Abstract
Discoveries in fruit flies have greatly contributed to our understanding of neuroscience. The use of an unparalleled wealth of tools, many of which originated between 1910–1960, has enabled milestone discoveries in nervous system development and function. Such findings have triggered and guided many research efforts in vertebrate neuroscience. After 100 years, fruit flies continue to be the choice model system for many neuroscientists. The combinational use of powerful research tools will ensure that this model organism will continue to lead to key discoveries that will impact vertebrate neuroscience.
Introduction
It was almost 100 years ago that Thomas Hunt Morgan reported the identification of the white gene in Drosophila melanogaster1. Hence, this is an appropriate time to reflect on the past and present contributions of fruit fly research to the field of neuroscience. Genetic approaches dominated the first 50 years of research in Drosophila (1910–1960), focusing on dissecting the principles of inheritance2. During this time period important concepts and tools were developed that allowed the study of many other biological processes between 1960–2010 (Timeline; Box 1). Indeed, investigators realized in the early fifties that genetic approaches could be used to study problems other than heredity. The continuous development of research tools between 1960–2010 has driven numerous new discoveries in fruit flies. This article highlights the many aspects of nervous system development and function that have been unraveled in fruit flies and how these studies have influenced neuroscience research in vertebrate species.
Box 1. Tools and principles developed between 1910–1960.
The most important tools and methods developed in this period include the balancer chromosomes178, 179. Balancer chromosomes allow investigators to maintain mutations in heterozygous stocks, without having to genotype the animals for further breeding. Hence, mutations in essential genes can easily be studied. Drosophila is still the only multicellular organism in which more than 95% of the mutations in essential genes can be maintained easily and effectively.
X-rays were found to be mutagenic and to induce chromosome rearrangements4 including deletions, duplications, and inversions. The ability to map these rearrangements on salivary gland polytene chromosomes180 allowed geneticists to physically map genes. Finally, the discovery of mitotic recombination181 laid the foundation to study the function of essential genes in mosaic animals. Many of the methodologies and reagents created between 1910–1960 have had a major influence on the approaches pursued since the following decades and have led to the discovery of numerous mutations in loci that are still being studied today.
Timeline.
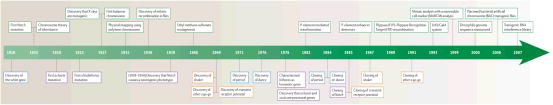
Boxes with green borders indicate the development of important tools and methods; boxes with purple borders indicate the discovery of genes involved in nervous system development; boxes with blue borders indicate events related to genes involved in behavior; and boxes with orange borders indicate events related to proteins that affect nervous system function. For more details see Box 1.
DEVELOPMENT OF THE NERVOUS SYSTEM
A pathway to Notch
Mutations in Notch were first identified in 1915 and reported in 1916 (Ref. 3) as mutations that result in the malformation of wings. It was Poulson who first documented the effects of Notch on embryonic development. Loss of Notch causes a so-called ‘neurogenic’ phenotype, characterized by presumptive hypoderm that differentiates into neuroblasts4, resulting in an embryo with a hypertrophied CNS at the expense of ventral hypoderm. A systematic search to identify other mutations with similar phenotypes led to the isolation of other key genes that control epidermal versus neuronal fate including neuralized, Delta, mastermind, big brain and Enhancer of split5. The cloning of Notch6 and its ligand Delta7 in the mid-eighties, as well as the cloning of other key players, helped delineate what is currently known as the Notch signaling pathway8, 9.
Evidence that Notch is conserved in vertebrates resulted from the cloning of the human NOTCH gene as the cause of a human leukemia10. In the 1990s it became apparent that the other core components of the Notch pathway that had been identified in Drosophila are conserved in vertebrates and that many have similar roles in vivo. All the Notch signaling components identified in flies and mammals have been recently compared in detail11. The Notch pathway has a seminal role in developmental neurobiology as it affects almost every aspect of neurogenesis and differentiation of neurons in vertebrates, in the developing as well as the adult brain, including neural stem cells12. However, the importance of Notch signaling stretches far beyond specifying neuronal versus epidermal cells. Some of its components, including neuralized, have now also been shown to have a role in learning and memory formation in adult flies13. More importantly, Notch signaling affects neuronal stem-cell specification, blood cell development, heart development, hematopoietic stem cell differentiation, bone and skin development and numerous other tissues. Mutations in the human NOTCH3 locus (there are four NOTCH loci in most vertebrates) cause a devastating neurological disorder named CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy14). Finally, aberrant Notch signaling causes several types of cancer11.
Homeotic genes and vertebrate nervous system segmentation
During the 1950s several scientists realized that existing spontaneous and X-ray induced mutations, such as achaete15 and Ultrabithorax16, which adversely affect the development of an organism can help unravel the principles of development and pattern formation. Lewis focused on the bithorax complex of genes and the polycomb gene, which are crucial to define the basic segmental identity of the larval and adult thorax and abdomen17. Sanchez-Herrero et al.18 showed that the bithorax complex contained three genes: Ultrabithorax, Abdominal-A and Abdominal-B. The work on the bithorax complex, as well as that on another complex of homeotic genes, the antennapedia complex19, 20, led to the discovery that both complexes contain genes encoding evolutionarily conserved homeobox-containing proteins21 that are involved in DNA-binding and function as transcription factors. Subsequently, the discovery of four large complexes of homeotic (Hox) genes in vertebrates, with many properties similar to those of Drosophila Hox clusters22, and an important role in patterning the hindbrain23, had a significant effect on vertebrate neurodevelopmental biology. Furthermore, the Hox genes seem to be key for defining the specificity of motor neuron–muscle connectivity24 and in the genetic program of neural crest migration25.
The proneural helix-loop-helix proteins
The achaete-scute complex26 contains a set of four genes (achaete, scute, lethal of scute and asense) that had a seminal role in the discovery of the basic-helix-loop-helix (bHLH) transcription factors. These proteins are typically expressed in neuronal precursors of the central and peripheral nervous system (PNS) and are often required to allow ectodermal cells to adopt a neural fate. Mutations in scute, which cause a loss of neurons and a loss of bristles or adult sensory structures, were isolated and studied between 1918–1940 (Ref. 27). However, in the late seventies, a key set of genetic and developmental observations suggested that achaete and scute are involved in the initial decision to specify a sensory organ, and not in the differentiation process itself28, 29. This analysis paved the way for the cloning30 and identification of proteins encoded by the achaete-scute complex. These turned out to correspond to bHLH transcription factors that are expressed in specific domains of ectodermal cells31 and are required to switch the fate of these cells to neuronal precursors of the PNS — this requirement was also observed for other bHLH proteins such as Atonal32. The homologues of these genes were then shown to have a key role in vertebrate neurogenesis33, specification of vertebrate inner ear hair cells34, touch receptors35, 36 and motorneurons37. In summary, the achaete-scute complex genes were the founders of an important family of genes required in neural development across phyla38. Moreover, apart from the discovery of the bHLH genes, numerous other key genes that affect the development of the external and internal sensory organs in fruit flies have been identified, including numb39, cut40, prospero41 and senseless42. Similarly, the vertebrate homologues of these genes (like Numb and Gfi1) have been shown to affect vertebrate neurogenesis43, 44, 45.
Neurogenesis, neuronal migration, and growth cone guidance
In the mid-seventies, the available genetic tools in Drosophila (Box 1) offered an opportunity to address how embryonic pattern formation is controlled and to determine which genes are involved46. By carrying out a systematic chemical mutagenesis screen47 on the different fly chromosomes and analyzing the larval cuticular patterns, Nüsslein-Volhard and Wieschaus identified 139 genes that affect the development of fly larvae48, 49. Although these screens were not designed to identify genes that affect the development or function of the nervous system, they identified many novel players that were later shown to be part of conserved signaling pathways, including genes in the Hedgehog, Wingless, Decapentaplegic or Tumor growth factor-β, and Notch pathways. These pathways are important in vertebrate neurogenesis50, neuronal migration51, growth cone guidance52 and maintenance and differentiation of neural stem cells53 (Table 1). These findings demonstrated the power of forward genetic approaches in solving complex development questions.
Table 1.
The roles of Hedgehog, Wingless, Dpp/TFGβ and Notch signaling in the nervous system
| Pathway | Neuronal specification | Neuronal migration | Growth cone guidance | Synapse formation | Neuronal stem cell maintenance |
|---|---|---|---|---|---|
| Hedgehog | Mammals182 and flies183 | ND | Mammals182 | Flies183 | Mammals182 and flies184 |
| Wingless | Mammals185 and flies186 | Mammals185 | Mammals185 | Mammals185, 187 and flies188 | Mammals185 |
| Dpp/TGFβ | Mammals189 and flies190, 191 | Mammals189 | Mammals189 and flies192, 193 | Mammals189 and flies194 | Mammals195, 196 and flies184 |
| Notch | Mammals197 and flies37, 198, 199 | Mammals197 | Flies200 | Flies201 | Mammals184 and flies184 |
Dpp, Decapentaplegic; ND, not determined; TGFβ, Tumor growth factor-β
Genetic screens in the early nineties also led to the identification of mutations that affect growth cone guidance54, leading to the discovery of the roundabout or Robo pathway55. This pathway controls the crossing of growth cones of pioneering neurons across the midline of the nervous system in flies and mice. Similarly, another set of growth cone guidance proteins that have a repulsive role, the semaphorins, were discovered simultaneously in flies56 and chick, where the founding member was named collapsin57. As is the case for many signaling pathways with pleiotropic roles, these signaling pathways do not only affect growth cone guidance but also other processes such as vascular development and tumor growth in vertebrates58.
THE MOLECULAR BASIS OF BEHAVIOR
The successful application of genetics to dissect the structure and function of prokaryotic genes in the fifties and sixties prompted Benzer to venture into a new area. He reasoned that as genetics could be used to dissect the principles of inheritance and development, a systematic genetic analysis of fly behavior should yield genes that control neuronal function. This simple but powerful idea, combined with an efficient protocol for chemical mutagenesis47, initiated the field of behavioral neurogenetics.
Circadian rhythms
Benzer ventured into this field in 1967 when he described a simple behavioral assay that is still used today: the light countercurrent assay59, a quantitative method to fractionate populations of flies according to their behavioral responses when exposed to light on repeated trials. He then used ethyl methane sulfonate (EMS) to induce mutations and screened for mutants with defective phototaxis. He isolated several X-chromosome mutations and argued that similar screens and assays could identify mutants that are impaired in gravity, odor or sound perception. This work convinced many of his students and contemporaries that genetics could be used to tackle questions regarding the molecular basis of behavior that were difficult, if not impossible, to address at the time.
In 1971, Benzer’s group published a seminal paper60 describing a forward genetic screen for defects in the daily rhythm of eclosion and locomotor activity of adult flies. They found three novel mutants affecting a single gene that they named period (per), which caused faster, slower or complete absence of rhythms (arrhythmic). The identification of the per gene took 13 years, largely because the work was published before recombinant DNA technology was a viable research tool61, 62, 63, 64. Another 13 years passed before human and mouse geneticists identified the corresponding homologues65. These studies, together with other forward genetic screens in flies66 and mice67, 68 led to the isolation of the timeless and clock genes respectively, laying the groundwork for unraveling the molecular mechanism of the circadian network that is conserved from flies to humans. Thins work also led to the discovery that mutations in these genes have a role in human disease. Indeed, familial advanced sleep phase syndrome is caused by mutations in the period homologue 2 (PER2) and casein kinase 1 delta (CSNK1D), two of the core clock genes in humans69.
On learning and memory
The development of an olfactory shock-avoidance learning assay in 1974 was another important contribution from the Benzer laboratory70, resulting in the isolation of the first learning mutant, dunce (dnc)71. Biochemical tests quickly provided compelling evidence that dnc mutants were deficient for cAMP phosphodiesterase activity72, 73 and shortly after, dnc was shown to encode this enzyme74. The role of cAMP in learning and memory was further substantiated with the cloning of the mutant gene of another learning mutant, rutabaga, which encodes an adenylate cyclase, an enzyme that produces cAMP75. This work laid the foundation for the isolation of many genes that are involved in olfactory learning in D. melanogaster and that affect cAMP levels in neurons76. A role for cAMP in learning and memory was also documented in Aplysia californica in the early eighties77, 78. This was later confirmed and expanded upon in vertebrates79. More recently fly biologists have started to identify changes in calcium dynamics in the olfactory circuitry that correspond well with the behavioral dynamics of olfactory memories80. This work is starting to reveal the neuronal mechanisms underlying the storage of memories.
PROTEINS THAT AFFECT THE FUNCTION OF THE NERVOUS SYSTEM
Tripping on TRP: the transient receptor potential channels
The screens performed by Benzer59 prompted others to look at EMS-induced mutant flies that had impaired vision. An electrophysiological assay that had originally been developed in other insects81 and that records the electrical potentials contributed by many different cell types in the retina upon light stimulation (the electroretinogram (ERG)), allowed the identification of numerous mutants with defects in their light responses82, 83. One of these mutants caused flies to only exhibit a short transient membrane potential in the ERG upon a flash of light. This mutant later became known as transient receptor potential or trp84.
In the early eighties, analysis of genes (and their products) that affect eye signal transduction (rhodopsins, trp)85, pigmentation (rosy, white)86 and development (sevenless, rough and many others)87 were greatly aided by the development of P-element-mediated transformation by Spradling and Rubin88. Indeed, Montell et al.89 used this technique to demonstrate that they had cloned the trp gene by rescuing the phenotype of mutants in vivo. Subsequent sequencing of trp revealed that it encoded a light-inducible calcium channel with six transmembrane domains expressed in photoreceptor cells90. The Montell laboratory then cloned the first vertebrate TRP channel in 1995 (Ref. 91), thereby establishing the presence of a large and interesting family of novel channels in vertebrates92.
TRP channels are expressed throughout the body and are activated and regulated by multiple stimuli including mechanical stretch, heat, touch and environmental chemicals93. TRPs have now been shown to mediate responses to nerve growth factor and pheromones, to affect proprioceptors and touch receptors, to be required for hearing and olfaction in flies, to transduce heat and pain perception, and to affect the transduction of other stimuli, such as osmolarity. Furthermore, mutations in several members of TRP-related channel proteins are responsible for neurodegenerative disorders: mutations in TRPML1 cause mucolipidosis type IV disease94, whereas mutations in TRPV4 cause hereditary motor and sensory neuropathy type IIC95, 96, 97.
Shaking it all: Shaker (Sh) and ether-a-go-go (eag)
Mutations in Sh cause flies to shake their legs when anesthetized with ether98. A detailed electrophysiological characterization of these mutants was initiated in the seventies99, 100. These studies showed that Sh mutations cause a prolonged release of neurotransmitters at the larval neuromuscular junction (NMJs) because motor neurons fail to repolarize, suggesting a defect in potassium channels100, 101, 102. This led to a race to clone and sequence the Sh gene10, 104, 105, 106. The cloning of Sh as the first potassium channel allowed its biochemical purification and molecular characterization. Subsequently, a family of at least four Sh-related potassium channel genes (Sh, Shab, Shal and Shaw) was identified in D. melanogaster and mammals107.
Another founding potassium channel member is encoded by the eag gene, which was also identified on the basis of its leg-shaking phenotype98. In eag mutants, neurotransmitter release is enhanced and more prolonged, and in the absence of nerve stimulation, there is a high frequency of spontaneous release101, 108. eag and Sh double mutants display a synergistic interaction, suggesting that two different types of potassium channels are involved in the repolarization of the nerve terminal102. Cloning and sequencing of eag109 verified this hypothesis, leading to the identification of another family of potassium channels, the so named EAG, ERG (eag-related gene) and ELK (eag-like potassium) channels110. The vertebrate homologue of ERG, HERG, was subsequently linked to a neurological heart disease (LQT syndrome)111. Moreover, it was discovered that many commonly used drugs like seldane and vicodin cause cardiac arrythmia by off-target effects on HERG. Obviously, potassium channels have a central role in all neurons112 and have been implicated in numerous human diseases113.
Synaptic transmission
The electrophysiological properties of the larval NMJ, a well-established model for studies of synaptic transmission, were first characterized in detail by Jan and Jan. The large size and accessibility of body wall muscles, make them most amenable to electrophysiological99, 114, immunohistochemical115 and microscopical116, 117 studies. By being able to manipulate the expression of genes pre- and postsynaptically, these technologies allow the dissection of protein function at an unparalleled level in vivo. For example, the study of the role of synaptotagmin at the fly NMJ was the first to provide compelling data in vivo that it functions as a calcium sensor for fast synaptic transmission118. This was also one of the first examples of using a reverse genetic approach to knockout a gene in Drosophila, as no P-element insertion, X-ray or EMS mutants for synaptotagmin were available at the time119, 120. Similarly, it was first discovered in fruit flies that dynamin, encoded by the fly shibire gene121, 122, has a crucial role in endocytosis. Again, the NMJ synapses were seminal in the in vivo dissection of the function of dynamin123, 124. The function of many other proteins required for exo- and endocytosis have been characterized using the fly NMJ, providing important information about the in vivo function of many important proteins required for synaptic transmission in vertebrates125, 126, 127. Table 2 details most of the presynaptic proteins that have been studied in fruit flies. Many of these studies were possible because of the coordinated efforts of the Drosophila Gene Disruption Project, which created a large collection of transposable elements that allowed the generation of mutations through imprecise excision, and therefore permitted the detailed functional investigation of many genes128.
Table 2.
Some of the synaptic vesicle exocytic and endocytic proteins studied in Drosophila melanogaster
| Protein | Function | Evoked response | Mini EJP amplitude | Ultrastructure | Refs | ||
|---|---|---|---|---|---|---|---|
| 1Hz | 10Hz | Normal SVs | Large SVs | ||||
| Exocytosis | |||||||
| Cacophony | Presynaptic calcium channel | Low | NA | Normal | ? | ? | 202 |
| Complexin | Clamp/fusion of SV | Low | NA | Normal | Normal | NA | 203 |
| CSP | Chaperone for SNAREs | Low | NA | Normal | ? | ? | 204 |
| Hip14 | Transport of CSP | Low | NA | Normal | Normal | NA | 205 |
| Rop | Docking and fusion of SV | Low | NA | Normal | ? | ? | 206, 159 |
| Straightjacket | Targeting of Ca2+ channels | Low | NA | Normal | Normal | NA | 207 |
| SNAP25 | Fusion of SV | Slight reduction | NA | Normal | ? | ? | 208 |
| Synaptobrevin | Fusion of SV | Low | NA | Normal | Normal | NA | 150, 209 |
| Synaptotagmin | Ca2+ sensor | Low | NA | Normal | Normal | NA | 119, 118 |
| Syntaxin | Fusion of SV | None | NA | None | Normal | NA | 210, 211, 209 |
| Unc-13 | Fusion of SV | Low | NA | Reduced | Normal | NA | 212 |
| Vha100-1a | Fusion of SV | Very low | NA | Normal/None | Normal | NA | 213 |
| Endocytosis | |||||||
| AP180 | Early adaptor | Normal | Low | High | NA | Large | 214 |
| Clathrin | SV coat | Normal | Low | Normal | NA | Large | 215, 216 |
| Dap160 | Scaffolding protein | Normal | Low | High | NA | Large | 217 |
| Dynamin | Fission of SV | Absent | Absent | Absent | Abnormal | ? | 218 |
| Endophilin | Recruitment of synaptojanin for uncoating | Normal | Very low | High | Normal | NA | 219 |
| Eps15 | Scaffolding protein | Normal | Low | Normal | NA | Large | 117 |
| Flower | Ca2+ channel that couples exocytosis to endocytosis | Normal | Low | High | NA | Large | 220 |
| Stoned A or B? | Sorting/recycling | Normal | Low | High | NA | Large | 221 |
| Synaptojanin | Clathrin uncoating | Normal | Very low | Not tested | Normal | NA | 222 |
| Tweek | PIP2 delivery | Normal | Low | High | NA | Large | 223 |
CSP, cysteine string protein; EJP, excitatory junction potential; NA, not applicable; PIP2, phosphatidylinositol bisphosphate; SV, synaptic vesicle; ?, unknown.
ADVANTAGES OF STUDYING FRUIT FLIES
Drosophila offers many unique advantages that will ensure that it is a premier research organism for many years to come. The sophisticated manipulations that can be carried out in flies are unsurpassed in any other multicellular model organism129, 130. These manipulations allow biologists to ask precise questions about behaviour, signalling processes, individual cell behaviours, organ development and adult behaviour. Two experimental key features, namely the successful and efficient removal or addition of single genes or gene products, are important for any model organism to be successfully used in the laboratory. In Drosophila, genes can be removed in a random fashion using chemical mutagenesis before screening for specific phenotypes, as already documented47. Current tools allow very rapid mapping of chemically-induced mutations that have robust phenotypes, permitting the isolation of null alleles, hypomorphs, hypermorphs, neomorphs and antimorphs as well as conditional alleles, vastly expanding the ability to assess gene function. It is possible to perform a chemical mutagenesis X-chromosome screen and map more than 50 genes in less than a year using duplications and deletions (HJB unpublished data), demonstrating that gene mapping has become almost trivial. Further improvements in speed and accessibility are expected from recent developments related to whole genome sequencing methodologies131. These will clearly have a major impact in the mapping of genes that control behaviour. In another approach, one can remove 65% of the fly genes in a targeted fashion using imprecise excisions of transposable elements128, 129. Alternatively, one can engineer mutations in the locus of interest through selective removal or replacement of sequences, the so-called targeted knockout technology developed originally by Golic and colleagues132. Yet another approach is to use RNA interference to reduce the expression of any gene. This methodology works well for some loci and less well for others, but it still offers many possibilities133, 134. Finally, other methods have been engineered and adapted to flies ensuring that Drosophila has the most complete arsenal of tools to knock out genes129.
Adding single genes or gene constructs in flies through P-element-mediated transformation has been available since 1982 (Ref. 88). This methodology has been extremely important as it allows the efficient transformation of flies with only a single copy of the DNA of interest — unlike in many other model species — and has permitted some of the most sophisticated manipulations in the animal world130. This transformation protocol has recently been improved significantly using P[acman] technology, allowing small to very large pieces of DNA to be inserted in specific docking sites spread throughout the genome135, 136. Efficient transformation has allowed the development of the flippase (FLIP)–flippase recognition target (FRT) recombination system137, 138 which enables the creation of mutant patches of tissues or cells in an otherwise heterozygous background. It also allowed the development of the UAS/Gal4 system, by which any gene can be expressed ectopically in almost any tissue or cell139. Finally, it also led to the development of a high efficiency mitotic recombination system that allows to knockout a gene in specific tissues, organs, cells or neurons and mark the mutant cells140. Finally, P[acman] technology allows the tagging of most genes in vivo, permitting sophisticated manipulations in a genomic context141.
In summary, these tools allow the dissection of the function of specific neurons at an unparalleled level of resolution. In addition, current electrophysiological methods allow the functional assessment of numerous different types of synapses including those at the NMJs of fly embryos142, larvae and adults99, 143, as well as synapses of photoreceptors81 and the giant fibre system144. In addition, several preparations have been developed to record specifically from central neurons145, 146, 147, 148. Moreover, thousands of UAS/Gal4 lines are now available149, which allow the modification of gene expression139, or to functionally150, 151, 152, 153 or physically ablate most neuronal populations in the brain. Finally, the availability of optogenetic tools154 allows to elicit innate behaviours under the control of a light source. These and many other methods that are currently being developed will ensure that the fruit fly stays at the forefront of neuroscience research for many years to come.
THE FUTURE OF RESEARCH IN THE FRUIT FLY
As outlined above, Drosophila has and will continue to contribute to many aspects of neuroscience. Current and future research in many areas of fly neurobiology will pave the way to new genes, new pathways and new approaches that will pioneer numerous fields of neurobiology, including vertebrate neurobiology. Obviously, the fruit fly is not suited to study vertebrate-specific issues, such as the development of specific brain structures, regulation of neural crest migration, the function and properties of hippocampal neurons, or to assess how the cerebellum controls motor outputs. However, the fly has the proven potential to provide information about the fundamental features of nervous system organization and function, how information is integrated and processed, how specific genes can cause neurodegeneration, how different brain areas are wired together, and what gene products and genetic cascades control behaviour. Below we list a small sample of recent exciting discoveries to illustrate that research in flies is continuing to be highly influential in neuroscience.
Flies, just like vertebrates, require sleep. Numerous aspects of the physiological properties of sleep are shared between Drosophila spp. and humans155, 156, and studies on sleep in flies are paving the way for a better understanding of sleep in vertebrates157. Recent genetic screens identified genes that affect the sleep cycle of flies, including mutations in sleepless158. Sleepless binds to Shaker, suggesting that it modulates Shaker activity by a direct interaction. It seems conceivable that proteins similar to Sleepless, that contain a Ly-6 domain and a glycosylphosphatidylinositol anchor will control sleep in vertebrates159. The sleep field is a nice example of how the mammalian community not only ‘accepts’ discoveries in D. melanogaster, but also of how investigators of mammalian systems have turned to flies to advance their research. Indeed, the first genetic screen for sleep genes was actually performed by a mammalian sleep laboratory in collaboration with the Ganetzky laboratory160. Similarly, some well-established vertebrate neuroscience laboratories have recently shifted their interest to solving important neuroscience questions in flies161, 162.
Another example of a recent contribution of research in flies relates to Parkinson’s disease, a CNS disease that leads to an impairment of motor skills, speech and other functions. The symptoms result from reduced activity of dopamine-secreting cells of the human substantia nigra163. Mutations in a number of genes including α-synuclein164, parkin165 and PTEN-induced putative kinase 1 (PINK1)166 cause Parkinson’s disease, but the mechanisms underlying the disease remain unknown. Studies with mammalian cells have indicated diverse pathogenic mechanisms and disease models, including protein misfolding, abnormal protein accumulation and mitochondrial dysfunction. However, no clear picture has emerged from these studies. The work on parkin and PINK1 mutations in D. melanogaster, however, has provided compelling evidence that PINK1 and Parkin are components of a pathway that is involved in regulating mitochondrial remodelling and that mitochondrial dysfunction is a cause of Parkinson’s disease167, 168. These, and many other neurodegenerative diseases, including ataxias caused by polyglutamine expansions169, 170, have and will continue to benefit from research in flies171.
Recently, many fly experts have focused their attention on dissecting the molecular and cellular basis of behavior. These include phototaxis, chemotaxis172, aggression173, physical response to mechanical stimuli174, escape behavior175 and sex176, 177. This focus is illustrated by the concerted efforts of researchers at Janelia Farm, Ashburn, Virginia, USA, who are trying to systematically dissect the origin of every fly neuron, identify different types of adult neurons, the nature of every synapse and the function of different neuronal populations. These studies will undoubtedly advance our understanding of how the nervous system of the fruit fly works and provide us with very valuable paradigms to study mammalian brain function.
As history tends to repeat itself, the main reason for predicting that studies in fruit flies will continue to reveal key aspects of nervous system function is simple: the fly toolbox has an unparalleled sophistication and precision that allows scientists to tackle almost any question in biology and answer it in a timely fashion129. Moreover, this toolbox continues to expand quickly133, 135, 141, 149, ensuring that D. melanogaster will remain a model organism of choice for neuroscientists.
Acknowledgments
We would like to thank B. Ganetzky, S. Yamamoto, M. Rasband, N. Giagtzoglou, M. Xue, J. Kiger, H. Dierick, K. Cook, K. Schulze and B. Hassan for reading the manuscript. We apologize to all our colleagues whose work was not cited because of space constraints. HJB is an investigator of the Howard Hughes Medical Institute, HT is supported by the Amyotrophic Lateral Sclerosis Association and CT is supported by a T32 from the National Institute of Neurological Disorders.
Biographies
Hugo J. Bellen
Hugo J. Bellen is an Investigator at the Howard Hughes Medical Institute at Baylor College of Medicine, Houston, Texas, USA, where he has been since 1989. After graduating as a veterinary doctor in 1983, he carried out his Ph.D. studies on learning in fruit flies. His own laboratory focuses on neural development and synaptic transmission in flies.
Chao Tong
Chao Tong is a postdoctoral Research Associate in Bellen’s laboratory. Chao obtained her Ph.D. at the University of Texas Southwestern, Dallas, Texas, USA, where she studied Hedgehog signalling in fly wing development. She is interested in the molecular aspects of synapse formation and the role of lysosomes in neurodegenerative disease in flies.
Hiroshi Tsuda
Hiroshi Tsuda is an Assistant Professor in the Department of Molecular and Human Genetics at Baylor College of Medicine, Houston, Texas, USA. He obtained his MD from Kobe University, Japan, and his board certification in neurology. Then he decided to work on the basic mechanisms of neurodegeneration and obtained his Ph.D. from Kyoto University, Japan. Currently he is focusing his research on amyotrophic lateral sclerosis using flies as disease models.
References
- 1.Morgan TH. Sex limited inheritance in Drosophila. Science. 1910;32:120–122. doi: 10.1126/science.32.812.120. [DOI] [PubMed] [Google Scholar]
- 2.Sturtevant AH. A History of Genetics. Cold Spring Harbor Laboratory Press; New York: 1966. [Google Scholar]
- 3.Morgan TH, Bridges CB. Sex-linked inheritance in Drosophila. Carnegie Institute of Washington Publication. 1916;237:1–88. [Google Scholar]
- 4.Poulson DF. In: Histogenesis, organogenesis and differentiation in the embryo of Drosophila melanogaster. Demerec M, editor. Hafner Publishing Co Ltd, Wiley; New York: 1950. [Google Scholar]
- 5.Campos-Ortega JA. Cellular interactions during early neurogenesis of Drosophila melanogaster. Trends Neurosci. 1988;11:400–405. doi: 10.1016/0166-2236(88)90077-x. [DOI] [PubMed] [Google Scholar]
- 6.Wharton KA, Johansen KM, Xu T, Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985;43:567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- 7.Vassin H, Bremer KA, Knust E, Campos-Ortega JA. The neurogenic gene Delta of Drosophila melanogaster is expressed in neurogenic territories and encodes a putative transmembrane protein with EGF-like repeats. EMBO J. 1987;6:3431–3440. doi: 10.1002/j.1460-2075.1987.tb02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 9.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 10.Ellisen LW, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 11.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlopoulos E, Anezaki M, Skoulakis EM. Neuralized is expressed in the alpha/beta lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc Natl Acad Sci USA. 2008;105:14674–14679. doi: 10.1073/pnas.0801605105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein A. Coincidence of crossing over in Drosophila melanogaster (Ampelophila) Genetics. 1918;3:135–172. doi: 10.1093/genetics/3.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridges CB, Morgan TH. The third-chromosome group of mutant characters of Drosophila melanogaster. Carnegie Institute of Washington Publication. 1923;327:1–251. [Google Scholar]
- 17.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Herrero E, Vernos I, Marco R, Morata G. Genetic organization of Drosophila bithorax complex. Nature. 1985;313:108–113. doi: 10.1038/313108a0. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman TC, Lewis R, Wakimoto B. Cytogenetic analysis of chromosome 3 in Drosophila melanogaster: the homoeotic gene complex in polytene chromosome interval 84a-B. Genetics. 1980;94:115–133. doi: 10.1093/genetics/94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garber RL, Kuroiwa A, Gehring WJ. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. EMBO J. 1983;2:2027–2036. doi: 10.1002/j.1460-2075.1983.tb01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGinnis W, Garber RL, Wirz J, Kuroiwa A, Gehring WJ. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984;37:403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- 22.Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- 23.Alexander T, Nolte C, Krumlauf R. Hox genes and segmentation of the hindbrain and axial skeleton. Annu Rev Cell Dev Biol. 2009;25:431–456. doi: 10.1146/annurev.cellbio.042308.113423. [DOI] [PubMed] [Google Scholar]
- 24.Dasen JS, Jessell TM. Hox networks and the origins of motor neuron diversity. Curr Top Dev Biol. 2009;88:169–200. doi: 10.1016/S0070-2153(09)88006-X. [DOI] [PubMed] [Google Scholar]
- 25.Dupin E, Creuzet S, Le Douarin NM. The contribution of the neural crest to the vertebrate body. Adv Exp Med Biol. 2006;589:96–119. doi: 10.1007/978-0-387-46954-6_6. [DOI] [PubMed] [Google Scholar]
- 26.Ghysen A, Dambly-Chaudiere C. From DNA to form: the achaete-scute complex. Genes Dev. 1988;2:495–501. doi: 10.1101/gad.2.5.495. [DOI] [PubMed] [Google Scholar]
- 27.Raffel D, Muller HJ. Position effect and gene divisibility considered in connection with three strikingly similar scute mutations. Genetics. 1940;25:541–583. doi: 10.1093/genetics/25.6.541a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Bellido A, Santamaria P. Developmental analysis of the Achaete-Scute system of Drosophila melanogaster. Genetics. 1978;88:469–486. doi: 10.1093/genetics/88.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Bellido A. Genetic analysis of the Achaete-Scute system of Drosophila melanogaster. Genetics. 1979;91:491–520. doi: 10.1093/genetics/91.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campuzano S, et al. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985;40:327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- 31.Cabrera CV, Martinez-Arias A, Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987;50:425–433. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- 32.Jarman AP, Grau Y, Jan LY, Jan Y. Natonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- 33.Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- 34.Bermingham NA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 35.Van Keymeulen A, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maricich SM, et al. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nature Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 38.Quan XJ, Hassan BA. From skin to nerve: flies, vertebrates and the first helix. Cell Mol Life Sci. 2005;62:2036–2049. doi: 10.1007/s00018-005-5124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58:349–360. doi: 10.1016/0092-8674(89)90849-0. [DOI] [PubMed] [Google Scholar]
- 40.Blochlinger K, Bodmer R, Jack J, Jan LY, Jan YN. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature. 1988;333:629–635. doi: 10.1038/333629a0. [DOI] [PubMed] [Google Scholar]
- 41.Vaessin H, et al. prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell. 1991;67:941–953. doi: 10.1016/0092-8674(91)90367-8. [DOI] [PubMed] [Google Scholar]
- 42.Nolo R, Abbott LA, Bellen HJ. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell. 2000;102:349–362. doi: 10.1016/s0092-8674(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 43.Roegiers F, Jan YN. Asymmetric cell division. Curr Opin Cell Biol. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 44.Wallis D, et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- 45.Zhong W, Chia W. Neurogenesis and asymmetric cell division. Curr Opin Neurobiol. 2008;18:4–11. doi: 10.1016/j.conb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 47.Lewis EB, Bacher F. Methods of feeding ethyl methane sulfonate (EMS) to Drosophila males. Dros Inf Serv. 1968;43:193. [Google Scholar]
- 48.Jurgens G, Wieschaus E, Nusslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Rouxs Arch Dev Biol. 1984;193:283–295. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- 49.Wieschaus E, Nüsslein-Volhard C, Jürgens G. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Rouxs Arch Dev Biol. 1984;193:296–307. doi: 10.1007/BF00848158. [DOI] [PubMed] [Google Scholar]
- 50.Ho KS, Scott MP. Sonic hedgehog in the nervous system: functions, modifications and mechanisms. Curr Opin Neurobiol. 2002;12:57–63. doi: 10.1016/s0959-4388(02)00290-8. [DOI] [PubMed] [Google Scholar]
- 51.Gaiano N. Strange bedfellows: Reelin and Notch signaling interact to regulate cell migration in the developing neocortex. Neuron. 2008;60:189–191. doi: 10.1016/j.neuron.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Charron F, Tessier-Lavigne M. The Hedgehog, TGF-β/BMP and Wnt families of morphogens in axon guidance. Adv Exp Med Biol. 2007;621:116–133. doi: 10.1007/978-0-387-76715-4_9. [DOI] [PubMed] [Google Scholar]
- 53.Pozniak CD, Pleasure SJ. A tale of two signals: Wnt and Hedgehog in dentate neurogenesis. Sci STKE. 2006;2006:pe5. doi: 10.1126/stke.3192006pe5. [DOI] [PubMed] [Google Scholar]
- 54.Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- 55.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 56.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 57.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 58.Eichmann A, Le Noble F, Autiero M, Carmeliet P. Guidance of vascular and neural network formation. Curr Opin Neurobiol. 2005;15:108–115. doi: 10.1016/j.conb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci USA. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bargiello TA, Jackson FR, Young MW. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984;312:752–754. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- 62.Reddy P, et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984;38:701–710. doi: 10.1016/0092-8674(84)90265-4. [DOI] [PubMed] [Google Scholar]
- 63.Zehring WA, et al. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984;39:369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]
- 64.Bargiello TA, Young MW. Molecular genetics of a biological clock in Drosophila. Proc Natl Acad Sci USA. 1984;81:2142–2146. doi: 10.1073/pnas.81.7.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun ZS, et al. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. [DOI] [PubMed] [Google Scholar]
- 66.Sehgal A, Price JL, Man B, Young MW. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- 67.Vitaterna MH, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.King DP, et al. The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of Kit. Genetics. 1997;146:1049–1060. doi: 10.1093/genetics/146.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981;289:79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- 73.Davis RL, Kiger JA., Jr Dunce mutants of Drosophila melanogaster: mutants defective in the cyclic AMP phosphodiesterase enzyme system. J Cell Biol. 1981;90:101–107. doi: 10.1083/jcb.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen CN, Denome S, Davis RL. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci USA. 1986;83:9313–9317. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 76.McGuire SE, Deshazer M, Davis RL. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- 78.Alberini CM. Genes to remember. J Exp Biol. 1999;202:2887–2891. doi: 10.1242/jeb.202.21.2887. [DOI] [PubMed] [Google Scholar]
- 79.Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 80.Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- 81.Pak WL, Grossfield J, White NV. Nonphototactic mutants in a study of vision of Drosophila. Nature. 1969;222:351–354. doi: 10.1038/222351a0. [DOI] [PubMed] [Google Scholar]
- 82.Pak WL, Grossfield J, Arnold KS. Mutants of the visual pathway of Drosophila melanogaster. Nature. 1970;227:518–520. doi: 10.1038/227518b0. [DOI] [PubMed] [Google Scholar]
- 83.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 84.Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 85.Zuker CS. The biology of vision of Drosophila. Proc Natl Acad Sci USA. 1996;93:571–576. doi: 10.1073/pnas.93.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levis R, Bingham PM, Rubin GM. Physical map of the white locus of Drosophila melanogaster. Proc Natl Acad Sci USA. 1982;79:564–568. doi: 10.1073/pnas.79.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zipursky SL, Rubin GM. Determination of neuronal cell fate: lessons from the R7 neuron of Drosophila. Annu Rev Neurosci. 1994;17:373–397. doi: 10.1146/annurev.ne.17.030194.002105. [DOI] [PubMed] [Google Scholar]
- 88.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 89.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 90.Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–268. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- 91.Wes PD, et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 94.Dong XP, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Landouré G, et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nature Genet. 2009;42:170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deng HX, et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nature Genet. 2009;42:165–169. doi: 10.1038/ng.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Auer-Grumbach M, et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nature Genet. 2009;42:160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaplan WD, Trout WE., 3rd The behavior of four neurological mutants of Drosophila. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jan YN, Jan LY, Dennis MJ. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977;198:87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- 101.Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogster. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu CF, Ganetzky B, Haugland FN, Liu AX. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983;220:1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- 103.Baumann A, et al. Molecular organization of the maternal effect region of the Shaker complex of Drosophila: characterization of an I(A) channel transcript with homology to vertebrate Na channel. EMBO J. 1987;6:3419–3429. doi: 10.1002/j.1460-2075.1987.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kamb A, Iverson LE, Tanouye MA. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987;50:405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- 105.Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–753. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 106.Tempel BL, Papazian DM, Schwarz TL, Jan YN, Jan LY. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987;237:770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- 107.Salkoff L, et al. An essential ‘set’ of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992;15:161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- 108.Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- 109.Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- 110.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Curran ME, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 112.Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annu Rev Neurosci. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- 113.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nature Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 114.Featherstone DE, Chen K, Broadie K. Harvesting and preparing Drosophila embryos for electrophysiological recording and other procedures. J Vis Exp. 2009 May 20; doi: 10.3791/1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brent J, Werner K, McCabe BD. Drosophila larval NMJ immunohistochemistry. J Vis Exp. 2009 Mar 28; doi: 10.3791/1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bellen H, Budnik V. In: The Neuromuscular Junction. Sullivan W, MAaRSH, editors. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- 117.Koh TW, et al. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J Cell Biol. 2007;178:309–322. doi: 10.1083/jcb.200701030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 120.DiAntonio A, Parfitt KD, Schwarz TL. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- 121.Poodry CA, Hall L, Suzuki DT. Developmental properties of Shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev Biol. 1973;32:373–386. doi: 10.1016/0012-1606(73)90248-0. [DOI] [PubMed] [Google Scholar]
- 122.van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 123.Poodry CA, Edgar L. Reversible alteration in the neuromuscular junctions of Drosophila melanogaster bearing a temperature-sensitive mutation, shibire. J Cell Biol. 1979;81:520–527. doi: 10.1083/jcb.81.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koenig JH, Kosaka T, Ikeda K. The relationship between the number of synaptic vesicles and the amount of transmitter released. J Neurosci. 1989;9:1937–1942. doi: 10.1523/JNEUROSCI.09-06-01937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Richmond JE, Broadie KS. The synaptic vesicle cycle: exocytosis and endocytosis in Drosophila and C. elegans. Curr Opin Neurobiol. 2002;12:499–507. doi: 10.1016/s0959-4388(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 126.Schwarz TL. Transmitter release at the neuromuscular junction. Int Rev Neurobiol. 2006;75:105–144. doi: 10.1016/S0074-7742(06)75006-1. [DOI] [PubMed] [Google Scholar]
- 127.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 128.Bellen HJ, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Venken KJ, Bellen HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nature Rev Genet. 2005;6:167–178. doi: 10.1038/nrg1553. [DOI] [PubMed] [Google Scholar]
- 130.Venken KJ, Bellen HJ. Transgenesis upgrades for Drosophila melanogaster. Development. 2007;134:3571–3584. doi: 10.1242/dev.005686. [DOI] [PubMed] [Google Scholar]
- 131.Hobert O. The impact of whole genome sequencing on model system genetics: get ready for the ride. Genetics. 2010;184:317–319. doi: 10.1534/genetics.109.112938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rong YS, et al. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 2002;16:1568–1581. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 134.Ni JQ, et al. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics. 2009;182:1089–1100. doi: 10.1534/genetics.109.103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 136.Groth AC, Calos MP. Phage integrases: biology and applications. J Mol Biol. 2004;335:667–678. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 137.Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 138.Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- 139.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 140.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 141.Venken KJ, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nature Methods. 2009;6:431–434. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Broadie K, Bate M. Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron. 1993;11:607–619. doi: 10.1016/0896-6273(93)90073-z. [DOI] [PubMed] [Google Scholar]
- 143.Broadie K. In: Drosophila Protocols. Sullivan W, Ashburner M, Hawley S, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2000. pp. 273–296. [Google Scholar]
- 144.Howlett IC, Tanouye MA. Neurocircuit assays for seizures in epilepsy mutants of Drosophila. J Vis Exp. 2009 Apr 15; doi: 10.3791/1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 146.Gu H, et al. Cav2-type calcium channels encoded by cac regulate AP-independent neurotransmitter release at cholinergic synapses in adult Drosophila brain. J Neurophysiol. 2009;101:42–53. doi: 10.1152/jn.91103.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rohrbough J, Broadie K. Electrophysiological analysis of synaptic transmission in central neurons of Drosophila larvae. J Neurophysiol. 2002;88:847–860. doi: 10.1152/jn.2002.88.2.847. [DOI] [PubMed] [Google Scholar]
- 148.Mamiya A, Beshel J, Xu C, Zhong Y. Neural representations of airflow in Drosophila mushroom body. PLoS ONE. 2008;3:e4063. doi: 10.1371/journal.pone.0004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci USA. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 151.Kitamoto T. Targeted expression of temperature-sensitive dynamin to study neural mechanisms of complex behavior in Drosophila. J Neurogenet. 2002;16:205–228. doi: 10.1080/01677060216295. [DOI] [PubMed] [Google Scholar]
- 152.Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 153.Luan H, et al. Functional dissection of a neuronal network required for cuticle tanning and wing expansion in Drosophila. J Neurosci. 2006;26:573–584. doi: 10.1523/JNEUROSCI.3916-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Miesenbock G. The optogenetic catechism. Science. 2009;326:395–399. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- 155.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 156.Harbison ST, Mackay TF, Anholt RR. Understanding the neurogenetics of sleep: progress from Drosophila. Trends Genet. 2009;25:262–269. doi: 10.1016/j.tig.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nature Rev Neurosci. 2009;10:549–560. doi: 10.1038/nrn2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Koh K, et al. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–376. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu MN, et al. SLEEPLESS, a Ly-6/neurotoxin family member, regulates the levels, localization and activity of Shaker. Nature Neurosci. 2010;13:69–75. doi: 10.1038/nn.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cirelli C, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 161.Axel R. Scents and sensibility: a molecular logic of olfactory perception (Nobel lecture) Angew Chem Int Ed Engl. 2005;44:6110–6127. doi: 10.1002/anie.200501726. [DOI] [PubMed] [Google Scholar]
- 162.Anderson DJ. Profile of David J. Anderson. Interview by Kaspar D. Mossman. Proc Natl Acad Sci USA. 2009;106:17623–17625. doi: 10.1073/pnas.0910003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lozano AM, Lang AE, Hutchison WD, Dostrovsky JO. New developments in understanding the etiology of Parkinson’s disease and in its treatment. Curr Opin Neurobiol. 1998;8:783–790. doi: 10.1016/s0959-4388(98)80122-0. [DOI] [PubMed] [Google Scholar]
- 164.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 165.Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 166.Valente EM, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 167.Greene JC, et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pesah Y, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 169.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 170.Lessing D, Bonini NM. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nature Rev Genet. 2009;10:359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Botas J. Drosophila researchers focus on human disease. Nature Genet. 2007;39:589–591. doi: 10.1038/ng0507-589. [DOI] [PubMed] [Google Scholar]
- 172.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 173.Iliadi KG. The genetic basis of emotional behavior: has the time come for a Drosophila model? J Neurogenet. 2009;23:136–146. doi: 10.1080/01677060802471650. [DOI] [PubMed] [Google Scholar]
- 174.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- 175.Fotowat H, Fayyazuddin A, Bellen HJ, Gabbiani F. A novel neuronal pathway for visually guided escape in Drosophila melanogaster. J Neurophysiol. 2009;102:875–885. doi: 10.1152/jn.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Manoli DS, Meissner GW, Baker BS. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 2006;29:444–451. doi: 10.1016/j.tins.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 177.Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- 178.Muller HJ. Genetic variability, twin hybrids and constant hybrids, in a case of balanced lethal factors. Genetics. 1918;3:422–499. doi: 10.1093/genetics/3.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muller HJ. Artificial transmutation of the gene. Science. 1927;66:84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- 180.Bridges CB. Salivary chromosome maps with a key to the banding of the chromosomes of Drosophila melanogaster. J Hered. 1935;26:60–64. [Google Scholar]
- 181.Stern C. Somatic crossing over and segregation in Drosophila melanogaster. Genetics. 1936;21:625–730. doi: 10.1093/genetics/21.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nature Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 183.Kunes S. Axonal signals in the assembly of neural circuitry. Curr Opin Neurobiol. 2000;10:58–62. doi: 10.1016/s0959-4388(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 184.Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- 185.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nature Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 186.Legent K, Treisman JE. Wingless signaling in Drosophila eye development. Methods Mol Biol. 2008;469:141–161. doi: 10.1007/978-1-60327-469-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nature Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 188.Korkut C, Budnik V. WNTs tune up the neuromuscular junction. Nature Rev Neurosci. 2009;10:627–634. doi: 10.1038/nrn2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nature Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- 190.Kaphingst K, Kunes S. Pattern formation in the visual centers of the Drosophila brain: wingless acts via decapentaplegic to specify the dorsoventral axis. Cell. 1994;78:437–448. doi: 10.1016/0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 191.Yoshida S, et al. DPP signaling controls development of the lamina glia required for retinal axon targeting in the visual system of Drosophila. Development. 2005;132:4587–4598. doi: 10.1242/dev.02040. [DOI] [PubMed] [Google Scholar]
- 192.Parker L, Ellis JE, Nguyen MQ, Arora K. The divergent TGF-beta ligand Dawdle utilizes an activin pathway to influence axon guidance in Drosophila. Development. 2006;133:4981–4991. doi: 10.1242/dev.02673. [DOI] [PubMed] [Google Scholar]
- 193.Serpe M, O’Connor MB. The metalloprotease tolloid-related and its TGF-b-like substrate Dawdle regulate Drosophila motoneuron axon guidance. Development. 2006;133:4969–4979. doi: 10.1242/dev.02711. [DOI] [PubMed] [Google Scholar]
- 194.Keshishian H, Kim YS. Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 2004;27:143–147. doi: 10.1016/j.tins.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 195.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFb/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 196.Ogawa K, et al. Activin-Nodal signaling is involved in propagation of mouse embryonic stem cells. J Cell Sci. 2007;120:55–65. doi: 10.1242/jcs.03296. [DOI] [PubMed] [Google Scholar]
- 197.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nature Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 198.Bardin AJ, Le Borgne R, Schweisguth F. Asymmetric localization and function of cell-fate determinants: a fly’s view. Curr Opin Neurobiol. 2004;14:6–14. doi: 10.1016/j.conb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 199.Carthew RW. Pattern formation in the Drosophila eye. Curr Opin Genet Dev. 2007;17:309–313. doi: 10.1016/j.gde.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Le Gall M, De Mattei C, Giniger E. Molecular separation of two signaling pathways for the receptor. Notch Dev Biol. 2008;313:556–567. doi: 10.1016/j.ydbio.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.de Bivort BL, Guo HF, Zhong Y. Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction. J Neurogenet. 2009;23:395–404. doi: 10.3109/01677060902878481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hou J, Tamura T, Kidokoro Y. Delayed synaptic transmission in Drosophila cacophony null embryos. J Neurophysiol. 2008;100:2833–2842. doi: 10.1152/jn.90342.2008. [DOI] [PubMed] [Google Scholar]
- 203.Xue M, et al. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron. 2009;64:367–380. doi: 10.1016/j.neuron.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bronk P, et al. The multiple functions of cysteine-string protein analyzed at Drosophila nerve terminals. J Neurosci. 2005;25:2204–2214. doi: 10.1523/JNEUROSCI.3610-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ohyama T, et al. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J Cell Biol. 2007;179:1481–1496. doi: 10.1083/jcb.200710061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schulze KL, et al. rop, a Drosophila homolog of yeast Sec1 and vertebrate n-Sec1/Munc-18 proteins, is a negative regulator of neurotransmitter release in vivo. Neuron. 1994;13:1099–1108. doi: 10.1016/0896-6273(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 207.Ly CV, Yao CK, Verstreken P, Ohyama T, Bellen HJ. straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel a1 subunit. J Cell Biol. 2008;181:157–170. doi: 10.1083/jcb.200712152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vilinsky I, Stewart BA, Drummond J, Robinson I, Deitcher DL. A Drosophila SNAP-25 null mutant reveals context-dependent redundancy with SNAP-24 in neurotransmission. Genetics. 2002;162:259–271. doi: 10.1093/genetics/162.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Broadie K, et al. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 210.Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 211.Wu MN, et al. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- 212.Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nature Neurosci. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- 213.Hiesinger PR, et al. The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell. 2005;121:607–620. doi: 10.1016/j.cell.2005.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang B, et al. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 215.Kasprowicz J, et al. Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake. J Cell Biol. 2008;182:1007–1016. doi: 10.1083/jcb.200804162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Heerssen H, Fetter RD, Davis GW. Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr Biol. 2008;18:401–409. doi: 10.1016/j.cub.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 218.Ramaswami M, Rao S, van der Bliek A, Kelly RB, Krishnan KS. Genetic studies on dynamin function in Drosophila. J Neurogenet. 1993;9:73–87. doi: 10.3109/01677069309083451. [DOI] [PubMed] [Google Scholar]
- 219.Verstreken P, et al. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- 220.Yao CK, et al. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–960. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Phillips AM, Ramaswami M, Kelly LE. Stoned Traffic. 11:16–24. doi: 10.1111/j.1600-0854.2009.00999.x. [DOI] [PubMed] [Google Scholar]
- 222.Verstreken P, et al. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 223.Verstreken P, et al. Tweek, an evolutionarily conserved protein, is required for synaptic vesicle recycling. Neuron. 2009;63:203–215. doi: 10.1016/j.neuron.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


