/kg BW, IV, and general anesthesia was induced with diazepam (Valium® 10 mg/2 mL, Roche, Boulogne-Billancourt, France), 0.05 mg/kg BW, IV, and ketamine (Imalgene® 1000; Merial, Villeurbanne, France), 2.2 mg/kg BW, IV. Anesthesia was maintained with isoflurane with the mare in dorsal recumbency and receiving Ringer’s lactate solution (Ringer® Lactate Aguettant; Coophavet, Ancenis, France) intravenously. A ventral laparotomy was performed. After opening the abdominal cavity, the ileum could not be exteriorized, the cecum appeared distended by gas and fluid and an intraluminal mass was palpated deep in the body of the cecum. A typhlotomy was performed to assess the cecal mass. The mass was bilobed, 15 to 20 cm in diameter, with an irregular surface, originating from a peduncle near the ileocaecal valve and obstructing the entrance to the right ventral colon (Figure 2). A ligature was tied around the peduncle before resecting the mass. After removal of the mass through the typhlotomy incision, 2 to 3 L of dark blood were removed from the cecum. Arterial blood pressure decreased but was quickly restored with dobutamine (Dobutamine® 250 mg/20 mL; Mylan, Saint-Priest, France), IV, and colloid (Plasmohes® 6%; Aguettant, Lyon, France), IV. The cecum was closed in 2 layers. The abdomen was lavaged with 20 L of sterile isotonic fluid, the abdominal wall was closed routinely and the mare recovered uneventfully.
Figure 2.
Macroscopic appearance of the mass after surgical resection.
The mare was given penicillin procaine (Dépocilline®; Intervet, Beaucouze, France), 22 000 UI/kg BW, IM, q12h, and gentamicin (G4®; Virbac, Carros, France), 6.6 mg/kg BW, IV, q24h. Flunixin meglumine (Flunixine 5% Norbrook®; Bayer Sante, Puteaux, France) was given intravenously 2 times a day for 3 d, then once a day for 3 d. Blood loss during surgery was significant based on pale mucous membranes, PCV of 18%, and a total protein concentration of 20 g/L. The mare received a 3.5 L whole blood transfusion.
After surgery the mare was tachycardic (80 bpm), showed mild abdominal pain, and was treated with butylscopolamine 0.2 mg/kg BW, and dypirone (Estocelan®; Boehringer Ingelheim, Paris, France) 25 mg/kg BW, IV. Over the next 2 d the mare improved and showed good appetite. However, 5 d after surgery, she developed a wound infection. Bacterial culture revealed Escherichia coli, resistant to most antimicrobials, including penicillin and gentamicin, except for colistin, some cephalosporins and neomycin. Antibiotic therapy (penicillin and gentamicin) was changed to a combination of colistin, 25 000 UI/kg BW and ampicillin, 10 mg/kg BW (Colampi; Biové, Saint Omer, France) intramuscularly q12h. After removal of skin clips, the wound was cleaned with 15 min hydrotherapy using a garden hose 4 times a day. Heart rate (52 bpm), PCV (22%), and total serum protein concentration (60 g/L) improved, but were still outside the reference ranges 20 d after surgery. The abdominal incision was draining less and the character of the discharge was improving but the mare developed muscular indurations due to the daily injections of the irritant colistin-ampicillin combination.
The mare was discharged with recommendations to continue hydrotherapy and administer cefquinome (Cobactan IV-IM 4.5%; Intervet), 1 mg/kg BW, IM, q12h for 10 d.
The wound healed well in a few weeks and 6 mo after discharge the mare was doing well and had regained her good body condition. No more signs of colic or weight loss were reported.
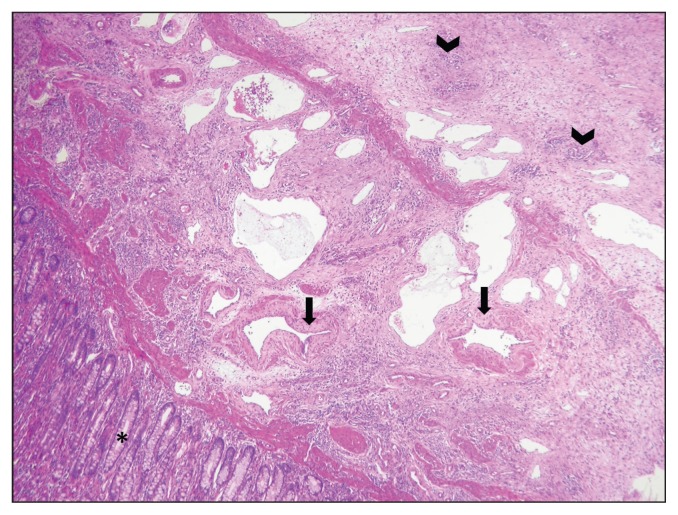
At histological examination, the mass displaced the submucosa and the muscularis, and was not encapsulated or demarcated. It consisted of a proliferation of well-organized, large, medium, and small arteries and dilated lymphatic vessels in a moderate amount of edematous connective tissue (Figure 3). Multifocally, tortuous new vessels with a muscular wall were observed. The described angiomatous lesion was consistent with a diagnosis of angiomatous hamartoma or vascular hamartoma.
Figure 3.
Microscopic appearance of the mass. A large number of vessels are present in the submucosa and the muscularis. They consisted in small (arrowheads), medium, and large arteries and ectatic lymphatic vessels. Medium and large arteries were often characterized by an incomplete muscular layer (arrows) replaced by abundant connective tissue. Intestinal mucosa (asterisk) is not affected. Hematoxylin and eosin 4×.
Discussion
Recurrent colic is frequently recorded in mature horses but this appears to be the first time that vascular hamartoma is reported to be the cause of such a problem. Vascular hamartoma is rare in all species. It has been described in the tongue of a horse (12), in the dorsal carpal region of 3 thoroughbreds (13), and in an ovary of a mare (14). In dogs, reported locations were the liver (15), and the lung and the brain (16), while in cats they were present in the gingiva and cervical spine (17). In cattle, vascular hamartoma were diagnosed in the liver (18), the ovary (19), the spinal cord (20), and the gingiva (21,22).
Vascular hamartomas are non-neoplastic proliferations of well-organized vascular tissue. They are congenital defects of vascular formation and development giving rise to normal tissue which is haphazardly arranged and often exuberant (17). In this case it is suspected that the cecal vascular hamartoma grew slowly, possibly since birth, without clinical signs until the mass was large enough to cause a colon obstruction.
Neoplastic vascular lesions generally originate from 1 cell type, thus they have the tendency to rebuild 1 type of vascular structure (small capillaries, arterioles, arteries, lymphatic vessels) which gives the name to the lesion. In our case, we observed different types of vessels (arterioles, large arteries, lymphatic vessels, capillaries). Moreover the architecture was not consistent with a benign or malignant tumor. A benign tumor is a well-delineated, often capsulated mass composed of normal-looking cells, while malignant neoplasm is highly infiltrative, not forming well-structured vessels and is composed of malignant cells. In our case the lesion was not well-delineated, it infiltrated, replaced, and displaced the normal architecture of the intestine, but it did not have cytological features of malignancy.
The blood vessels had a normal structure (endothelium, connective tissue, muscle tissue), while hemangiomas lack the muscular part of the vessels (15). Thus, a diagnosis of vascular hamartoma was made.
Other common developmental lesions with the appearance of a tumor are dermoid and teratoma. Dermoid is a tumor composed of skin in an abnormal location, while teratoma is an uncommon neoplasm composed of abnormal tissue derived from at least 2 of the 3 germ cell layers (endoderm, mesoderm, ectoderm), thus it is composed of different tissue (e.g., epithelial, nervous, renal, respiratory, digestive) (23).
Gastrointestinal stromal tumors (GIST) are rare, discrete, encapsulated, non-ulcerated, benign mesenchymal tumors made of spindle cells (6). They were part of our differential diagnosis, but were excluded after histological examination.
The classification of vascular proliferations is confusing, occasionally mixing tumoral and non-tumoral proliferations in the same categories. Angiomatosis is a multifocal proliferation of tumoral or non-tumoral vessels in 1 or multiple organs. Intestinal angiomatosis has been described twice in horses. In the first case it was an incidental finding at necropsy, lesions being located in the ovary and intestine of a 21-year-old mare (24). In the second case, angiomatous lesions were described in the skin of 9 horses. One of them also had a large intestinal mass, diagnosed as hemangioma (25). In other animals, angiomatosis or hemangiomatosis is usually described as skin lesions (26). Cardiac angiomatosis is described in young cattle and is called juvenile bovine angiomatosis (27).
Recurrent colic was considered secondary to obstruction of the ceco-colonic orifice (7).
The mare’s hypoproteinemia can be explained by a combination of reduced production of proteins secondary to malnutrition, protein-losing enteropathy caused by the mass, peritoneal inflammation due to surgery and intestinal parasitism, and bleeding during surgery.
In conclusion, the case reported here suggests that vascular hamartoma, although rare, should be considered among the potential causes of recurrent colic and weight loss in horses and can have a good prognosis after surgical removal. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Patterson-Kane JC, Sanchez LC, MacKay RJ, Sundberg JP, Homer BL. Small intestinal adenomatous polyposis resulting in protein-losing enteropathy in a horse. Vet Pathol. 2000;37:82–85. doi: 10.1354/vp.37-1-82. [DOI] [PubMed] [Google Scholar]
- 2.Saulez MN, Cebra CK, Snyder SP. Small colon polyp with peritonitis in an Arabian yearling filly. Equine Vet Educ. 2004;16:184–187. [Google Scholar]
- 3.Purcell KL, Johnson PJ, Kreeger JM, Wilson DA. Jejunal obstruction caused by a Pythium insidiosum granuloma in a mare. J Am Vet Med Assoc. 1994;205:337–339. [PubMed] [Google Scholar]
- 4.Pascoe PJ. Colic in a mare caused by a colonic neurofibroma. Can Vet J. 1982;23:24–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Edens LM, Taylor DD, Murray MJ, Spurlock GH, Anver MR. Intestinal myxosarcoma in a thoroughbred mare. Cornell Vet. 1992;82:163–167. [PubMed] [Google Scholar]
- 6.Hafner S, Harmon BG, King T. Gastrointestinal stromal tumors of the equine cecum. Vet Pathol. 2001;38:242–246. doi: 10.1354/vp.38-2-242. [DOI] [PubMed] [Google Scholar]
- 7.Munoz MJA, Lemberger K, Cadoré J-L, Lepage O. Small intestine adenocarcinoma in conjunction with multiple adenomas causing acute colic in a horse. J Vet Diagn Invest. 2008;20:121–124. doi: 10.1177/104063870802000128. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhof N, Steinhauer D, Fey K. Equine adenocarcinomas of the large intestine with osseous metaplasia. J Comp Path. 1996;114:451–456. doi: 10.1016/s0021-9975(96)80020-x. [DOI] [PubMed] [Google Scholar]
- 9.Harvey-Micay J. Intestinal adenocarcinoma causing recurrent colic in the horse. Can Vet J. 1999;40:729–730. [PMC free article] [PubMed] [Google Scholar]
- 10.Honnas CM, Snyder JR, Olander HJ, Wheat JD. Small intestinal adenocarcinoma in a horse. J Am Vet Med Assoc. 1987;191:845–846. [PubMed] [Google Scholar]
- 11.Wright JA, Edwards GB. Adenocarcinoma of the intestine in a horse: An unusual occurrence. Equine Vet J. 1984;16:136–137. doi: 10.1111/j.2042-3306.1984.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 12.Brunson BL, Taintor J, Newton J, Schumacher J, Christman U. Vascular hamartoma in the tongue of a horse. J Equine Vet Sc. 2006;26:275–277. [Google Scholar]
- 13.Colbourne CM, Yovich JV, Richards RB, Rose KJ, Huxtable CR. Vascular hamartomas of the dorsal carpal region in three young thoroughbred horses. Aust Vet J. 1997;75:20–23. doi: 10.1111/j.1751-0813.1997.tb13821.x. [DOI] [PubMed] [Google Scholar]
- 14.Rhyan JC, Dandrea GH, Smith LS. Congenital ovarian vascular hamartoma in a horse. Vet Pathol. 1981;18:131. doi: 10.1177/030098588101800118. [DOI] [PubMed] [Google Scholar]
- 15.Gualtieri M, Cocci A, Monti S, Olivero D. Surgical removal of a localised vascular hepatic hamartoma in a dog. Aust Vet J. 2009;87:360–362. doi: 10.1111/j.1751-0813.2009.00472.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith SH, Van Winkle T. Cerebral vascular hamartomas in five dogs. Vet Pathol. 2001;38:108–112. doi: 10.1354/vp.38-1-108. [DOI] [PubMed] [Google Scholar]
- 17.Parkes JD, Kline KL, Riedesel EA, Haynes JS. A vascular hamartoma arising from the cervical spine of a cat. J Feline Med Surg. 2009;11:724–727. doi: 10.1016/j.jfms.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braum U, Trosch L, Gerspach C, Brosinski K, Hilbe M. Ultrasonographic findings in a cow with vascular hamartoma of the liver: Case report. BMC Vet Res. 2011;7:52. doi: 10.1186/1746-6148-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoit J-M, Lefebvre RC, Mulon P-Y, Raggio I, Dore M. Ovarian vascular hamartoma in a cow. Can Vet J. 2005;46:1026–1028. [PMC free article] [PubMed] [Google Scholar]
- 20.Cho CY, Cook JE, Leipold HW. Angiomatous vascular malformation in the spinal cord of a Hereford calf. Vet Pathol. 1979;16:613–616. doi: 10.1177/030098587901600516. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi GR, Maleki M, Sardaroi K. Gingival vascular hamartoma in a young Holstein calf. Comp Clin Pathol. 2007;16:73–75. [Google Scholar]
- 22.Sheahan BJ, Donnelly WJC. Vascular hamartomas in the gingiva of two calves. Vet Pathol. 1981;18:562–564. doi: 10.1177/030098588101800415. [DOI] [PubMed] [Google Scholar]
- 23.Mannan AA, Sharma MC, Singh MK, Bahadur S, Hatimota P. Vascular hamartoma of the paranasal sinuses: Report of 3 rare cases and a short review of the literature. Ear Nose Throat J. 2009;88:740–743. [PubMed] [Google Scholar]
- 24.Lamm CG, Njaa BL. Ovarian and intestinal angiomatosis in a horse. Vet Pathol. 2007;44:386–388. doi: 10.1354/vp.44-3-386. [DOI] [PubMed] [Google Scholar]
- 25.Platt H. Vascular malformations and angiomatous lesions in horses: A review of 10 cases. Equine Vet J. 1987;19:500–504. doi: 10.1111/j.2042-3306.1987.tb02658.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Reinecke S, Malarkey DE. Cutaneous angiomatosis in a young dog. Vet Pathol. 2005;42:378–381. doi: 10.1354/vp.42-3-378. [DOI] [PubMed] [Google Scholar]
- 27.Watson TD, Thompson H. Juvenile bovine angiomatosis: A syndrome of young cattle. Vet Rec. 1990;127:279–282. [PubMed] [Google Scholar]