Abstract
Background
Two large surveillance studies in adults with asthma have found an increased risk of asthma‐related mortality in those who took regular salmeterol as monotherapy in comparison to placebo or regular salbutamol. No similar sized surveillance studies have been carried out in children with asthma, and we remain uncertain about the comparative safety of regular combination therapy with either formoterol or salmeterol in children with asthma.
Objectives
We have used the paediatric trial results from Cochrane systematic reviews to assess the safety of regular formoterol or salmeterol, either as monotherapy or as combination therapy, in children with asthma.
Methods
We included Cochrane reviews relating to the safety of regular formoterol and salmeterol from a search of the Cochrane Database of Systematic Reviews conducted in May 2012, and ran updated searches for each of the reviews. These were independently assessed. All the reviews were assessed for quality using the AMSTAR tool. We extracted the data relating to children from each review and from new trials found in the updated searches (including risks of bias, study characteristics, serious adverse event outcomes, and control arm event rates).
The safety of regular formoterol and salmeterol were assessed directly from the paediatric trials in the Cochrane reviews of monotherapy and combination therapy with each product. Then monotherapy was indirectly compared to combination therapy by looking at the differences between the pooled trial results for monotherapy and the pooled results for combination therapy. The comparative safety of formoterol and salmeterol was assessed using direct evidence from trials that randomised children to each treatment; this was combined with the result of an indirect comparison of the combination therapy trials, which represents the difference between the pooled results of each product when randomised against inhaled corticosteroids alone.
Main results
We identified six high quality, up to date Cochrane reviews. Four of these related to the safety of regular formoterol or salmeterol (as monotherapy or combination therapy) and these included 19 studies in children. We added data from two recent studies on salmeterol combination therapy in 689 children which were published after the relevant Cochrane review had been completed, making a total of 21 trials on 7474 children (from four to 17 years of age). The two remaining reviews compared the safety of formoterol with salmeterol from trials randomising participants to one or other treatment, but the reviews only included a single trial in children in which there were 156 participants.
Only one child died across all the trials, so impact on mortality could not be assessed.
We found a statistically significant increase in the odds of suffering a non‐fatal serious adverse event of any cause in children on formoterol monotherapy (Peto odds ratio (OR) 2.48; 95% confidence interval (CI) 1.27 to 4.83, I2 = 0%, 5 trials, N = 1335, high quality) and smaller increases in odds which were not statistically significant for salmeterol monotherapy (Peto OR 1.30; 95% CI 0.82 to 2.05, I2 = 17%, 5 trials, N = 1333, moderate quality), formoterol combination therapy (Peto OR 1.60; 95% CI 0.80 to 3.28, I2 = 32%, 7 trials, N = 2788, moderate quality) and salmeterol combination therapy (Peto OR 1.20; 95% CI 0.37 to 2.91, I2 = 0%, 5 trials, N = 1862, moderate quality).
We compared the pooled results of the monotherapy and combination therapy trials. There was no significant difference between the pooled ORs of children with a serious adverse event (SAE) from long‐acting beta2‐agonist beta agonist (LABA) monotherapy (Peto OR 1.60; 95% CI 1.10 to 2.33, 10 trials, N = 2668) and combination trials (Peto OR 1.50; 95% CI 0.82 to 2.75, 12 trials, N = 4,650). However, there were fewer children with an SAE in the regular inhaled corticosteroid (ICS) control group (0.7%) than in the placebo control group (3.6%). As a result, there was an absolute increase of an additional 21 children (95% CI 4 to 45) suffering such an SAE of any cause for every 1000 children treated over six months with either regular formoterol or salmeterol monotherapy, whilst for combination therapy the increased risk was an additional three children (95% CI 1 fewer to 12 more) per 1000 over three months.
We only found a single trial in 156 children comparing the safety of regular salmeterol to regular formoterol monotherapy, and even with the additional evidence from indirect comparisons between the combination formoterol and salmeterol trials, the CI around the effect on SAEs is too wide to tell whether there is a difference in the comparative safety of formoterol and salmeterol (OR 1.26; 95% CI 0.37 to 4.32).
Authors' conclusions
We do not know if regular combination therapy with formoterol or salmeterol in children alters the risk of dying from asthma.
Regular combination therapy is likely to be less risky than monotherapy in children with asthma, but we cannot say that combination therapy is risk free. There are probably an additional three children per 1000 who suffer a non‐fatal serious adverse event on combination therapy in comparison to ICS over three months. This is currently our best estimate of the risk of using LABA combination therapy in children and has to be balanced against the symptomatic benefit obtained for each child. We await the results of large on‐going surveillance studies to further clarify the risks of combination therapy in children and adolescents with asthma.
The relative safety of formoterol in comparison to salmeterol remains unclear, even when all currently available direct and indirect trial evidence is combined.
Keywords: Child; Humans; Albuterol; Albuterol/administration & dosage; Albuterol/adverse effects; Albuterol/analogs & derivatives; Anti‐Asthmatic Agents; Anti‐Asthmatic Agents/administration & dosage; Anti‐Asthmatic Agents/adverse effects; Asthma; Asthma/drug therapy; Asthma/mortality; Drug Therapy, Combination; Drug Therapy, Combination/methods; Ethanolamines; Ethanolamines/administration & dosage; Ethanolamines/adverse effects; Formoterol Fumarate; Randomized Controlled Trials as Topic; Review Literature as Topic; Salmeterol Xinafoate
Plain language summary
Overview of the safety of regular formoterol or salmeterol in children with asthma
Asthma is a common condition that affects the airways, the small tubes that carry air in and out of the lungs. People can have underlying inflammation in their lungs and sticky mucus or phlegm may build up, which can narrow the airways. When a person with asthma comes into contact with an irritant (an asthma trigger), the muscles around the walls of the airways tighten, the airways become narrower, and the lining of the airways becomes inflamed and starts to swell. This leads to the symptoms of asthma, which are wheezing, coughing and difficulty in breathing. There is no cure for asthma; however, there are medications that allow most people to control their asthma so they can get on with daily life. People with asthma are generally advised to take inhaled corticosteroids to combat the underlying inflammation in their lungs. If asthma is still not controlled, current clinical guidelines recommend the introduction of an additional medication to help. One type of additional medication is the long‐acting beta2‐agonists, such as formoterol and salmeterol, which work by reversing the narrowing of the airways that occurs during an asthma attack. These drugs, taken by inhaler, are known to improve lung function, symptoms, quality of life and to reduce the number of asthma attacks. However, the evidence for the usefulness of long‐acting beta2‐agonists is more limited in children than adults, and there are concerns about the safety of these drugs in both adults and children. We did this overview to take a closer look at the safety of formoterol or salmeterol, either alone or given in combination with corticosteroid therapy, in children with asthma.
We looked at previous Cochrane reviews on long‐acting beta2‐agonists and also searched for additional trials on long‐acting beta2‐agonists in children. We found a total of 21 trials involving 7318 children that provided information on the safety of formoterol or salmeterol given alone or combined with corticosteroids. We also found one trial on 156 children which directly compared formoterol to salmeterol.
There were more non‐fatal serious adverse events in children taking formoterol or salmeterol compared to those on placebo; for every 1000 children treated with formoterol or salmeterol over six months, 21 extra children suffered a non‐fatal event in comparison with placebo. There was a smaller and non‐significant increase in serious adverse events in children on formoterol or salmeterol and corticosteroids compared to corticosteroids alone: for every 1000 children treated with combination therapy over three months, three extra children suffered a non‐fatal event in comparison with corticosteroids alone. This number illustrates the average difference between combination therapy and corticosteroids. Our analyses showed that in fact the true answer could be between 1 fewer and 12 more children who would experience a non‐fatal event.
We did not have enough numbers from the small trial comparing formoterol to salmeterol, or from information in the other trials, to tell whether one long‐acting beta2‐agonist treatment is safer than the other. There was only one death across all the trials, so we did not have enough information to tell whether formoterol or salmeterol increases the risk of death.
Background
Description of the condition
Despite efforts to define asthma over the past 30 years, there is “still no specific definition or validated diagnostic algorithm for the disease” (Anderson 2008). The definition of asthma in the Global Initiative for Asthma (GINA) guidelines (GINA 2011) is therefore functional:
“Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyper‐responsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread, but variable, airflow obstruction within the lung that is often reversible either spontaneously or with treatment.”
The main cause of short‐term asthma symptoms (wheezing and shortness of breath) is contraction of the smooth muscle around the airways (bronchoconstriction). Children with asthma show airways hyper‐responsiveness to inhaled allergens (Cockcroft 2006) and a variety of chemical stimuli (Boushey 1980). It is by no means clear how airway hyper‐responsiveness relates to the inflammatory changes seen in asthma, or the inflammatory pathways that mediate these changes (Anderson 2008).
In clinical practice, most children with asthma are treated in primary care and never suffer from life‐threatening exacerbations. However, there remains a minority of children who continue to be at risk of hospital admission and even death from their asthma. This remains true today, even with the advances in available treatment.
In life‐threatening asthma, mucus plugging and oedema of the airways accompany smooth muscle contraction. Although the relative contribution of these elements to deaths from asthma is not clear, it is potentially dangerous to relieve bronchoconstriction without treating the underlying inflammatory changes.
Description of the interventions
Inhaled selective beta2‐agonists were introduced in 1969 to reduce bronchoconstriction (Phillips 1990). These were followed in 1974 by the introduction of inhaled corticosteroids (ICS), and regular ICS treatment has remained the basis of the treatment of the inflammation in asthma since the early 1990s. The original beta2‐agonists were short‐acting and had a duration of action of four to six hours. Long‐acting beta2‐agonists (salmeterol and formoterol) were introduced in the 1990s; these only needed to be inhaled twice daily since they had a duration of action of 12 hours or more. Of these, salmeterol has a slower onset of action than formoterol (Van Noord 1996). The long‐acting beta2‐agonists were first introduced as monotherapy inhalers and then later combined with an ICS in combination inhalers (such as formoterol/budesonide or salmeterol/fluticasone).
The beta2‐agonists relax the airways smooth muscle and relieve bronchoconstriction, and they are recommended as intermittent first‐step treatment for children with asthma (SIGN/BTS 2012). In children who require treatment (or who have asthma symptoms) more than twice a week, the second step in treatment is to add ICS to reduce inflammation in the airways. The addition of a regular long‐acting beta2‐agonist (LABA) to an ICS is the current recommended next step for adults and children over five years of age whose asthma symptoms are not controlled with regular ICS alone (SIGN/BTS 2012). For children under five years of age who are not controlled with regular ICS alone, the addition of oral leukotriene receptor antagonists to ICS is recommended.
How the intervention might work
The mechanism by which beta2‐agonists might cause harm is not currently known. There are several theories (Tattersfield 2006) that include the possibility of direct toxicity of beta2‐agonists due to adverse cardiac effects, tolerance induced by regular use of beta2‐agonists so that they become less effective bronchodilators in acute asthma exacerbations (Weinberger 2006), delay in seeking medical help (if the beta2‐agonists mask the severity of an attack) or reduced use of corticosteroids (which are needed to treat bronchial oedema and excess mucus production due to increased inflammation during exacerbations).
Why it is important to do this overview
The evidence for the benefit of LABAs in children remains weaker than in adults (Ducharme 2010; Ducharme 2011; Ni Chroinin 2009), and in 2007 the Pediatric Advisory Committee of the Food and Drug Administration (FDA) reviewed the safety of regular salmeterol in children. As a result, a meta‐analysis of individual patient data was carried out by the FDA to assess outcomes in different age‐groups (McMahon 2011). The analysis found that children aged four to 11 years on LABA monotherapy were the age‐group with the largest increase in the risk of serious asthma events (using a composite index of hospitalisation, intubation or asthma‐related mortality). In 2008 the Advisory Committee voted to restrict the use of LABAs to combination ICS/LABA products for children and adults. At a further meeting in 2010, labelling changes were made including a recommendation that, for children, LABAs should be used as combination ICS and LABA products (McMahon 2011).
Regular treatment with LABA is not recommended without regular ICS (Lougheed 2010; SIGN/BTS 2012), but the FDA advice to use regular LABA for "the shortest duration possible to achieve control of asthma symptoms and then be discontinued" has been challenged as not evidence‐based by the Canadian Thoracic Society Asthma Committee group (Lougheed 2010).
Two spikes in the rate of global asthma deaths have been linked to the use of short‐acting beta2‐agonists, isoprenaline forte in the 1960s and fenoterol in the 1980s (Tattersfield 2006). Subsequently two large surveillance studies and a meta‐analysis have reported an increased risk of death from asthma with regular use of salmeterol in adults with asthma (Castle 1993; Nelson 2006; Salpeter 2006). Given the results of these surveillance studies in adults, the safety of both regular formoterol and salmeterol, with and without ICS, needs to be compared in children with asthma. The evidence that is available from children also needs to be set against the results from these large surveillance studies of the safety of salmeterol in adults with asthma.
Serious adverse events are uncommon and although they are routinely recorded in randomised trials, individual clinical trials are not usually powered to detect small but potentially important differences in the risk of serious adverse events. Moreover, the reporting of serious adverse events in journal articles based on the trials is likely to be incomplete (Cates 2008). Systematic reviews increase the statistical power to detect rare events, but there is a particular challenge in that there are many ways in which serious adverse events can be described and reported in medical journals (Ioannidis 2001), and only a part of the picture may be seen if the analysis of serious adverse events is restricted to those that the investigators considered to be related to treatment. There is evidence that selective reporting does occur, both in relation to efficacy outcomes and adverse events (Chan 2004; Chan 2004a; Whittington 2004), and there has been a call for better reporting of harms in trial reports in journals (Ioannidis 2004). In view of these difficulties, we have sought to summarise evidence from Cochrane systematic reviews that included clinical trial data on serious adverse events reported on manufacturers' websites and in FDA submissions in addition to events reported in medical journals.
Objectives
We have used the paediatric trial results from Cochrane systematic reviews to assess the safety of regular formoterol or salmeterol, either as monotherapy or as combination therapy, in children with asthma.
Methods
Criteria for considering reviews for inclusion
Types of reviews
Cochrane systematic reviews of randomised trials published in the Cochrane Database of Systematic Reviews (CDSR) that have a primary focus on adverse events.
Participants
Children with asthma. We included reviews of both adults and children but only analysed the results from the trials in children.
Interventions
Regular formoterol monotherapy versus placebo
Regular salmeterol monotherapy versus placebo
Regular formoterol in combination with ICS versus the same dose of ICS
Regular salmeterol in combination with ICS versus the same dose of ICS
Regular formoterol versus regular salmeterol
Regular formoterol in combination with ICS versus regular salmeterol in combination with ICS
We did not include reviews of formoterol used for maintenance and relief of symptoms, or relief of symptoms alone.
Outcome measures
Primary outcomes: all‐cause mortality and non‐fatal serious adverse events
Secondary outcomes: asthma‐related deaths and asthma‐related non‐fatal serious adverse events
Search methods for identification of reviews
We searched the Cochrane Database of Systematic Reviews (CDSR) in The Cochrane Library (2012, Issue 5 of 12) in May 2012. We did not apply any date restrictions. We did not search for non‐Cochrane reviews. See Appendix 1 for the search strategy.
We conducted updated literature searches for each identified adverse event review to search for any new trials that may not yet have been incorporated into the Cochrane reviews, using the search strategy published in each review.
Data collection and analysis
Selection of reviews
Two review authors independently assessed Cochrane reviews for inclusion. There was no disagreement, so discussion with a third person was not needed.
Data extraction and management
We extracted data from studies included in the existing Cochrane reviews in relation to the characteristics, risks of bias and data for the outcomes specified above.
We also extracted data from the reviews on control group event rates (both as a proportion of the total number of participants and then adjusted for the duration of each trial).
We extracted data from new trials that had not been included in the published version of the included reviews and incorporated the data into our overview.
All data were extracted independently by two reviewers.
Assessment of methodological quality of included reviews
Quality of included reviews
Two review authors independently assessed the included reviews for methodological quality, with particular emphasis on potential bias in the review process of each review, using the AMSTAR tool (Shea 2007). We assessed the incorporation of the risk of bias into each review, and planned to carry out a sensitivity analysis based on the results of studies at low or unclear risk of bias for each outcome. We considered the risks of bias in relation to the selection of studies, ascertainment of serious adverse events, and method of analysis of the results.
Quality of evidence in included reviews
We assessed whether the included reviews relied merely on evidence from reports of trial results published in journals or looked more widely at manufacturers' trial reports and submissions to the FDA (in order to reduce the risk of publication bias).
Two review authors independently assessed the quality of evidence in the included reviews using the 'Risk of bias' tables in the included reviews (for the trials that were on children). We also assessed the limitations of the evidence found in the reviews for the trials for children using the 'Summary of findings' tables from the included reviews, and independently reassessed the downgrading decisions made in each review using the GRADE process.
Data synthesis
Direct randomised comparison data
We extracted data from two new trials, which were included after we ran the updated search, and analysed them together with data from the relevant included systematic review using Review Manager 5 (RevMan 5); the results are summarised in Forest plots and tables of pooled results.
We analysed serious adverse event data as odds ratios (OR) and as risk differences using RevMan 5 . Where there were zero cells in any of the studies the Peto OR was preferred as it requires no zero cell adjustment (Bradburn 2007). Whilst the risk difference analysis has the advantage of including data from trials with no events in either arm, there is usually higher heterogeneity than using ORs. The risk differences were used to compare all‐cause events and asthma‐related events on the same scale, since ORs would not be expected to be the same if the ratio of all‐cause events was driven by the increase in asthma‐related events.
We preferred ORs to risk ratios as there are two separate risk ratios for participants who suffer an adverse event and participants who do not, and the choice between these two risk ratios, which cannot be made on good empirical grounds, could alter the point estimates and statistical significance of the pooled results.
Since the dose‐response curves for each product and formulation may not have been the same (Cates 2011; Senn 1997), we also looked at subgroups of trials using different products and doses of formoterol, and assessed heterogeneity of the ORs using the I2 statistic in RevMan 5 from the data sets in the existing reviews.
We converted the pooled ORs (and 95% confidence interval (CI)) into absolute differences for the summary of findings table and the Cates plots with Visual Rx 2012 (using the mean control arm event rates from the trials).
Indirect comparison of monotherapy and combination therapy
We explored the safety interaction with ICS by comparing the treatment effects of formoterol or salmeterol versus placebo (diagonal green lines in Figure 1A) and the difference from the treatment effect of formoterol or salmeterol with ICS versus the same dose of ICS (corresponding vertical green lines in Figure 1B) using the method described in Altman 2003 and Bucher 1997. This comparison was carried out by entering the monotherapy and combination therapy trial results as different subgroups in RevMan 5, and the results were displayed as a forest plot. The test for interaction between subgroups was generated for the Peto ORs using RevMan 5 and is displayed on the forest plots.
1.
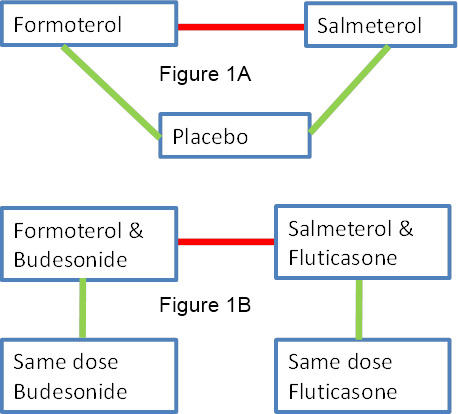
Network of comparisons of serious adverse events with regular formoterol and salmeterol (with or without regular inhaled corticosteroids (ICS)). Red lines show direct comparisons between formoterol and salmeterol. Green lines show direct comparisons for each drug with placebo (Figure 1A) or ICS (Figure 1B), and can be compared (horizontally) with each other to make indirect comparisons of formoterol and salmeterol. The placebo comparison results (Figure 1A) can also be compared (vertically) to the ICS comparison results (Figure 1B) to indirectly assess the impact of ICS on the serious adverse events with formoterol and salmeterol.
Direct and indirect comparisons of regular formoterol and salmeterol
We first considered formoterol and salmeterol separately and then compared them to each other using direct and indirect comparisons. In Figure 1A and Figure 1B, the direct comparisons between formoterol and salmeterol are shown as red lines on the network diagram, and the indirect comparisons are shown as vertical or sloping green lines, comparing each drug with placebo or ICS.
Methods used to calculate indirect comparisons
For results analysed as Peto ORs, the indirect comparison was generated by taking the natural logarithm of the pooled OR from the salmeterol combination therapy versus ICS trials and subtracting this from the natural logarithm of the pooled OR from the formoterol combination therapy versus ICS trials. The variance of the difference in the log ORs is the sum of the variance of each log OR. The indirect difference in log ORs and its standard error were then entered into RevMan 5 (using the generic inverse variance method) and could be combined with the log OR from the trial that directly randomised children to regular formoterol or salmeterol.
Control group event rates
Major differences between control group event rates present a threat of confounding to indirect comparisons between the results from different reviews, as we would not expect risk differences to be the same across widely different control group risks. We therefore extracted control group events from each review and compared the mean event rates both as proportions of the total number in the control groups and as weekly rates.
Results
We have created a new summary of findings table for this overview (Table 1). The table summarises the relative and absolute impact of regular formoterol or salmeterol (as monotherapy and combination therapy) on non‐fatal serious adverse events of any cause in children with asthma in the upper half; and the lower half summarises the children with asthma‐related serious adverse events.
1. Summary of findings ‐ children with a serious adverse event.
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular LABA (salmeterol or formoterol) | |||||
| Children with a fatal serious adverse event of any cause | ||||||
| All comparisons | see comment | see comment | see comment | see comment | see comment | There was only a single child who died in all the studies so mortality could not be assessed. |
| Children with a non‐fatal serious adverse event of any cause | ||||||
| Regular formoterol versus placeboCates 2012a Follow‐up: mean 27 weeks | 12 per 1000 | 30 per 1000 (15 to 56) | OR 2.48 (1.27 to 4.83) | 1335 (5 studies) | ⊕⊕⊕⊕ high | |
| Regular salmeterol versus placeboCates 2008 Follow‐up: mean 31 weeks | 56 per 1000 | 72 per 1000 (46 to 108) | OR 1.3 (0.82 to 2.05) | 1333 (5 studies) | ⊕⊕⊕⊝ moderate1 | |
|
Regular formoterol & ICS versus ICSCates 2009b Follow‐up: mean 13 weeks |
8 per 1000 | 14 per 1000 (7 to 27) | OR 1.62 (0.80 to 3.28) | 2788 (7 studies) |
⊕⊕⊕⊝ moderate1 | |
| Regular salmeterol & ICS versus ICSCates 2009a Follow‐up: mean 15 weeks | 5 per 1000 | 6 per 1000 (2 to 19) | OR 1.20 (0.37 to 3.91) | 1862 (5 studies) | ⊕⊕⊕⊝ moderate1 | |
| Regular formoterol versus regular salmeterolCates 2012b Follow‐up: 13 weeks |
13 per 1000 (on salmeterol) |
12 per 1000
(1 to 168) (on formoterol) |
OR 0.95 (0.06 to 15.33) | 156 (1 study) | ⊕⊕⊝⊝ low1,2 | Formoterol was considered the active treatment and salmeterol the control treatment for this comparison |
| Children with a non‐fatal serious adverse event related to asthma | ||||||
| Regular formoterol versus placeboCates 2012a Follow‐up: mean 27 weeks | 2 per 1000 | 8 per 1000 (4 to 18) | OR 4.06 (1.78 to 9.22) | 1335 (5 studies) | ⊕⊕⊕⊕ high | |
| Regular salmeterol versus placeboCates 2008 Follow‐up: mean 31 weeks | 33 per 1000 | 55 per 1000 (33 to 92) | OR 1.72 (1 to 2.98) | 1333 (5 studies) | ⊕⊕⊕⊕ high | |
| Regular formoterol & ICS versus ICSCates 2009b Follow‐up: mean 13 weeks | 4 per 1000 | 6 per 1000 (2 to 17) | OR 1.49 (0.48 to 4.61) | 2788 (7 studies) | ⊕⊕⊝⊝ low1,3 | |
| Regular salmeterol & ICS versus ICSCates 2009a Follow‐up: mean 15 weeks | 1 per 1000 | 1 per 1000 (0 to 17) | OR 0.99 (0.06 to 15.85) | 1862 (5 studies) | ⊕⊕⊝⊝ low1,3 | |
| *The basis for the assumed risk (was the mean control group risk across studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SAE: serious adverse event | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Confidence intervals include the possibility of an increase and a decrease in SAEs on regular LABA
2. Single unblinded study
3. Considerable heterogeneity between trial results
Description of included reviews
Our search of the CDSR retrieved 25 reviews. Figure 2 shows further details of the inclusion and exclusion process. Six Cochrane reviews on serious adverse events associated with LABA treatment in asthma were included:
2.
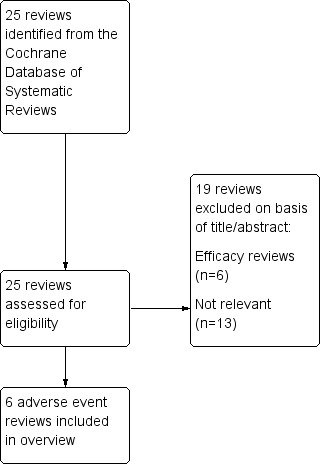
Review selection flow diagram.
Regular treatment with formoterol for chronic asthma: serious adverse events (Cates 2012a),
Regular treatment with salmeterol for chronic asthma: serious adverse events (Cates 2008),
Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events (Cates 2009b),
Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events (Cates 2009a)
Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events (Cates 2012b),
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events (Cates 2010).
The characteristics of the included reviews are summarised in Table 2. All the reviews used the same inclusion criteria (randomised controlled trials in patients of any age with a diagnosis of asthma) and outcome measures (all‐cause mortality, all‐cause non‐fatal serious adverse events, asthma‐related mortality and serious adverse events). The included studies were not restricted to products approved for children by the FDA. The definition of serious adverse events was uniform across the reviews (see Appendix 2). The latest search dates in the reviews ranged from 2008 to 2012. Our updated literature searches for each review found an additional two studies including 689 children (Li 2010; NCT01192178) meeting the inclusion criteria for Cates 2009a, and the results of these studies have been incorporated into this overview. We did not find any additional studies meeting the criteria for the other five reviews.
2. Characteristics of included reviews.
| Inclusion criteria | ||||||||
| Review title | Studies | Participants | Intervention | Comparison | Primary outcome measures | Date of search | No. included studies (all) | No. included studies (children only) |
| 1. Regular treatment with formoterol for chronic asthma: serious adverse events Cates 2012a |
Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled formoterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or short‐acting beta2‐agonists | All‐cause mortality All‐cause non‐fatal SAEs |
January 2012 | 22 | 5 |
| 2. Regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2008 | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled salmeterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or short‐acting beta2‐agonists | All‐cause mortality All‐cause non‐fatal SAEs |
August 2011 | 32 | 5 |
| 3. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events Cates 2009b | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled corticosteroids and formoterol once or twice/day; at least least 12 weeks duration; any dose; any single or separate device | Same dose and type of inhaled corticosteroids | All‐cause mortality All‐cause non‐fatal SAEs |
October 2008 | 21 | 7 |
| 4. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events Cates 2009a | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled corticosteroids and salmeterol once or twice/day; at least least 12 weeks duration; any dose; any single or separate device | Same dose and type of inhaled corticosteroids | All‐cause mortality All‐cause non‐fatal SAEs |
October 2008 | 33 | 3 (2 additional studies identified by updated search) |
| 5. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2012b | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled formoterol; at least 12 weeks duration; not randomised with inhaled corticosteroids | Inhaled salmeterol; at least 12 weeks duration; not randomised with inhaled corticosteroids | All‐cause mortality All‐cause non‐fatal SAEs |
January 2012 | 4 | 1 |
| 6. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events Cates 2010 | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled formoterol with an inhaled steroid; at least 12 weeks duration; any dose; any single or separate delivery device | Inhaled salmeterol with an inhaled steroid; at least 12 weeks duration; any dose; any single or separate delivery device | All‐cause mortality All‐cause non‐fatal SAEs |
August 2011 | 7 | 0 |
Including the new studies, there are a total of 21 studies on 7318 children in the first four reviews, and a single study on 156 children in the sixth review comparing regular formoterol with regular salmeterol. There were no studies found in children comparing formoterol and salmeterol combination therapy against each other. The studies in children from four to 17 years of age are from a range of settings and conducted between 1992 and 2010. Separate data from children above or below the age of 12 years were not available. The early studies primarily randomised children between monotherapy LABAs with or without ICS as background therapy. In later years studies standardised ICS treatment in control and intervention groups, perhaps in response to concerns over the use of LABAs without concurrent ICS. The characteristics of the included studies in children in each of the reviews are summarised in Table 3, Table 4, Table 5 , Table 6 and Table 7, respectively.
3. Regular formoterol v placebo trial details.
|
Duration (weeks) |
% children on ICS background Rx |
Formoterol 48 mcg/day (N) |
Formoterol 24 mcg/day (N) |
Formoterol 12 mcg/day (N) |
Placebo (N) | Brand | Age Ranges | |
| Bensch 2002 | 52 | 69 | 171 | 171 | 176 | Foradil | 5 to 12 | |
| Corren 2007 | 12 | 0 | 9 | 9 | Oxis | 6 to 11 | ||
| Levy 2005 | 12 | 72 | 127 | 122 | Foradil | 5 to 13 | ||
| Von Berg 2003 | 12 | 82 | 83 | 81 | 84 | Oxis | 6 to 17 | |
| Zimmerman 2004 | 12 | 100 | 94 | 105 | 101 | Oxis | 6 to 11 | |
| Total | mean = 27 weeks | 171 | 475 | 186 | 492 | |||
| All trials were sponsored by AstraZeneca or Novartis, and contributed data on all‐cause mortality and non‐fatal serious adverse events, except Levy 2005 for which mortality data was not available. | ||||||||
4. Regular salmeterol v placebo trial details.
|
Duration (weeks) |
% children on ICS background Rx | Salmeterol 100 mcg/day (N) | Salmeterol 50 mcg/day (N) | Placebo(N) | Age Ranges | |
| Russell 1995 | 12 | 100 | 99 | 107 | 4 to 16 | |
| Simons 1997 | 52 | 0 | 80 | 80 | 6 to 14 | |
| SLGA3014 | 12 | 50 | 109 | 115 | 110 | 4 to 11 |
| von Berg 1998 | 52 | 52 | 220 | 206 | 5 to 15 | |
| Weinstein 1998 | 12 | 57 | 102 | 105 | 4 to 11 | |
| TOTAL | mean =31 weeks | 610 | 115 | 608 | ||
| All trials were sponsored by GSK and contributed data on all‐cause mortality and non‐fatal serious adverse events | ||||||
5. Regular formoterol & ICS v ICS trial details.
| Children and Adolescents | Duration (weeks) | Formoterol and ICS (N) | ICS Alone (N) | Daily Dose Budesonide (mcg ) | Daily Dose Formoterol (mcg ) | Combined Inhaler | Age Ranges |
| Morice 2008 | 12 | 415 | 207 | 200 | 24 | ✓ | 6 to 11 |
| Pohunek 2006 | 12 | 417 | 213 | 400 | 24 | ✓ | 4 to 11 |
| SD‐039‐0714 | 12 | 136 | 134 | 400 | 12 | ✓ | 11 to 17 |
| SD‐039‐0718 | 12 | 128 | 145 | 200 | 24 | ✓ | 6 to 15 |
| SD‐039‐0719 | 26 | 123 | 63 | 400 | 24 | ✓ | 6 to 11 |
| SD‐039‐0725 | 12 | 352 | 169 | 200 | 12 or 24 | ✓ | 6 to 15 |
| Tal 2002 | 12 | 148 | 138 | 400 | 24 | ✓ | 4 to 17 |
| Total | mean = 13 weeks | 1,719 | 1,069 | ||||
| All trials were sponsored by AstraZeneca and contributed data on fatal and non‐fatal serious adverse events | |||||||
6. Regular salmeterol & ICS v ICS trial details.
| Duration (Weeks) | Salmeterol & ICS | ICS alone | Dose of Fluticasone mcg/day | Dose of Salmeterol mcg/day | Combined Inhaler | Age Ranges | |
| Li 2010 | 12 | 173 | 177 | 200 | 100 | ✓ | 4 to 11 |
| Malone 2005 | 12 | 101 | 102 | 200 | 100 | ✓ | 4 to 11 |
| NCT01192178 | 16 | 171 | 168 | 200 | 100 | ✓ | 4 to 11 |
| SAM40012 | 24 | 181 | 181 | 200 | 100 | ✓ | 4 to 11 |
| SAS30021 | 12 | 304 | 304 | 100 | 50 | ✓ | 4 to 11 |
| Total | mean = 15 weeks | 930 | 932 | ||||
| All trials were sponsored by GSK and contributed data on all‐cause mortality and non‐fatal serious adverse events | |||||||
7. Regular formoterol versus regular salmeterol.
| Duration (Weeks) | % children on ICS background Rx | Formoterol 24 mcg/day | Salmeterol 100 mcg/day | Formoterol device | Salemterol device | Sponsors | Age Ranges | |
| Everden 2004 | 12 | 100% | 80 | 76 | Oxis Turbohaler | Salmeterol Accuhaler | AstraZeneca | 6 to 17 |
Methodological quality of included reviews
Quality of the included reviews
The methods used in the reviews were assessed using the AMSTAR tool (Shea 2007). As all the included reviews were Cochrane reviews, they were conducted according to the rigorous methods in the Cochrane Handbook for Systematic Reviews of Interventions, and therefore the AMSTAR ratings were high (all achieved a score of at least 9 out of a possible 11). The review authors sought additional data from the manufacturers' websites and from FDA reports for each individual review to minimise publication bias.
Because one of the authors of this overview (CJC) is also the lead author of all the included reviews, the quality assessments were conducted by ES and another person not associated with the reviews (Susan Wieland). There was complete agreement between the assessors and our full quality assessment is summarised in Table 8.
8. AMSTAR ratings.
| AMSTAR Criteria | Cates 2008 | Cates 2012a | Cates 2009a | Cates 2009b | Cates 2012b | Cates 2010 |
| 1. Was an 'a priori' design provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2a. Was there duplicate study selection? (0.5 point) | Yes | Yes | Yes | Yes | Yes | No |
| 2b. Was there duplicate data extraction? (0.5 point) | No | No | Yes | Yes | Yes | No |
| 3. Was a comprehensive literature search performed? | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | No | No | No | No | No | No |
| 5. Was a list of studies (included and excluded) provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Were the characteristics of the included studies provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was the scientific quality of the included studies assessed and documented? | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the methods used to combine the findings of studies appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Was the likelihood of publication bias assessed? | Yes | Yes | Yes | Yes | Not applicable | Not applicable |
| 11. Was the conflict of interest stated? | Yes | Yes | Yes | Yes | Yes | Yes |
| Total criteria met: | 10.5 | 10.5 | 11 | 11 | 10 | 9 |
|
(item 4 is met with the assessment 'NO', all others 'YES') |
||||||
| Note: we felt that item 2 was 2 separate questions, so we split it into two parts and awarded half a point for each. This differs from the published version of the tool. | ||||||
Risk of bias of the included studies in each review
Each review assessed the risk of bias for the included studies relating to children suffering an all‐cause serious adverse event (SAE) and asthma‐related SAE, and the results are summarised in Table 9. Although reporting of sequence generation and allocation concealment was patchy in the trial reports, discussion with the trial sponsors indicated that standard procedures adopted in the trials would lead to a low risk of selection bias. The included studies were also all double‐blind in design (with the exception of one study from Cates 2009b and the single trial comparing formoterol with salmeterol in Cates 2012b, as shown in Table 9). Complete SAE outcome data were obtained with the exception of mortality data from a single study in Cates 2012a. We have summarised the assessments of the risks of bias in the included studies in each review in Table 9, The primary outcome results were not downgraded due to risks of bias in any of the reviews.
9. Risks of Bias for the included trials in each Cochrane review.
| Cochrane review | trial ID | sequence generation | allocation concealment | blinding | incomplete outcome data | selective reporting |
| "Regular tretatment with formoterol for chronic asthma: SAE" Cates 2012a | Bensh 2002 | unclear | unclear | low | low | low |
| Levy 2005 | unclear | unclear | low | low | unclear1 | |
| von Berg 2003 | low | unclear | low | low | low | |
| Zimmenman 2004 | unclear | unclear | low | unclear | low | |
| Corren 2002 | low | unclear | low | unclear | low | |
| "regular treatment with salmeterol for chronic asthma: SAE" Cates 2008 | Lenny 1995a | n/a | unclear | low | n/a | low |
| Lenny 1995b | n/a | unclear | low | n/a | low | |
| Russel 1995 | n/a | unclear | low | n/a | low | |
| Simons 1997 | n/a | unclear | low | n/a | low | |
| SLGA 3014 | n/a | unclear | low | n/a | low | |
| von Berg 1998 | n/a | unclear | low | n/a | low | |
| Weinstein 1998 | n/a | unclear | low | n/a | low | |
| "Regular treatment with formoterol and ICS for chronic asthma: SAE" Cates 2009b | Morice 2008 | low | unclear | low | low | low |
| Pohunek 2006 | unclear | unclear | low | low | low | |
| SD‐039‐0714 | unclear | unclear | low | low | low | |
| SD‐039‐0718 | low | unclear | low | low | low | |
| SD‐039‐0719 | unclear | unclear | high | low | low | |
| SD‐039‐0725 | unclear | unclear | low | low | low | |
| Tal 2002 | low | unclear | low | low | low | |
|
"Regular treatment with salmeterol and ICS for chronic asthma: SAE" Cates 2009a |
Li 2010 | unclear | unclear | low | low | low |
| Malone 2005 | unclear | unclear | low | low | low | |
| NCT01192178 | unclear | unclear | low | low | low | |
| SAM40012 | unclear | unclear | low | low | low | |
| SAS30021 | unclear | unclear | low | low | low | |
| "Regular treatment with formoterol versus regular treatment with satmeterol for chronic asthma: SAE" Cates 2012b | Everden 2004 | low | unclear | high | low | low |
1. No mortality data was available from this trial
There was, however, no independent assessment of the causation of SAEs in any of the studies. This means that the trials were not clearly protected from ascertainment bias for asthma‐related events. Even with double‐blinding, if the threshold was high for assessing any SAE as being asthma‐related across all the participants in a trial, this could reduce the numbers of events deemed to be asthma‐related and introduce bias by reducing the apparent difference between the groups for asthma‐related events.
Effect of interventions
Mortality
There was only one death in a child across all the reviews. Correspondence with the trialist confirmed that the child concerned died from a sub‐arachnoid haemorrhage whilst taking formoterol monotherapy (Cates 2012a.
All‐cause serious adverse events (SAE)
How does regular formoterol compare with placebo?
The review comparing regular formoterol with placebo (Cates 2012a), in five trials including 1335 children, showed a significant increase in the odds ratio (OR) of children suffering an SAE of any cause (Peto OR 2.48; 95% CI 1.27 to 4.83, I2 = 0% as shown in the top forest plot in Figure 3). We did not downgrade this result in the 'Summary of findings' table (high quality evidence; see Table 1).
3.
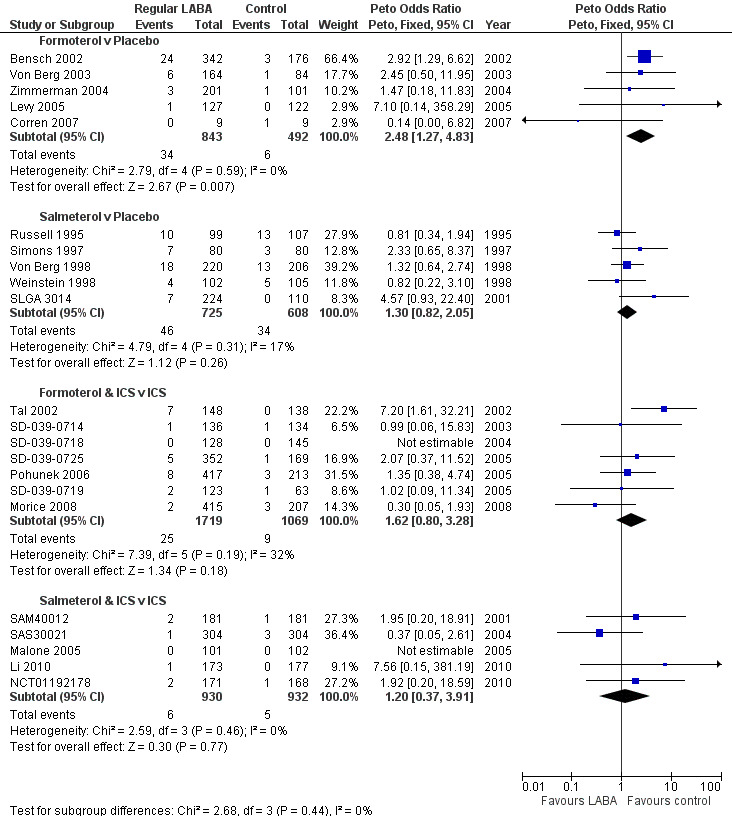
Children with all‐cause SAEs compared using Peto ORs
There might be differences between the ORs from different brands and doses of formoterol (see Table 3) as the differences between the formulation for each product means that we cannot assume that they all have the same safety profiles, so these have been shown as separate subgroups in Figure 4. The test for subgroup differences was not significant (Chi² = 1.18, df = 3 (P = 0.76), I² = 0%), so although the ORs in the Foradil trials were numerically larger than in the Oxis trials, the difference between the Foradil and Oxis trials was not statistically significant. However, we cannot infer that the safety of Foradil and Oxis is equivalent as the CIs were too wide to draw such conclusions.
4.
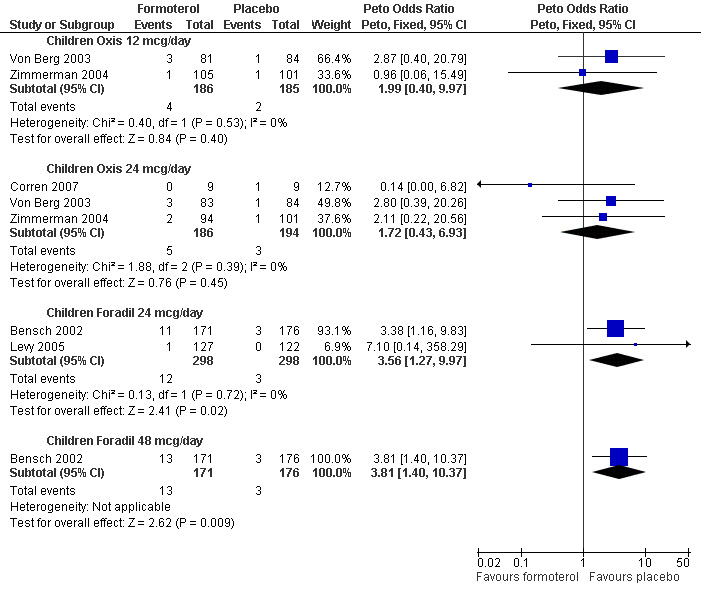
Children with an all‐cause SAE: formoterol versus placebo subgrouped by brand and dose
There was much more heterogeneity when risk differences were used to combine the trial results (I2 = 55%). The pooled risk difference using a fixed‐effect model was an increase of 26 children per 1000 over 27 weeks (95% CI 9 more to 42 more per 1000), whilst a random‐effects model which incorporates the heterogeneity had a wider CI and showed an increase of 20 children per 1000 over 27 weeks (95% CI 3 fewer to 43 more per 1000) which was not statistically significant. The pooled risk differences analysed with a random‐effects model are shown in Table 10.
10. Risk differences for children with SAE of any cause.
| Children with an all‐cause SAE (pooled risk differences, M‐H Random) | |||||
| Formoterol monotherapy | Placebo | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 34 | 843 | 6 | 492 | 0.0195 (‐0.0034, 0.0425) | 55% |
| Salmeterol monotherapy | Placebo | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 46 | 725 | 34 | 608 | 0.0225 (0.0023, 0.0426) | 0% |
| Formoterol combination therapy | ICS | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 25 | 1719 | 9 | 1069 | 0.0034 (‐0.0062, 0.0131) | 34% |
| Salmeterol combination therapy | ICS | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 6 | 930 | 5 | 932 | 0.0008 (‐0.0067, 0.0082) | 0% |
How does regular salmeterol compare with placebo?
The review comparing regular salmeterol with placebo (Cates 2008), in five trials including 1333 children, found an increase in the OR of children suffering an SAE of any cause that was not statistically significant (Peto OR 1.30; 95% CI 0.82 to 2.05, I2 = 17%, as shown in the second forest plot in Figure 3). We downgraded this result to moderate quality as the CI included the possibility of both increased and decreased odds of an SAE. These trials were all carried out in an earlier time‐period than any of the others (1992 to 2001) and had a notably higher rate of children with an SAE than the trials in any of the other reviews, as shown in Table 11. The higher placebo arm event rates combined with a lower OR means that the pooled risk differences from the trials in children on salmeterol and formoterol were almost identical (see Table 10).
11. Mean event rates in control arms of included trials (SAE of any cause).
| Comparison | Children with an event (n) | Total number of children (N) | SAE per 10,000 children (95% CI) | Mean duration of trials (weeks) | SAE per 10,000 children per week (95% CI) |
| Formoterol v Placebo | 6 | 492 | 122 (56 to 263) | 27 | 5 (2 to 10) |
| Salmeterol v Placebo | 34 | 608 | 559 (403 to 771) | 31 | 18 (13 to 25) |
| Formoterol & ICS v ICS | 9 | 1069 | 84 (44 to 159) | 13 | 6 (3 to 12) |
| Salmeterol & ICS v ICS | 5 | 932 | 54 (23 to 125) | 15 | 4 (2 to 8) |
Almost all the children were given the same dose of salmeterol (50 µg twice daily, see Table 4), so no subgrouping by dose was attempted for these trials.
How does combination therapy with regular formoterol and ICS compare with the same dose of ICS?
The review comparing regular formoterol in combination with ICS versus the same dose of ICS (Cates 2009b), in seven trials on 2788 children, also found an increase in the OR of children suffering an SAE of any cause that was not statistically significant (Peto OR 1.60; 95% CI 0.80 to 3.28, I2 = 32%, as shown in the third forest plot in Figure 3). We downgraded this result to moderate quality as the CI included the possibility of both increased and decreased odds of an SAE. The heterogeneity found in this set of trials appeared to arise from the earliest trial, Tal 2002, which showed a large increase in children with an SAE of any cause on regular formoterol and ICS. All the trials used a single combination inhaler to deliver formoterol and ICS, and the summary of the characteristics of the studies (Table 5) does not highlight any obvious differences between Tal 2002 and the other studies, so the heterogeneity was unexplained.
In contrast to the placebo controlled review results, the pooled risk difference was smaller in this review showing an increase of 3 per 1000 over 13 weeks (95% CI 6 fewer to 13 more) as shown in Table 10. A possible explanation for the risk differences from this review being smaller than in the monotherapy review, whilst the ORs are similar, is that the risk of an SAE of any cause in the control groups given ICS was much smaller than in the placebo arms of the previous reviews (see Table 11). This may be partly explained by the shorter duration of the trials (average 13 weeks) than in the placebo controlled trials (average 27 weeks) or, possibly, a protective effect of ICS.
How does combination therapy with regular salmeterol and ICS compare with the same dose of ICS?
The review comparing regular salmeterol in combination with ICS versus the same dose of ICS (Cates 2009a), in five trials on 1862 children, also found an increase in the OR of children suffering an SAE of any cause which was not statistically significant (Peto OR 1.20; 95% CI 0.37 to 2.91, I2 = 0%, as shown in the fourth forest plot in Figure 3). We downgraded this result to moderate quality as the CI included the possibility of both increased and decreased odds of an SAE.
In keeping with the combination therapy results from the previous review, the risk differences were very small with an increase of one per 1000 over 15 weeks (95% CI 7 fewer to 8 more) as shown in the bottom section of Table 10. Again the risk of having an SAE on the control ICS arm was lower than in the placebo arms of the monotherapy trials (see Table 11).
Is treatment with regular LABA safer when used in combination with regular ICS treatment?
To address this question, Figure 5 shows the ORs from the trials subgrouped into trials with monotherapy and placebo comparisons from the first two reviews and then trials in which LABA was given in combination with ICS (in a single inhaler) and compared to the same dose of ICS from the third and fourth reviews. We used these subgroups to indirectly compare the results of the monotherapy and combination therapy trials.
5.
Interaction between randomised use of ICS and children with all‐cause SAE on regular LABA using ORs
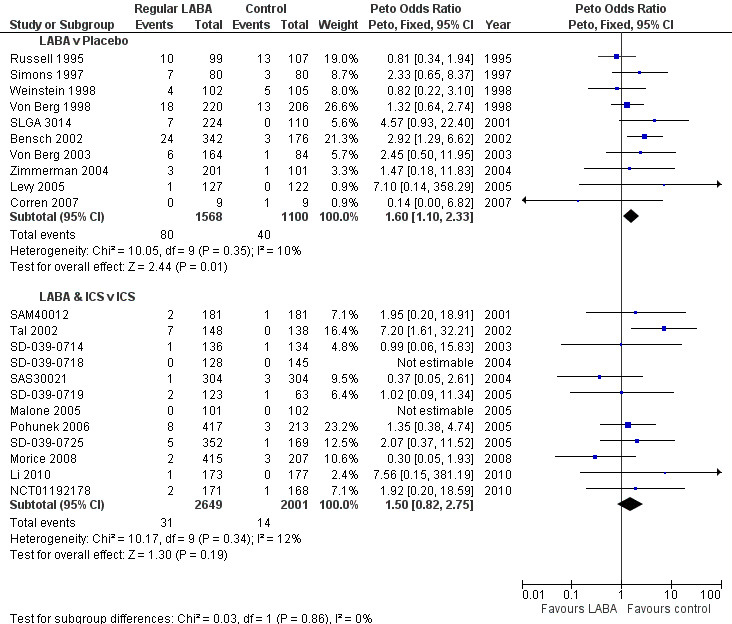
There were more children with an all‐cause SAE on LABA monotherapy compared to those children on placebo and the difference was statistically significant (Peto OR 1.60; 95% CI 1.10 to 2.33, 10 studies, 2668 children; Figure 5). The comparison between children on combination therapy compared with children on ICS showed a very similar OR that was not statistically significant (Peto OR 1.50; 95% CI 0.82 to 2.75, 12 studies, 4650 children; Figure 5). However, the test of statistical significance for each subgroup on its own cannot be used to compare the relative safety of monotherapy and combination therapy trials (Altman 2003). When the results were subgrouped in this way, a statistical test for the difference between subgroups gives an indication as to whether combination therapy is safer than monotherapy. When the trials were analysed using Peto ORs there was no significant test for interaction (Chi2 = 0.03, df = 1, P = 0.86) between monotherapy (Peto OR 1.60; 95% CI 1.10 to 2.33) and combination therapy (Peto OR 1.50; 95% CI 0.82 to 2.75; Figure 5).
However, there was a marked difference in the proportion of children with an SAE in the placebo arms of the monotherapy trials and in the ICS arms of the combination therapy trials (see Table 11). We therefore converted the Peto OR into an absolute difference using Visual Rx 2012. The OR and its 95% CI were applied to the baseline risk from the trials of LABA monotherapy (3.6% over 29 weeks). The Cates plot in Figure 6 demonstrated that for every 1000 children treated with placebo over a 29 week period, there were 36 who suffered from an SAE (shown as red faces). In contrast, if all 1000 had been treated with regular LABA monotherapy this would have resulted in 57 children suffering an adverse event (95% CI 40 to 81 children with an SAE). So for every 1000 children given regular LABA monotherapy for 27 weeks, there were 21 more who suffered an SAE (95% CI 4 to 45 more), and these are shown as crossed‐out green faces in Figure 6.
6.
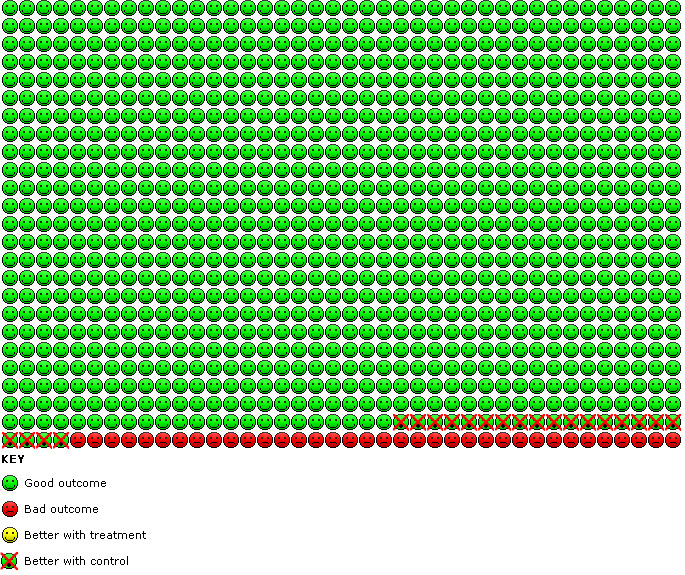
Cates plot of monotherapy versus placebo trials: In the placebo group 36 people out of 1000 had non‐fatal serious adverse events of any cause over 29 weeks, compared to 57 (95% CI 40 to 81) out of 1000 for the LABA monotherapy group. The crossed‐out faces show that there were 21 additional children suffering an SAE for every 1000 treated with LABA monotherapy.
In the same way, we converted the Peto OR from the combination therapy trials using the lower baseline risk for children on ICS (0.7% over 14 weeks). The Cates plot in Figure 7 demonstrated that for every 1000 children treated with placebo over a 29 week period, there were 7 who suffered from an SAE. In contrast, if all 1000 had been treated with regular LABA monotherapy this would have resulted in 10 children suffering an adverse event (95% CI 6 to 19 children with an SAE). So for every 1000 children given regular LABA monotherapy for 14 weeks, there were 3 more who suffered an SAE (95% CI 1 less to 12 more). So in absolute terms, the impact of LABA on the risk of an SAE in the combination therapy trials is much smaller than in the monotherapy trials.
7.
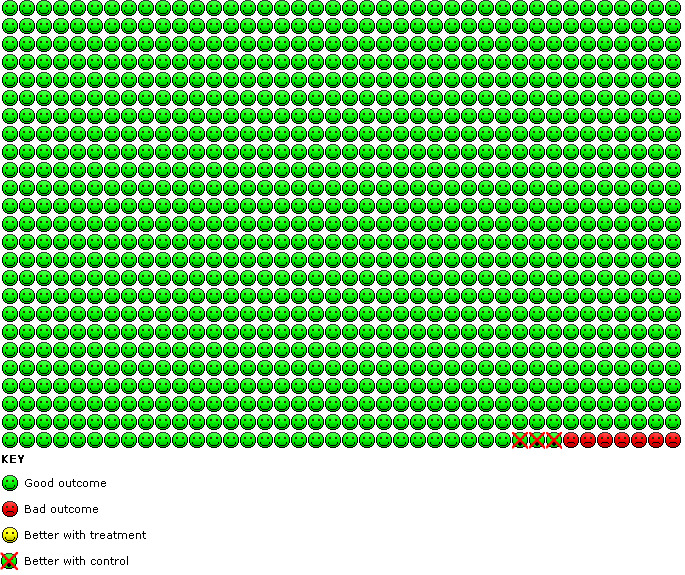
Cates plot of combination therapy versus ICS trials: In the ICS group 7 people out of 1000 had non‐fatal serious adverse events of any cause over 14 weeks, compared to 10 (95% CI 6 to 19) out of 1000 for the combination therapy group. The crossed‐out faces show that there were 3 additional children suffering an SAE for every 1000 treated with LABA combination therapy.
This comparison between the subgroups of trials using monotherapy and combination therapy was an indirect comparison and needs to be interpreted cautiously. The risks of suffering an SAE in the control arms of the trials in each review was not uniform (Table 11), and we do not know whether the lower risks on regular ICS reflected differences in the study design, behaviour of the children in the trials or whether the lower risks were due to the presence of the ICS treatment as part of the study medication. Therefore, although the risk differences in the combination therapy trials were smaller than in the monotherapy trials (Table 12), we cannot be sure that this was due to the ICS given to all the children.
12. Monotherapy versus combination therapy risk differences for children with SAE of any cause.
| Children with an all‐cause SAE (pooled risk differences, M‐H Random) | |||||
| LABA monotherapy | Placebo | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 80 | 1568 | 40 | 1100 | 0.0191 (0.0061, 0.0321) | 15% |
| LABA combination therapy | ICS | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 31 | 2649 | 14 | 2001 | 0.0017 (‐0.0037, 0.0070) | 2% |
Is there a difference in safety between regular salmeterol and regular formoterol?
The final two reviews (Cates 2010 and Cates 2012b) looked for evidence from trials that randomised children to receive either regular formoterol or salmeterol (with or without combination ICS). Between the two reviews there was only a single open trial (Everden 2004) in 156 children comparing monotherapy with formoterol to salmeterol, and in this trial one child in each arm suffered an SAE (neither of which was asthma‐related). This was not sufficient evidence to draw any conclusions about the relative safety of regular formoterol and salmeterol, as demonstrated by the very wide CI (OR 0.95; 95% CI 0.06 to 15.36) for Everden 2004 (Figure 8). There were no trials making direct comparisons between combination inhalers in children.
8.

Indirect comparison of formoterol and budesonide with salmeterol and fluticasone
Indirect comparisons can be made by contrasting the pooled results of the trials which compared formoterol to placebo with the pooled results of the trials which compared salmeterol with placebo (as shown in Figure 1A). These were not randomised comparisons and were based on the assumption that the trials on formoterol were sufficiently similar to those on salmeterol in terms of participants, outcome assessment, co‐interventions etc. In this instance the wide discrepancies between the event rates in the control arms of the placebo controlled trials (Table 11) suggested that there were important differences between the groups of trials. Moreover, the trials on formoterol monotherapy used a wide variety of doses and formulations, so there was also considerable clinical heterogeneity within the formoterol monotherapy trials. We therefore decided not to carry out an indirect comparison of the placebo controlled trials on formoterol and salmeterol.
The combination therapy trials comparing formoterol and budesonide (BDF) with the same dose of budesonide showed a similar enough control arm event rate to the trials comparing salmeterol and fluticasone (FPS) with the same dose of fluticasone (Table 11), so an indirect comparison was made between these sets of trials. The indirect comparison in which the log OR of the pooled FPS versus fluticasone results was subtracted from the log OR of the pooled BDF versus budesonide results is shown in the second line of Figure 8. The indirect OR of the comparative impact of BDF to FPS on children with an SAE of any cause was 1.35 (95% CI 0.34 to 5.34). Even with the addition of indirect comparisons to the direct comparison, the CI remained wide (OR 1.26; 95% CI 0.37 to 4.32; Figure 8) so we are still very uncertain about the comparative safety of formoterol and salmeterol in children.
Asthma‐related serious adverse events (SAE)
The findings of the systematic reviews in relation to asthma‐related SAEs were in line with the results of all‐cause events described above (Table 1).
The reviews showed significant increases in the Peto OR for asthma‐related SAEs with formoterol versus placebo (Peto OR 4.06; 95% CI 1.78 to 9.22, I2 = 0%; Figure 9) and salmeterol versus placebo (Peto OR 1.72; 95% CI 1.00 to 2.98, I2 = 0%; Figure 9). Together the pooled OR from all the monotherapy trials showed a significant increase (Peto OR 2.24; 95% CI 1.42 to 3.54, I2 = 0%; Figure 10).
9.
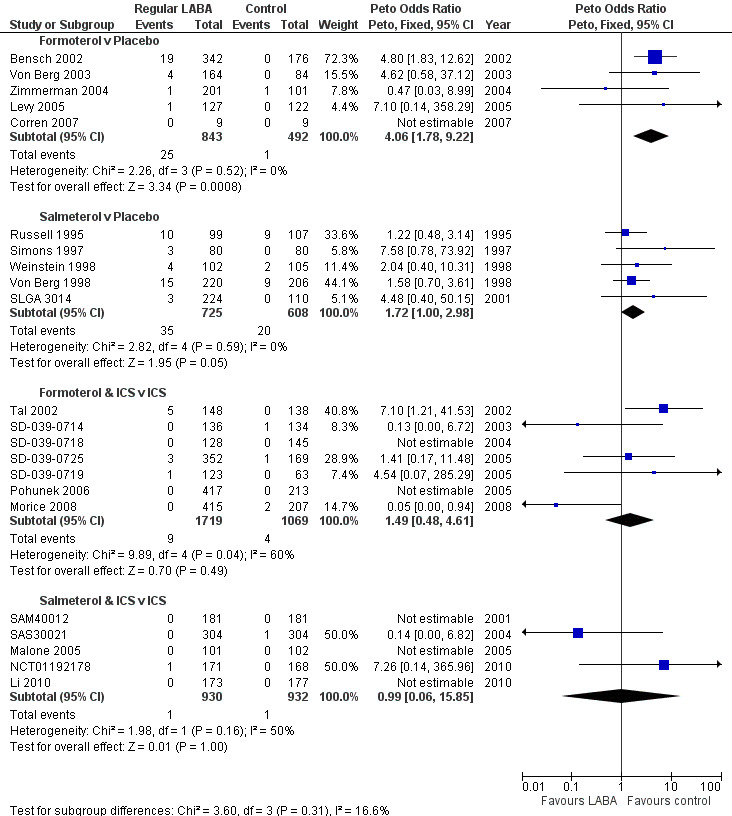
Children with asthma‐related SAE compared using Peto OR
10.
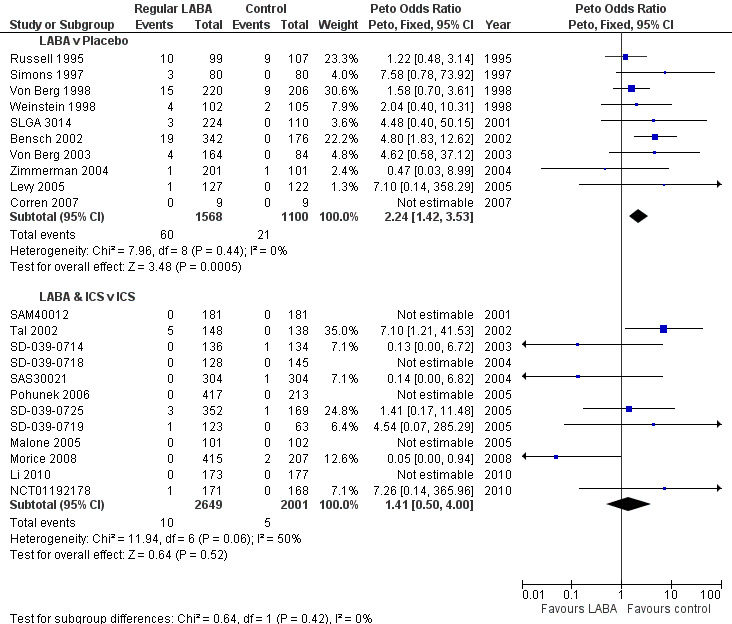
Interaction between randomised use of ICS and children with asthma‐related SAE on regular LABA using ORs
For the reviews of combination therapy the pooled ORs had more heterogeneity and wider CIs than the monotherapy results. Forest plots for formoterol combination therapy versus ICS (Peto OR 1.49; 95% CI 0.48 to 4.61, I2 = 60%) and salmeterol combination therapy versus ICS (Peto OR 0.99; 95% CI 0.06 to 15.85, I2 = 50%) are shown in Figure 9. The pooled results from all the combination therapy results showed a lower OR than for monotherapy (Peto OR 1.41; 95% CI 0.50 to 4.00, I2 = 50%) as shown in Figure 10. However the pooled odds ratio from the combination therapy trials was not significantly different from the monotherapy reviews (test for subgroup differences: Chi2 = 0.64, df = 1, P = 0.42; Figure 10).
Analysed as risk differences, the increased risk was of the order of 19 additional children with an asthma‐related SAE for every 1000 treated with LABA monotherapy (Table 13). These risk differences were very similar to those found for children with SAE of any cause in Table 10. The risk differences on combination therapy were lower than for monotherapy, at 0.3 less per 1000 (95% CI ‐4 to 3 per 1000), see Table 14. Again, this was very much in line with the results found for all‐cause SAEs, and there were fewer children with an asthma‐related SAE on ICS than on placebo, as shown in Table 15.
13. Risk differences for children with SAE related to asthma.
| Children with an asthma related SAE (pooled risk differences, M‐H Random) | |||||
| Formoterol monotherapy | Placebo | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 25 | 843 | 1 | 492 | 0.0196 (‐0.0071, 0.0463) | 75% |
| Salmeterol monotherapy | Placebo | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 35 | 725 | 20 | 608 | 0.0185 (0.0027, 0.0343) | 0% |
| Formoterol combination therapy | ICS | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 9 | 1719 | 4 | 1069 | 0.0000 (‐0.0064, 0.0064) | 19% |
| Salmeterol combination therapy | ICS | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 1 | 930 | 1 | 932 | ‐0.0005 (‐0.0058, 0.0048) | 0% |
14. Monotherapy versus combination therapy risk differences for children with SAE related to asthma.
| Children with an asthma related SAE (pooled risk differences, M‐H Random) | |||||
| LABA monotherapy | Placebo | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 60 | 1568 | 21 | 1100 | 0.0197 (0.0055, 0.0339) | 44% |
| LABA combination therapy | ICS | Risk Difference (95% CI) | Heterogeneity (I‐squared) | ||
| Children with SAE | Total | Children with SAE | Total | ||
| 10 | 2649 | 5 | 2001 | ‐0.0003 (‐0.0040, 0.0034) | 0% |
15. Mean event rates in control arms of included trials (SAE related to asthma).
| Comparison | Children with an event (n) | Total number of children (N) | SAE per 10,000 children (95% CI) | Mean duration of trials (weeks) | SAE per 10,000 children per week (95% CI) |
| Formoterol v Placebo | 1 | 492 | 20 (4 to 114) | 27 | 1 (0 to 4) |
| Salmeterol v Placebo | 20 | 608 | 329 (214 to 503) | 31 | 11 (7 to 16) |
| Formoterol & ICS v ICS | 4 | 1069 | 37 (15 to 96) | 13 | 3 (1 to 7) |
| Salmeterol & ICS v ICS | 1 | 932 | 11 (2 to 61) | 15 | 1 (0 to 4) |
We have not made indirect comparisons between formoterol and salmeterol for asthma‐related events as none of the trials had independent assessment of the causation of SAEs, and there was considerable heterogeneity in the results of the combination therapy trials.
Discussion
Summary of main results
We found six reviews including 22 randomised trials, on a total of 7474 children, of regular LABA monotherapy or combination therapy. This is a much smaller number than the equivalent trials in adults (65,000 adults), and there is insufficient evidence to assess whether there is any impact of regular formoterol or salmeterol combination therapy on mortality in children. In particular, we cannot rule out the increased asthma mortality risk identified in adults on salmeterol monotherapy.
We have created a new 'Summary of findings' table for this overview in relation to the primary outcome of children suffering an SAE of any cause (see upper half of Table 1). This shows a statistically significant increase in the odds of suffering a SAE on formoterol monotherapy (Peto OR 2.48; 95% CI 1.27 to 4.83, I2 = 0%, 5 trials, N = 1335) and smaller increases in odds, which are not statistically significant, for salmeterol monotherapy (Peto OR 1.30; 95% CI 0.82 to 2.05, I2 = 17%, 5 trials, N = 1333), formoterol combination therapy (Peto OR 1.60; 95% CI 0.80 to 3.28, I2 = 32%, 7 trials, N = 2788) and salmeterol combination therapy (Peto OR 1.20; 95% CI 0.37 to 2.91, I2 = 0%, 5 trials, N = 1862). Similarly, a summary of the results for children suffering an SAE related to asthma in each review are summarised in the lower half of Table 1. The results are very similar to those for an SAE of any cause.
We made indirect comparisons between the pooled results of the monotherapy and combination therapy trials (versus placebo and ICS respectively). There was no significant difference between the pooled odds ratios of children with a serious adverse event from LABA monotherapy and combination trials (Figure 3). There was an absolute increase in risk of an additional 21 children (95% CI 4 to 45) suffering such an SAE of any cause for every 1000 children treated over six months with either regular formoterol or salmeterol monotherapy (Figure 6), whilst for combination therapy the increased risk was three children (95% CI 1 fewer to 12 more) per 1000 over three months (Figure 7).
The absolute increase in the monotherapy trials in children was larger than that found in the equivalent trials in adults from the same Cochrane reviews (of around four per 1000 over a similar time period).
The absolute increases in children with an asthma‐related serious adverse event on LABA monotherapy or combination therapy are very similar in size to the increase for all‐cause events (Table 10; Table 13).
We combined direct estimates from a monotherapy comparison study with indirect comparisons from studies comparing formoterol and salmeterol in combination with ICS against ICS alone. We elected not to make indirect comparisons between the results of the placebo controlled formoterol and salmeterol monotherapy studies due to systematic differences in the control group risks of SAEs. We explore possible reasons for this below in Potential biases in the overview process. Even with the combined direct and indirect comparisons from these reviews, it is not possible to decide whether or not there is a difference in safety between regular formoterol and regular salmeterol.
Overall completeness and applicability of evidence
The key question for people making decisions about treating asthma in children is how each individual child will respond to different treatment regimens. In some instances immediate symptom relief can act as a guide to management, but for each child the balance between the longer‐term risks and benefits of treatment are unknown. The risk of asthma exacerbations, hospitalisation or death cannot be judged from the symptomatic impact of treatment for an individual child in the short‐term. Evidence from systematic reviews of randomised trials on large populations of children over a prolonged period of time is needed to assess such risks and potentially allow the patient or family to balance potential risks and benefits of treatment.
The number of children who have been studied in randomised trials of regular treatment with formoterol or salmeterol is much smaller than the numbers of adults (7463 children and 65,000 adults respectively). Although many of the existing trials in adults also recruited adolescents, down to 12 years of age, no separate results have yet been published in trial reports for the adolescent age‐group.
None of the studies recruited children younger than four years of age, so we have no safety information for LABA treatment in children with asthma who are less than four years old.
Chowdhury 2011 highlights a number of on‐going safety trials of combination therapy with regular LABA and ICS, which have been made a requirement by the FDA. Four of these trials will each aim to recruit 11,700 adults and adolescents over 12 years of age. These trials will last for six months and will study budesonide and formoterol (NCT01444430), mometasone and formoterol (NCT01471340), fluticasone and salmeterol (NCT01475721), and Foradil. It has been stipulated that 10% of participants recruited to these trials must be under 18 years of age and we believe that it is important that data from the adolescent population are reported separately. There will be a further trial in 6200 children aged 4 to 11 years on fluticasone and salmeterol (NCT01462344).
These trials will potentially contribute 10,000 children and adolescents (up to 18 years of age) to the results of this overview and should help clarify the risks of salmeterol combination therapy in children and LABA combination therapy in adolescents. They are expected to be completed in 2016 to 2017.
Quality of the evidence
All the included reviews were Cochrane reviews and judged to be of good quality with high AMSTAR scores. The quality of individual studies was assessed in the reviews using the Cochrane risk of bias tool. Although sequence generation and the method of allocation concealment were not clearly reported in most of the trials in the reviews, we judged that there was low risk of selection bias as all the trials were sponsored by the manufacturers and used standard methods designed for regulatory purposes. Almost all the trials were double‐blind in design, and the reviews included data on mortality and non‐fatal serious adverse events (SAEs) from all the trials (with a single exception as shown in Table 3). The reviews sought data from manufacturers' websites and FDA reports. The review results therefore were not downgraded due to risks of bias in the included trials.
We chose all‐cause SAEs as the primary outcome for this overview because ascertainment bias is a concern for the asthma‐related events. Even in double‐blind trials, if there is a high threshold for labelling events as being asthma‐related, this could lead to an underestimation of the true effect of treatment on such events. Moreover a patient with an SAE may have this recorded under more than one category (leading to double‐counting of individual patients) whereas data on the number of participants with at least one DAE of any cause is more reliably available from the manufacturers' trial reports on their websites.
Potential biases in the overview process
This overview has found that the absolute increase in the risk of children suffering an SAE is smaller on LABA combination therapy (compared to ICS alone) than on LABA monotherapy (compared to placebo). Whilst it is tempting to think that this difference is caused by the presence of ICS treatment in the combination inhaler, it is important to recognise that this is not necessarily the case.
The comparison between the results of the monotherapy and combination therapy reviews is an indirect observational comparison and is not protected from bias by the randomisation that was carried out in the individual trials. There may have been other differences between the monotherapy and combination therapy trials, and to investigate this further we looked at the duration of the trials and their respective control arm event rates. The results are shown in Table 11 and Table 15, which show that the combination therapy trials were carried out over an average period of three months, in comparison to six months for the monotherapy trials.
Absolute differences are likely to be dependent on the duration of the trials and are expected to be larger for trials of longer duration. This means that even if the odds ratios (ORs) were actually the same for LABA combination therapy versus ICS, and LABA monotherapy versus placebo, the risk differences would be expected to be twice as large for the monotherapy trials because they lasted twice as long.
Furthermore, after adjusting for trial duration, Table 11 and Table 15 still show much higher weekly event rates in the placebo arms of the trials in the salmeterol monotherapy review than in any of the other reviews. This is suggestive of other differences between the salmeterol monotherapy trials and the rest of the included trials (such as asthma severity, co‐interventions, outcome ascertainment and the level of supervision of trial participants).
We therefore remain uncertain whether the lower risk differences in the combination therapy trials (compared to the monotherapy trials) were due to the presence of ICS in the combination inhaler or other confounding factors (such as those listed above).
Finally, we are unable to assess the relevance of the background ICS treatment given to more than half the children in the monotherapy trials because we have no information about whether the individual children who suffered an SAE were prescribed or actually taking ICS, or not.
Agreements and disagreements with other studies or reviews
There is insufficient information from this overview to come to any conclusions in relation to the risks of mortality in children on regular formoterol or salmeterol. In particular, we do not know whether children on regular formoterol or salmeterol (as either monotherapy or combination therapy) might be exposed to the risk of increased mortality of one per 1000 asthma deaths over 28 weeks on regular salmeterol monotherapy that was found in adults (Cates 2008).
The FDA have reviewed individual patient data obtained from the sponsors of all randomised controlled trials of LABA formulations that are approved in the United States for asthma. They were able to break down the results by age‐group and by the use of concomitant or assigned inhaled corticosteroids (ICS) (McMahon 2011). The outcome measure used was a composite of asthma‐related mortality, hospitalisation or intubation. The FDA review found a higher incidence difference in children than adults overall. The majority of these children included in the composite measure were hospitalised for asthma. The increased incidence of this composite outcome for children aged 4 to 11 years of age was 30.4 (95% CI 5.7 to 55.1) per 1000 patient‐years. This result is in keeping with the monotherapy asthma‐related SAE findings from this overview, showing an increase of 20 children (95% CI 6 to 34) per 1000 over six months.
There was a significant age‐related trend when the results of participants with concomitant (background) ICS treatment were analysed, with the highest incidence difference in the 4 to 11 age‐group of 48.54 (95% CI 7.2 to 89.7) per 1000 patient‐years. There were fewer children in trials assigned to ICS treatment (in other words ICS given as part of the randomised treatment regimen) and no significant trend for age was found in this case.
Authors' conclusions
Implications for practice.
We do not know if regular combination therapy with formoterol or salmeterol in children alters the risk of dying from asthma.
Monotherapy with regular formoterol or salmeterol is no longer advocated in clinical guidelines. If separate inhalers are used to deliver LABA and ICS, this runs the risk of children defaulting on their ICS treatment whilst continuing to take LABA.
Regular combination therapy is likely to be safer than monotherapy in children with asthma, but we cannot say that combination therapy is risk free. There are probably an additional three children per 1000 over three months who suffer a non‐fatal serious adverse events on combination therapy in comparison to ICS. This is currently our best estimate of the risk of using LABA combination therapy in children and has to be balanced against the symptomatic benefit obtained for each child.
The relative safety of formoterol and salmeterol remains unclear, even when direct and indirect evidence is combined.
Implications for research.
Large surveillance trials of combination therapy in adults and children have been mandated by the FDA. The safety results of regular salmeterol and fluticasone combination therapy in children with asthma from these trials are awaited. The adult trials will also contain at least 10% of participants who are adolescents under 18 years of age, so safety data on both salmeterol and formoterol combination therapy will be available for these adolescents.
What's new
| Date | Event | Description |
|---|---|---|
| 3 March 2014 | Amended | Funder acknowledgement added |
Acknowledgements
We are grateful to Lorne Becker for advice on the review. We would like to thank Susan Wieland for her assistance with quality assessing the included Cochrane reviews and composing the plain language summary. Toby Lasserson, Francine Ducharme and Emma Welsh all provided helpful editorial advice.
Appendices
Appendix 1. Cochrane Library search strategy
#1 MeSH descriptor Asthma explode all trees #2 (asthma*):ti,ab,kw #3 (#1 OR #2) #4 (formoterol):ti,ab,kw #5(salmeterol):ti,ab,kw #6 MeSH descriptor Adrenergic beta‐2 Receptor Agonists explode all trees #7 LABA:ti,ab #8 ((long‐acting or "long acting") NEAR/3 beta*):ti #9 (#4 OR #5 OR #6 OR #7 OR #8) #10 (#2 AND #9)
[Restrict to Cochrane Database of Systematic Reviews]
Appendix 2. Definition of serious adverse events
The Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) define serious adverse events as follows (ICH E2A 1995):
"A serious adverse event (experience) or reaction is any untoward medical occurrence that at any dose:
results in death,
is life‐threatening,
requires inpatient hospitalization or prolongation of existing hospitalization,
results in persistent or significant disability/incapacity, or
is a congenital anomaly/birth defect.
NOTE: The term "life‐threatening" in the definition of "serious" refers to an event in which the patient was at risk of death at the time of the event; it does not refer to an event which hypothetically might have caused death if it were more severe."
Contributions of authors
Chris Cates, Elizabeth Stovold and Marta Oleszczuk wrote the protocol and search strategy together. Elizabeth Stovold carried out the searches for Cochrane reviews (which were independently assessed for quality by Elizabeth Stovold and Susan Wieland). Elizabeth Stovold also carried out the updated searches for each included review. Chris Cates and Marta Oleszczuk independently assessed the search results and extracted data from the new trials. Chris Cates carried out the statistical analyses. All authors contributed to the final version of the review.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Sources of support
Internal sources
St George's University of London, UK.
External sources
-
NIHR, UK.
Programme grant funding
Declarations of interest
Chris Cates authored the included systematic reviews on the adverse events of long‐acting beta2‐agonists in adults and children, and was not involved in the assessment of the quality of the reviews.
Edited (no change to conclusions)
References
References to included reviews
Cates 2008
- Cates Christopher J, Cates Matthew J. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2008, issue 3. [DOI: 10.1002/14651858.CD006363.pub2; CD006363] [DOI] [PMC free article] [PubMed]
Cates 2009a
Cates 2009b
Cates 2010
- Cates Christopher J, Lasserson Toby J. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2010, issue 1. [DOI: 10.1002/14651858.CD007694.pub2; CD007694] [DOI] [PMC free article] [PubMed]
Cates 2012a
- Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2012, issue 4. [DOI: 10.1002/14651858.CD006923.pub3] [DOI] [PMC free article] [PubMed]
Cates 2012b
- Cates CJ, LJ. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2012, issue 3. [DOI: 10.1002/14651858.CD007695.pub3] [DOI] [PMC free article] [PubMed]
Additional references
Altman 2003
- Altman DG, Bland JM. Statistics Notes: Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2008
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008;372(9643):1107‐19. [DOI] [PubMed] [Google Scholar]
Boushey 1980
- Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. American Review of Respiratory Disease 1980;121(2):389‐413. [DOI] [PubMed] [Google Scholar]
Bradburn 2007
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Castle 1993
- Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ 1993;306(6884):1034‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2011
- Cates CJ. Safety of tiotropium. BMJ 2011;342:d2970. [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan A‐W, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [DOI] [PubMed] [Google Scholar]
Chan 2004a
- Chan A‐W, Krleza‐Jeric K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. Canadian Medical Association Journal 2004;171(7):735‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chowdhury 2011
- Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. The New England Journal of Medicine 2011;364(26):2473‐5. [DOI] [PubMed] [Google Scholar]
Cockcroft 2006
- Cockcroft D. Airway hyper‐responsiveness as a determinant of the early asthmatic response to inhaled allergen. Journal of Asthma 2006;43(3):175‐8. [DOI] [PubMed] [Google Scholar]
Ducharme 2010
- Ducharme Francine M, Ni Chroinin M, Greenstone I, Lasserson Toby J. Addition of long‐acting beta2‐agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2010, issue 5. [DOI: 10.1002/14651858.CD005535.pub2] [DOI] [PMC free article] [PubMed]
Ducharme 2011
- Ducharme Francine M, Lasserson Toby J, Cates Christopher J. Addition to inhaled corticosteroids of long‐acting beta2‐agonists versus anti‐leukotrienes for chronic asthma. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2011, issue 5. [DOI: 10.1002/14651858.CD003137.pub4] [DOI] [PubMed]
GINA 2011
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. Available from: http://www.ginasthma.org/. 2011.
ICH E2A 1995
- Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Clinical safety data management: definitions and standards for expedited reporting. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073087.pdf (accessed July 2012). 1995.
Ioannidis 2001
- Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001;285(4):437‐43. [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JPA, Evans SJW, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [DOI] [PubMed] [Google Scholar]
Li 2010
- Li JS, Qaqundah PY, Weinstein SF, LaForce CF, Ellsworth AV, Ortega HG, et al. Fluticasone propionate/salmeterol combination in children with asthma: Key cardiac and overall safety results. Clinical Research and Regulatory Affairs 2010;27(3):87‐95. [Google Scholar]
Lougheed 2010
- Lougheed MD, Lemiere C, Dell SD, Ducharme FM, Fitzgerald JM, Leigh R, et al. Canadian Thoracic Society Asthma Committee commentary on long‐acting beta‐2 agonist use for asthma in Canada. Canadian Respiratory Journal 2010;17(2):57‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
McMahon 2011
- McMahon AW, Levenson MS, McEvoy BW, Mosholder AD, Murphy D. Age and risks of FDA–approved long‐acting β2‐adrenergic receptor agonists. Pediatrics 2011;128(5):e1147‐54. [DOI] [PubMed] [Google Scholar]
NCT01192178
- GlaxoSmithKline. A Randomized, Double‐Blind, Parallel Group Study of FSC 100/50 and FP 100, both twice daily, in a Pediatric Population during the Fall Viral Season [ADA113872]. http://www.gsk‐clinicalstudyregister.com/.
NCT01444430
- AstraZeneca. A 6 Month Safety Study Comparing Symbicort With Inhaled Corticosteroid Only in Asthmatic Adults and Adolescents. ClinicalTrials.gov 2012 (accessed June 2012).
NCT01462344
- GlaxoSmithKline. 6‐month Safety and Benefit Study of ADVAIR in Children 4‐11 Years Old (VESTRI). ClinicalTrials.gov 2012 (accessed 29/06/2012).
NCT01471340
- Merck. A Serious Asthma Outcome Study With Mometasone Furoate/Formoterol Versus Mometasone Furoate in Asthmatics 12 Years and Over (P06241 AM3) (SPIRO). ClinicalTrials.gov 2012 (accessed June 2012).
NCT01475721
- GlaxoSmithKline. SAS115359: A 6‐month Study to Assess the Safety and Benefit of Inhaled Fluticasone Propionate/Salmeterol Combination Compared With Inhaled Fluticasone Propionate in the Treatment of Adolescents and Adults (12 Years of Age and Older) With Asthma. (AUSTRI). ClinicalTrials.gov 2012 (accessed June 2012).
Nelson 2006
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129(1):15‐26. [DOI] [PubMed] [Google Scholar]
Ni Chroinin 2009
- Ni Chroinin M, Lasserson Toby J, Greenstone I, Ducharme Francine M. Addition of long‐acting beta‐agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2009, issue 3. [DOI: 10.1002/14651858.CD007949] [DOI] [PMC free article] [PubMed]
Phillips 1990
- Phillips GD, Finnerty JP, Holgate ST. Comparative protective effect of the inhaled beta‐2‐agonist salbutamol (albuterol) on bronchoconstriction provoked by histamine, methacholine, and adenosine 5'‐monophosphate in asthma. Journal of Allergy and Clinical Immunology 1990;85(4):755‐62. [DOI] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Salpeter 2006
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma‐related deaths. Annals of Internal Medicine 2006;144(12):904‐12. [DOI] [PubMed] [Google Scholar]
Senn 1997
- Senn SJ, Lillienthal J, Patalano F, Till D. An incomplete blocks cross‐over in asthma: a case study in collaboration. In: Vollmar J, Hothorn LA editor(s). Cross‐over Clinical Trials. Stuttgart: Fischer, 1997:3‐26. [Google Scholar]
Shea 2007
- Shea B, Grimshaw J, Wells G, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI: 10.1186/1471-2288-7-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN/BTS 2012
- Scottish Intercollegiate Guidelines Network/British Thoracic Society. British guideline on the management of asthma: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2008 (revised 2012). [Google Scholar]
Tattersfield 2006
- Tattersfield AE. Current issues with beta2‐adrenoceptor agonists: historical background. Clinical Reviews in Allergy and Immunology 2006;31(2‐3):107‐18. [DOI] [PubMed] [Google Scholar]
Van Noord 1996
- Noord JA, Smeets JJ, Raaijmakers JAM, Bommer AM, Maesen FPV. Salmeterol versus formoterol in patients with moderately severe asthma: onset and duration of action. European Respiratory Journal 1996;9(8):1684‐8. [DOI] [PubMed] [Google Scholar]
Visual Rx 2012
- Visual Rx. www.nntonline.net/visualrx/ Accessed June 2012.
Weinberger 2006
- Weinberger M, Abu‐Hasan M. Life threatening asthma during treatment with salmeterol. The New England Journal of Medicine 2006;355:852‐3. [DOI] [PubMed] [Google Scholar]
Whittington 2004
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363(9418):1341‐5. [DOI] [PubMed] [Google Scholar]


