Abstract
Background.
The mechanisms by which testosterone increases hemoglobin and hematocrit remain unclear.
Methods.
We assessed the hormonal and hematologic responses to testosterone administration in a clinical trial in which older men with mobility limitation were randomized to either placebo or testosterone gel daily for 6 months.
Results.
The 7%–10% increase in hemoglobin and hematocrit, respectively, with testosterone administration was associated with significantly increased erythropoietin (EPO) levels and decreased ferritin and hepcidin levels at 1 and 3 months. At 6 months, EPO and hepcidin levels returned toward baseline in spite of continued testosterone administration, but EPO levels remained nonsuppressed even though elevated hemoglobin and hematocrit higher than at baseline, suggesting a new set point. Consistent with increased iron utilization, soluble transferrin receptor (sTR) levels and ratio of sTR/log ferritin increased significantly in testosterone-treated men. Hormonal and hematologic responses were similar in anemic participants. The majority of testosterone-treated anemic participants increased their hemoglobin into normal range.
Conclusions.
Testosterone-induced increase in hemoglobin and hematocrit is associated with stimulation of EPO and reduced ferritin and hepcidin concentrations. We propose that testosterone stimulates erythropoiesis by stimulating EPO and recalibrating the set point of EPO in relation to hemoglobin and by increasing iron utilization for erythropoiesis.
Key Words: Testosterone, Erythropoietin, Hepcidin, Ferritin.
Testosterone use in men has increased markedly over the past 15 years—reaching nearly $1.7 billion in prescription sales in 2012—due to numerous factors, including the increased awareness of androgen deficiency syndromes in men and the growing off-label use of testosterone for age-related decline in testosterone levels (1). Increased red blood cell mass (erythrocytosis) is the most common adverse event associated with testosterone therapy in clinical practice and in testosterone trials (2,3). Thus, understanding the mechanism of testosterone-induced erythrocytosis is important within the context of its safety, especially in older men, who experience greater increments in hemoglobin and hematocrit in response to testosterone administration than young men (4).
Historical studies in preclinical models had suggested that testosterone induces an erythropoiesis-stimulating factor, which was measured in these early studies by a bioassay using a polycythemic mouse model (5). In retrospect, this erythropoiesis-stimulating activity of plasma from testosterone-treated animals may not only have reflected the activity of erythropoietin (EPO) but may also have reflected other circulating factors that are regulated by testosterone and which modulate erythropoiesis or systemic iron bioavailability (5–7). However, human studies have not provided clear evidence that testosterone stimulates EPO secretion. For example, administration of graded doses of testosterone to healthy young and older men, whose endogenous testosterone production was suppressed by administration of a long-acting gonadotropin releasing hormone agonist, dose dependently increased hemoglobin and hematocrit but did not change EPO levels consistently after 20 weeks even at supraphysiologic doses of testosterone (4). Erythrocytosis in some other testosterone trials also was not associated with increased EPO levels, although these studies were likely underpowered to detect an effect given the interindividual variability in EPO levels (8–10). Furthermore, testosterone fails to directly activate EPO transcription in Hep3B cells, an EPO-secreting cell line that is highly sensitive to hypoxic induction (11), thus suggesting that any putative EPO-dependent mechanism for testosterone-induced erythrocytosis may be indirect, of modest magnitude, and/or transient. Accordingly, alternative mechanisms of testosterone-induced erythrocytosis have been suggested, including direct effects on bone marrow erythroblasts and on red cell survival (5,6). We have reported that administration of supraphysiologic doses of testosterone in healthy men suppresses the iron regulatory peptide hepcidin while EPO levels remained unchanged after 20 weeks of treatment (12). This observation raises the possibility that unchanged EPO levels reflect a higher biological activity of EPO, via increased iron bioavailability, which drives erythrocytosis. Considering these historic yet contrasting data, one hypothesis is that testosterone stimulates EPO transiently, along with suppression of hepcidin, and these two mechanisms result in a new EPO “set point” at a higher physiologic level of hemoglobin.
Here, we characterized the temporal changes in circulating EPO and hepcidin levels in relation to changes in hematological and hormonal parameters, in a randomized, placebo-controlled trial in which elderly men with low testosterone levels and mobility limitation were assigned to receive either testosterone or placebo gel daily for 6 months (13). The primary aim of The Testosterone in Older Men with Mobility Limitation Trial was to evaluate the effects of testosterone on muscle performance and physical function (13). Unlike many earlier trials, which included healthy older men, the Testosterone in Older Men with Mobility Limitation trial’s participants had physical dysfunction and high burden of comorbid conditions, such as diabetes, hypertension, obesity, and heart disease. Testosterone administration raised mean testosterone levels into the mid-normal range for young men and resulted in an increase in red blood cells that was accompanied by an increase in serum EPO, suppression of hepcidin and ferritin levels, and an increase in soluble transferrin receptor (sTR). To determine whether testosterone also stimulates EPO and erythropoiesis in older men with anemia, we performed sensitivity analyses in men who were anemic at baseline. Taken together with historical data, these observations provide important leads to the mechanism by which testosterone induces erythrocytosis.
Materials and Methods
The Testosterone in Older Men with Mobility Limitation trial was a randomized, double-blind, placebo-controlled trial whose design and primary results have been published (13). Eligible participants were randomized to placebo or testosterone gel based on concealed, computer-generated randomization tables, using a block size of 6. Randomization was stratified by age: (a) 65–74 and (b) 75 and older. Study personnel and participants were unaware of intervention assignment.
The enrollment in the trial was halted by the trial’s Data and Safety Monitoring Board due to an increase in cardiovascular events in the testosterone arm (13). At the time of trial’s cessation, 209 men had been randomized and 166 had completed 6 months of study intervention; these 166 men constituted the analytic sample for this investigation.
The eligibility criteria for the trial have been described (13). Briefly, ambulatory, community-dwelling men, aged 65 years and older, with mobility limitation, total testosterone 100–350ng/dL, or free testosterone <50 pg/mL, who had no contraindication to testosterone administration, were randomized to placebo or 10g testosterone gel daily for 6 months. Mobility limitation was defined as difficulty walking two blocks on a level surface or climbing 10 steps, and a score of 4–9 on the Short Physical Performance Battery indicating moderate degree of physical dysfunction. The primary outcome was the change from baseline in leg press strength at 6 months.
Testosterone levels were measured by a radioimmunoassay that has been validated against liquid chromatography tandem mass spectrometry (13) and has a sensitivity of 10ng/dL. Sex hormone-binding globulin (SHBG) was measured using an immunofluorometric assay with sensitivity 0.5 nmol/L (14). Free testosterone was calculated from total testosterone and SHBG concentrations using a published law-of-mass-action equation (14). Complete blood counts and red cell indices, ferritin, serum iron, iron binding capacity, and percentage saturation were measured at Quest Diagnostics (Cambridge, MA). Serum hepcidin was measured by enzyme-linked immunosorbent assay (Intrinsic Life Sciences) with sensitivity of 5ng/mL, interassay coefficient of variation 12%, and range 29–254ng/mL in men (12,15). Serum EPO was measured by a two-site immunoenzymatic sandwich assay on the DxI 800 system (Beckman Instruments, Chaska, MN). Intraassay coefficients of variation were 2.9% at 8.1 mIU/mL, 3.3% at 54.0 mIU/mL, and 4.4% at 340.0 mIU/mL. Interassay coefficients of variation were 3.7% at 14.3 mIU/mL, 2.6% at 55.1 mIU/mL, and 2.5% at 81.7 mIU/mL.
Statistical Analysis
Baseline variables were described using summary statistics and graphical assessments of trends. Changes in outcomes with time were modeled using Generalized Estimating Equations with unstructured correlation matrix and controlling for baseline outcome levels. We anticipated that trajectories with time would be nonlinear; therefore treatment effects were allowed to vary by visit. For each time point (1, 3, and 6 months postrandomization), the testosterone-vs-placebo effect was estimated using treatment contrasts. The statistical significance of effects was evaluated using Wald tests.
To characterize association between measured quantities (hemoglobin, hematocrit, etc.), we used generalized additive models. These models were used to evaluate trends in EPO with change in hemoglobin and hematocrit at multiple time points, stratified by treatment arm.
Results
Baseline Characteristics of the Participants
Among the 209 randomized men, 166 men, who completed the 6-month intervention, were evaluated at baseline, after 1, 3, and 6 months of intervention and 3 months after discontinuation of study intervention (Figure 1). The majority of participants had hypertension, approximately one half had heart disease, and one quarter had diabetes (13). The two groups were generally similar with respect to their baseline characteristics (Table 1). Serum hepcidin and ferritin levels were within the reported reference ranges for men and similar between groups (15). Compared with the testosterone group, slightly more participants in the placebo group were former smokers. A small percentage of men (<10%) in both groups were current smokers. Thirty-three participants were anemic based on the World Health Organization criteria (<13g/dL) (16) (Figure 1).
Figure 1.
CONSORT Diagram depicting the flow of participants, treatment arms, and attribution of nonanemic and anemic participants.
Table 1.
Baseline Characteristics of Study Subjects.
| Variable | Testosterone (82) | Placebo (84) |
|---|---|---|
| Age (y) | 74±6 | 74±5 |
| BMI (kg/m2) | 29.8±4.1 | 30.1±4.4 |
| Diabetes | 21 (26%) | 26 (31%) |
| Hyperlipidemia | 53 (65%) | 42 (50%) |
| Statin use | 53 (65%) | 39 (46%) |
| Smoking status | ||
| Current | 6 (7.4%) | 6 (7.1%) |
| Former | 55 (68%) | 58 (69%) |
| Never | 20 (25%) | 20 (24%) |
| Hemoglobin (g/dL) | 14.0±1.2 | 13.9±1.3 |
| Hematocrit (%) | 41.1±3.5 | 40.8±3.6 |
| Hepcidin (ng/mL) | 89±54 | 102±65 |
| Iron (mcg/dL) | 150±72 | 154±88 |
| IBC (mcg/dL) | 392±85 | 391±107 |
| TSAT (%) | 37±12 | 38±11 |
| Ferritin (ng/dL) | 115±79 | 132±97 |
| EPO (mIU/mL) | 13.5±11.9 | 11.7±6.6 |
| Total testosterone (ng/dL) | 252±56 | 236±63 |
| Free testosterone (ng/dL) | 4.8±2.6 | 4.3±1.7 |
| Estrone (pg/mL) | 35±18 | 31±13 |
| Estradiol (pg/mL) | 22±10 | 21±9 |
| Interleukin-6 (pg/mL) | 3.2±2.4 | 4.2±4.3 |
| sTR (nmol/L) | 19.2±5.2 | 21.9±10.4 |
| WBC (1000/µL) | 7.0±1.5 | 7.1±1.8 |
| MCV (fL) | 93.0±4.2 | 91.6±6.3 |
| MCHC (g/dL) | 34±0.9 | 34±0.8 |
| Lymphocytes (%) | 23.4±9.2 | 26.9±12.2 |
| Platlet (1000/µL) | 214±63 | 209±57 |
| RDW (%) | 13.9±1.1 | 14.0±1.5 |
| RBC (million/μL) | 4.42±0.43 | 4.43±0.72 |
| sTR/log hepcidin | 10.6±3.3 | 11.3±4.7 |
| sTR/log ferritin | 10.1±3.0 | 10.7±4.6 |
Notes: BMI = body mass index; EPO = erythropoietin; IBC = iron binding capacity; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; RBC = red blood cell; RDW = red cell distribution width; sTR = soluble transferrin receptor; TSAT = transferrin saturation; WBC = white blood cell.
The Effects of Testosterone on Hematocrit and Hemoglobin Levels, and Red Cell Indices in Elderly Men with Mobility Limitations
Testosterone administration increased mean testosterone levels (Figure 2) from low levels at baseline (252 ± 56ng/dL) into the mid-normal range (reference range 300–1000ng/dL) for healthy young men at 3 months (708 ± 327ng/dL) and 6 months (633 ± 419ng/dL), whereas testosterone levels remained unchanged in the placebo group (236 ± 63, 282 ± 126, and 290 ± 157ng/dL at baseline, 3 months, and 6 months, respectively). Calculated free testosterone also rose significantly in testosterone-treated men with a similar time course and also reaching a plateau at 3 months (Figure 2). In contrast, serum levels of E2 and E1 peaked at 6 months (Figure 2). As expected, hemoglobin and hematocrit increased in men assigned to testosterone group by an average 1.1g/dL and 4.4%, respectively (Table 2), representing 7% and 10% increase, respectively, from baseline. Hemoglobin and hematocrit levels in testosterone-treated men peaked at month 3 in most participants and remained at these elevated levels for the remainder of the intervention period. The increases in hemoglobin and hematocrit levels in men assigned to the testosterone arm were similar in magnitude to those reported in other testosterone replacement studies (17–20). Three months after discontinuation of testosterone administration, hemoglobin and hematocrit had returned to normal. Mean on-treatment hematocrit levels in men who experienced cardiovascular adverse events were similar to those without adverse events. Testosterone stimulated erythropoiesis specifically, as the red blood cell count increased (Table 2), while platelet counts showed small increases relative to placebo (Table 2). There was a small decrease in mean corpuscular volume and mean corpuscular hemoglobin concentration (Table 2) but no change in red cell distribution width or serum iron, iron-binding capacity, or percentage iron saturation (data not shown) in either group.
Figure 2.
Change in total (A) and free (B) testosterone levels and serum estrone (C) and estradiol (D) levels during the 6-month intervention period according to randomized assignment. Means and 95% CIs are depicted. *p < .05 for comparison between the placebo and testosterone groups.
Table 2.
Profile of Blood Parameters by Study Time, Mean (95% CI)
| One Month | Three Months | Six Months | Nine Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testosterone | Placebo | p-Value* | Testosterone | Placebo | p-Value | Testosterone | Placebo | p-Value | Testosterone | Placebo | p-Value | |
| Hemoglobin (g/dL) | 0.53 (−0.25, 1.31) | 0 (−0.60, 0.60) | .23 | 1.05 (0.80, 1.30) | 0.04 (−0.11, 0.18) | <.01 | 0.78 (0.52, 1.03) | −0.05 (−0.21, 0.12) | <.01 | 0.07 (−0.17, 0.31) | 0.05 (−0.17, 0.28) | .94 |
| Hematocrit (%) | 3.18 (0.39, 5.96) | −0.19 (−1.89, 1.52) | .03 | 3.59 (2.84, 4.34) | 0.18 (−0.26, 0.61) | <.01 | 2.96 (2.21, 3.71) | 0.07 (−0.43, 0.58) | <.01 | 0.22 (−0.52, 0.96) | 0.44 (−0.17, 1.05) | .65 |
| WBC (1000/µL) | 0.03 (−0.66, 0.71) | −0.50 (−1.44, 0.44) | .32 | −0.11 (−0.33, 0.12) | −0.57 (−0.86, −0.27) | .01 | −0.21 (−0.45, 0.02) | −0.62 (−0.91, −0.32) | .04 | −0.29 (−0.64, 0.07) | −0.35 (−0.76, 0.06) | .82 |
| MCV (fL) | 0.09 (−2.88, 3.06) | 0.75 (−2.17, 3.66) | .72 | −0.71 (−1.41, −0.01) | 0.64 (0.04, 1.24) | <.01 | −0.59 (−1.40, 0.22) | 0.63 (0.09, 1.16) | .01 | 0.78 (−0.01, 1.56) | 0.79 (−0.32, 1.90) | .99 |
| MCHC (g/dL) | −0.45 (−1.59, 0.69) | 0.01 (−0.49, 0.51) | .38 | −0.31 (−0.56, −0.07) | −0.10 (−0.32, 0.11) | .20 | −0.53 (−0.83, −0.23) | −0.09 (−0.30, 0.13) | .02 | −0.01 (−0.32, 0.30) | 0.39 (−1.17, 1.94) | .62 |
| Platelet (1000/µL) | −12.7 (−61.4, 36.0) | −15.5 (−32.1, 1.0) | .90 | 8.8 (0.8, 16.7) | −7.9 (−17.6, 1.8) | <.01 | 1.3 (−11.8, 14.4) | −6.8 (−13.4, −0.2) | .28 | −2.7 (−9.8, 4.4) | −4.1 (−12.7, 4.6) | .81 |
| RBC (million/uL) | 0.24 (−0.04, 0.52) | −0.05 (−0.23, 0.13) | .07 | 0.43 (0.35, 0.50) | 0.06 (−0.08, 0.19) | <.01 | 0.34 (0.25, 0.44) | 0.04 (−0.09, 0.17) | <.01 | 0.02 (−0.07, 0.12) | 0.07 (−0.11, 0.24) | .68 |
Notes: MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; RBC = red blood cell; WBC = white blood cell.
*Student’s t-tests.
Testosterone Administration Stimulates EPO and Shifts the EPO–Hemoglobin Relationship to the Right
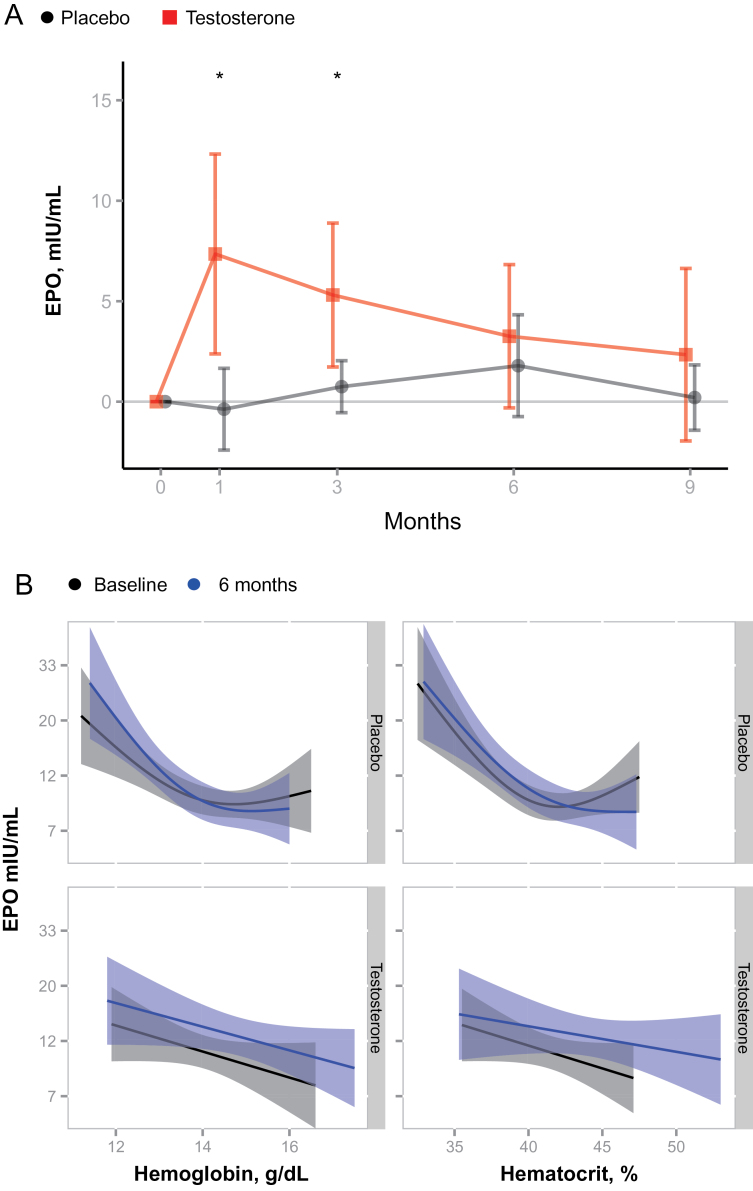
Testosterone administration significantly increased serum EPO level into the high normal range (13.5 ± 12 to 21.3 ± 17 mIU/mL) at 1 month; this 58% increase from baseline was statistically significant and remained significant at 3 months (Figure 3A). The placebo group showed no significant change in serum EPO level. Serum EPO levels trended toward baseline by 6 months in spite of continued testosterone administration, but remained nonsuppressed in spite of elevated levels of hemoglobin and hematocrit in testosterone-treated men.
Figure 3.
(A) Changes in erythropoietin (EPO) levels during testosterone or placebo administration. EPO levels increased significantly during treatment with testosterone compared with placebo. Mean and 95% CIs are shown. *p < .05 for comparison between the placebo and testosterone groups. (B) Testosterone administration shifts the log EPO to hemoglobin and log EPO to hematocrit curves, whereas placebo had no effect on this relationship. Fitted curves and 95% confidence regions are depicted (obtained by generalized additive models). The vertical shift in the top two panels indicates increased EPO per hemoglobin or hematocrit at end of testosterone treatment. No such shift is observed for placebo.
Steady-state levels of hemoglobin and hematocrit in healthy adults typically correlate negatively with log10 EPO levels (21). Thus, increased hemoglobin and hematocrit would normally be expected to suppress serum EPO levels. However, this linear-log relationship between hemoglobin levels and serum EPO levels was shifted to the right after 6 months of testosterone administration (Figure 3B); at each level of hemoglobin, serum EPO levels were greater by about 30% after testosterone administration than they had been at baseline. This rightward shift in the EPO–hemoglobin relationship curve suggests that testosterone administration had reset the “set point” for EPO in relation to hemoglobin.
Effects of Testosterone Administration on Hepcidin and Biomarkers of Iron Bioavailability
Testosterone administration resulted in a significant suppression of serum hepcidin levels (49%) (Figure 4A). The decrease in serum hepcidin levels in testosterone-treated men was greater than that in placebo-treated men at 1 and 3 months (Figure 4A). By the sixth month of treatment, hepcidin levels in men assigned to the testosterone arm tended to fall back toward baseline and were no longer significantly different from those in participants assigned to placebo arm. Three months after treatment discontinuation, hepcidin levels had rebounded slightly above baseline. The changes in circulating testosterone, estradiol, and estrone levels from baseline to 6 months were all significantly and negatively associated with changes in hepcidin concentrations. A difference of 100ng/dL total testosterone was associated with a mean (95% CI) difference of −2.8 (−5.6, −0.08) ng/mL in 6-month change in hepcidin. After controlling for testosterone changes, 1 pg/mL differences in change in E2 or E1 were associated with −0.93 (−1.37, −0.48) and −1.04 (−1.47, −0.61) differences in change in hepcidin, respectively. The variability in the circulating levels of hepcidin, a major iron regulating protein, was substantially greater than that in hemoglobin, which is the major iron-containing protein in the body. Hepcidin and hemoglobin levels were measured in the morning throughout the study to minimize variability due to the diurnal variation in testosterone and hepcidin. The coefficient of variation for hepcidin, hematocrit, and hemoglobin was 0.47 (0.41, 0.53), 0.04 (0.04, 0.05), and 0.04 (0.04, 0.04) SDs, respectively. Thus, in this sample of elderly men with mobility limitation, hepcidin displayed 10 times greater variability than hemoglobin.
Figure 4.
Changes in (A) serum hepcidin, (B) soluble transferrin receptor (sTR), (C) ferritin, (D) ferritin index (sTR/log ferritin), and (E) interleukin-6 (IL-6) in testosterone and placebo groups. Means and 95% CIs are depicted. *p < .05 for comparison between the placebo and testosterone groups.
Serum sTR concentration reflects total erythroid activity in rats and humans and has been shown to reflect plasma iron turnover and erythroid transferrin uptake very closely if iron deficiency is not present (22). Levels of sTR increased markedly in the testosterone group at 1 and 3 months and then returned toward baseline but remained higher than placebo at 6 months (Figure 4B). There were no significant changes in sTR in the placebo group. The changes in serum iron, total iron binding capacity, and transferrin saturation did not differ significantly between groups (data not shown). Serum ferritin levels decreased in the testosterone group in parallel with hepcidin but remained unchanged in the placebo group (Figure 4C). In parallel with hepcidin levels, a rebound increase in ferritin was observed after testosterone treated was stopped at 9 months. The ratio of sTR to log10 ferritin, which has been described as an index of iron-dependent erythropoietic activity, increased significantly in men assigned to the testosterone arm but did not change in those assigned to the placebo arm (Figure 4D). Taken together, these changes in hematologic parameters support the hypothesis that testosterone increases iron utilization for erythropoiesis. To explain the observed suppression of hepcidin, we considered the possibility that testosterone suppresses the cytokine interleukin-6, a known positive regulator of hepcidin (23). However, serum interleukin-6 levels were similar between groups at baseline and did not change significantly in either group (Figure 4E). Other markers of inflammation, C-reactive protein and tumor necrosis factor-α, also did not differ between groups.
Sensitivity Analyses in Older Men with Anemia
Anemia is commonly seen in older adults; it is not known whether anemic participants respond differently to testosterone than nonanemic participants. We therefore performed subgroup analyses on nonanemic (hemoglobin > 13g/dL, n = 133) and anemic (hemoglobin < 13g/dL, n = 33) men, using World Health Organization criteria (16). Evidence for occult iron deficiency was lacking in this population, as indicated by normal ferritin, serum iron and transferrin saturation, and normal mean corpuscular volume.
The hematologic changes in response to testosterone administration in anemic men were generally similar to those observed in nonanemic men and in the entire cohort. The changes in hemoglobin and hematocrit during testosterone administration did not differ significantly from those in the entire cohort (n = 166) as well as in nonanemic participants (Figure 5). After 6 months of testosterone administration, 64% of men who were anemic at baseline were no longer anemic.
Figure 5.
Changes in hemoglobin (A, B) and hematocrit (C, D) levels in response to testosterone or placebo administration in men who were anemic (hemoglobin <13g/dL) and in those who were not anemic at baseline. Means and 95% CIs are depicted. *p < .05 for comparison between the placebo and testosterone groups.
Discussion
We found that testosterone administration was associated with a consistent and significant elevation in EPO levels that was most pronounced at 1 and 3 months, along with suppression of hepcidin levels that also were most pronounced in the first 3 months. The strengths of this study include its prospective, randomized, placebo- controlled trial design, the relatively large patient population, and a sufficient study duration to assess the chronic effects of testosterone on numerous hematologic parameters. The limitations of this study include relatively infrequent analyses (monthly) and lack of analyses of changes in and response to hypoxia inducible factor (HIF), Von Hippel Lindau (VHL), and prolyl hydroxylase (PHD) levels/ activity that would allow for a more mechanistic interpretation. Hematologic changes (sTR/log ferritin) were consistent with the expected increased iron utilization to meet the demands of increased erythropoiesis. Previous studies of the effects of testosterone on EPO levels have been inconsistent. Many studies report no increase in EPO levels in testosterone-treated men, likely reflecting small sample sizes, variable treatment durations, and the failure to measure EPO longitudinally during testosterone administration (8–10). Even though serum EPO levels trended toward baseline by 6 months, they remained inappropriately elevated in relation to the elevated hemoglobin and hematocrit levels. The typical log-linear, negative correlation between EPO and hemoglobin levels was shifted to the right by testosterone administration (21). The EPO levels were higher than baseline at each hemoglobin level after testosterone administration; this was true even in participants who developed erythrocytosis. These observations are similar to those reported in Chuvash polycythemia due to homozygosity for VHL R200W, in which EPO levels are often in the normal range but inappropriately increased in the context of polycythemia (24). Our findings indicate that testosterone administration recalibrates the set point for EPO in relation to the prevalent hemoglobin concentrations resulting in higher EPO levels.
We also show that testosterone administration is associated with lower hepcidin levels in older men with mobility limitation and high burden of chronic diseases, confirming our previous observations in healthy younger and older men and demonstrating a similar magnitude of hepcidin suppression (12). Several observations reported in this article suggest that testosterone administration is associated with increased iron utilization. Serum sTR concentration reflects total erythroid activity and iron status and has been shown to reflect plasma iron turnover and erythroid transferrin uptake if iron deficiency is not present (22,25). The increased sTR levels in association with the reduced serum ferritin concentration during testosterone administration indicate that storage iron is being utilized to support the observed increase in hemoglobin concentration. We have reported recently that testosterone regulates hepcidin transcription in mice through regulation of bone morphogenetic protein (BMP) signaling mechanisms (26). Our studies in mice indicate that testosterone stimulates the incorporation of intravenously administered transferrin-bound diferric 58Fe into red blood cells (26). Furthermore, we have shown that testosterone increases hemoglobin accumulation in differentiating erythroleukemia cells (26). These observations from mice, when taken together with data from this trial, suggest that testosterone increases iron utilization for erythropoiesis.
The suppression of hepcidin alone cannot fully explain the polycythemia associated with testosterone administration because hemochromatosis patients with inactivating mutations in the hepcidin gene (HAMP) and mice with disruption of the hepcidin gene expression do not display a phenotype of polycythemia (27,28). Nonetheless, genetic alterations in iron regulatory genes in mice (Hamp knockout, ferroportin deletion mutant pcm mice) alone are sufficient to cause transient polycythemia, thus supporting the hypothesis that suppression of hepcidin by itself may play a contributory role in testosterone-induced erythrocytosis (28,29). Therefore, it is likely that increased EPO levels lead to increased erythropoiesis and increased iron utilization to support the increase in hemoglobin levels. Furthermore, erythroid progenitor cells in mice treated with testosterone have been reported to exhibit increased proliferative response to EPO (30,31), suggesting that testosterone may not only increase EPO levels but may also heighten the sensitivity of erythroid progenitor cells to EPO resulting in increased red cell production.
A potential mechanism for testosterone-induced increase in EPO levels could be via induction of hypoxia or hypoxic sensing, which could increase EPO secretion (32). However, many of the adaptations induced by testosterone—increased hemoglobin and hematocrit, increased red cell 2,3-bisphosphoglycerate, and increased muscle capillarity—would be expected to increase net oxygen delivery to the tissue. These observations do not preclude the possibility of a direct oxygen-independent effect of testosterone on the expression of hypoxia-inducible factors or on upstream regulators (VHL, PHD) or downstream regulators (HIFs primarily) of EPO transcription and secretion. Alternatively, testosterone could potentially exert direct effects on renal physiology that could alter EPO secretion from renal peritubular fibroblasts (33), thus establishing a new “set point” as seen in posttransplant erythrocytosis (34). Increased EPO levels, reduced iron stores due to increased utilization, or increased erythropoiesis all could secondarily affect hepcidin levels.
Our studies in mice suggest that testosterone directly suppresses hepcidin transcription independently of its effects on EPO levels (26). Recent studies have demonstrated that estradiol, which is the product of aromatase-catalyzed testosterone metabolism, regulates hepcidin transcription and systemic levels of hepcidin in vitro and in vivo, respectively (35–37). In our studies, testosterone and estradiol levels increased during treatment, both correlated negatively and similarly with change in serum hepcidin levels, although there was direct concordance between the temporal relationship of testosterone increase and hepcidin suppression. Serum iron concentrations did not change significantly in response to testosterone administration, thus making it unlikely that testosterone suppresses hepcidin levels by modulating systemic iron. A model for testosterone-induced erythrocytosis is proposed (Figure 6) that involves both EPO secretion and increased iron bioavailability (suppressed hepcidin) and that has experimental support from our work and that of others. Future studies are warranted to define the role that specific sex steroids play in the regulation of hepcidin and systemic iron homeostasis.
Figure 6.
Model illustrating the currently established mechanisms by which testosterone increases red cell mass: (1) testosterone directly inhibits BMP-Smad signaling in hepatocytes leading to suppression of hepcidin transcription (26), (2) testosterone stimulates renal secretion of EPO through unknown mechanisms that stimulates erythropoiesis, which could suppress hepcidin (this manuscript), (3) testosterone upregulates the expression of GATA-1- and GATA-dependent genes, which could increase EPO sensitivity and stimulate stress erythropoiesis (38), and (4) testosterone is converted to estradiol (E2), which can regulate hepcidin transcription (35–37).
The clinical consequences of erythrocytosis in testosterone-treated men have not been clarified but are of great interest given the increasing use of testosterone. It is not known whether erythrocytosis in response to testosterone administration in elderly men with mobility limitation poses risks or provides benefit. Large epidemiologic studies show that increased hematocrit levels are associated with increased risk of adverse cardiovascular events (39). The meta-analyses of randomized testosterone trials have, however, reported very low frequency of neuro-occlusive events (2,3,20). One potential explanation for these contrasting effects could be adaptation to erythrocytosis. EPO transgenic mice, for example, despite achieving hematocrit levels of ~90%, fail to develop thrombosis ostensibly due to lower than predicted blood viscosity, increased erythrocyte elasticity and deformability, and increased synthesis and release of nitric oxide from the endothelium and possibly from erythrocytes (40). An important clinical question will be the relative effect of testosterone at higher altitude as a contributor to excessive erythrocytosis (41). The clinical consequences of testosterone-induced erythrocytosis in men remain to be elucidated.
In summary, the increases in hemoglobin and hematocrit during testosterone administration to older men with mobility limitation and low testosterone levels were associated with increased EPO levels, a rightward shift in EPO–hemoglobin relationship curve, suppressed hepcidin levels, and increased iron utilization for erythropoiesis. Similar increases in hemoglobin and hematocrit were observed in men who were anemic and in those who were not anemic, raising the possibility that androgens may be useful in the treatment of anemia of aging. These results support the hypothesis that EPO secretion reaches a new, chronic physiologic “set point” in response testosterone administration. This new equilibrium set point for EPO/hemoglobin has precedent in the observation that men and women have different hemoglobin reference ranges yet similar EPO levels (42). In addition, patients with posttransplant erythrocytosis, renal dysfunction, and some populations who live at high elevation show evidence of a new EPO/hemoglobin set point (34,43,44). In the case of testosterone therapy or supplementation, this new set point appears become established after 3 months of treatment (4,10). Further studies are needed to test the hypothesis that testosterone stimulates erythropoiesis by increasing EPO secretion and resetting its relationship to hemoglobin and by increasing iron utilization for erythropoiesis.
Funding
This research was supported by the National Institute on Aging through a cooperative agreement 1UO1AG14369 and an RO1. Additional support was provided by the Boston Claude D. Pepper Older Americans Independence Center for Function promoting Anabolic Therapies.
Conflict of Interest
The authors report no conflicts of interest.
Acknowledgments
We thank Mark Westerman at Intrinsic Life Sciences for measuring hepcidin, and Drs. Victor Gordeuk and Joseph Prchal for helpful discussions. E.B. and S.B. (S. Bhasin) designed the experiments, E.B., S.B. and S.Ba (S. Basaria) collected the data, T.G.T. and M.N.D. analyzed the data, V.G. participated in discussions related to interpretation of data, and E.B. and S.B. wrote the manuscript.
References
- 1. Liverman CT, Blazer DG. Testosterone and Aging: Clinical Research Directions. Washington, DC: Joseph Henry Press; 2004 [PubMed] [Google Scholar]
- 2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559 [DOI] [PubMed] [Google Scholar]
- 3. Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–1457 [DOI] [PubMed] [Google Scholar]
- 4. Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab. 2008;93:914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mirand EA, Gordon AS, Wenig J. Mechanism of testosterone action in erythropoiesis. Nature. 1965;206(981):270–272 [DOI] [PubMed] [Google Scholar]
- 6. Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest. 2009;32(8):704–716 [DOI] [PubMed] [Google Scholar]
- 7. Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360 [DOI] [PubMed] [Google Scholar]
- 8. Ip FF, di Pierro I, Brown R, Cunningham I, Handelsman DJ, Liu PY. Trough serum testosterone predicts the development of polycythemia in hypogonadal men treated for up to 21 years with subcutaneous testosterone pellets. Eur J Endocrinol. 2010;162(2):385–390 [DOI] [PubMed] [Google Scholar]
- 9. Rochira V, Zirilli L, Madeo B, Maffei L, Carani C. Testosterone action on erythropoiesis does not require its aromatization to estrogen: insights from the testosterone and estrogen treatment of two aromatase-deficient men. J Steroid Biochem Mol Biol. 2009;113:189–194 [DOI] [PubMed] [Google Scholar]
- 10. Maggio M, Snyder PJ, Ceda GP, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1(1):24–28 [DOI] [PubMed] [Google Scholar]
- 11. Blanchard KL, Acquaviva AM, Galson DL, Bunn HF. Hypoxic induction of the human erythropoietin gene: cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Mol Cell Biol. 1992;12:5373–5385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bachman E, Feng R, Travison T, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95:4743–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74:512–519 [DOI] [PubMed] [Google Scholar]
- 15. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297 [DOI] [PubMed] [Google Scholar]
- 16. Izaks GJ, Westendorp RG, Knook DL. The definition of anemia in older persons. JAMA. 1999;281:1714–1717 [DOI] [PubMed] [Google Scholar]
- 17. Jockenhövel F, Vogel E, Reinhardt W, Reinwein D. Effects of various modes of androgen substitution therapy on erythropoiesis. Eur J Med Res. 1997;2:293–298 [PubMed] [Google Scholar]
- 18. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677 [DOI] [PubMed] [Google Scholar]
- 19. Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650 [DOI] [PubMed] [Google Scholar]
- 20. Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575 [DOI] [PubMed] [Google Scholar]
- 21. Beguin Y, Clemons GK, Oris R, Fillet G. Circulating erythropoietin levels after bone marrow transplantation: inappropriate response to anemia in allogeneic transplants. Blood. 1991;77:868–873 [PubMed] [Google Scholar]
- 22. Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057 [PubMed] [Google Scholar]
- 23. Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordeuk VR, Sergueeva AI, Miasnikova GY, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924–3932 [DOI] [PubMed] [Google Scholar]
- 25. Huebers HA, Beguin Y, Pootrakul P, Einspahr D, Finch CA. Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood. 1990;75:102–107 [PubMed] [Google Scholar]
- 26. Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12(2):280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pietrangelo A. Hemochromatosis: an endocrine liver disease. Hepatology. 2007;46(4):1291–1301 [DOI] [PubMed] [Google Scholar]
- 28. Lesbordes-Brion JC, Viatte L, Bennoun M, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108(4):1402–1405 [DOI] [PubMed] [Google Scholar]
- 29. Mok H, Jelinek J, Pai S, et al. Disruption of ferroportin 1 regulation causes dynamic alterations in iron homeostasis and erythropoiesis in polycythaemia mice. Development. 2004;131(8):1859–1868 [DOI] [PubMed] [Google Scholar]
- 30. Moriyama Y, Fisher JW. Effects of testosterone and erythropoietin on erythroid colony formation in human bone marrow cultures. Blood. 1975;45:665–670 [PubMed] [Google Scholar]
- 31. Malgor LA, Valsecia M, Vergés E, De Markowsky EE. Blockade of the in vitro effects of testosterone and erythropoietin on Cfu-E and Bfu-E proliferation by pretreatment of the donor rats with cyproterone and flutamide. Acta Physiol Pharmacol Ther Latinoam. 1998;48:99–105 [PubMed] [Google Scholar]
- 32. Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27(1):41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan X, Suzuki N, Hirano I, Yamazaki S, Minegishi N, Yamamoto M. Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS One. 2011;6(10):e25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vlahakos DV, Marathias KP, Agroyannis B, Madias NE. Posttransplant erythrocytosis. Kidney Int. 2003;63:1187–1194 [DOI] [PubMed] [Google Scholar]
- 35. Yang Q, Jian J, Katz S, Abramson SB, Huang X. 17β-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology. 2012;153(7):3170–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ikeda Y, Tajima S, Izawa-Ishizawa Y, et al. Estrogen regulates hepcidin expression via GPR30-BMP6-dependent signaling in hepatocytes. PLoS One. 2012;7(7):e40465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hou Y, Zhang S, Wang L, et al. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511(2):398–403 [DOI] [PubMed] [Google Scholar]
- 38. Guo W, Li M, Bhasin S. Testosterone supplementation improves anemia in aging male mice. J Gerontol A Biol Sci Med Sci. 2013. [Epub ahead of print]. PMID: 23974081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21(7):515–520 [DOI] [PubMed] [Google Scholar]
- 40. Schuler B, Arras M, Keller S, et al. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Natl Acad Sci U S A. 2010;107:419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzales GF, Gasco M, Tapia V, Gonzales-Castañeda C. High serum testosterone levels are associated with excessive erythrocytosis in chronic mountain sickness in men. Am J Physiol Endocrinol Metab. 2009;296(6):E1319–E1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murphy WG, Tong E, Murphy C. Why do women have similar erythropoietin levels to men but lower hemoglobin levels? Blood. 2010;116:2821–2862 [DOI] [PubMed] [Google Scholar]
- 43. Fehr T, Ammann P, Garzoni D, et al. Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int. 2004;66:1206–1211 [DOI] [PubMed] [Google Scholar]
- 44. van Patot MC, Gassmann M. Hypoxia: adapting to high altitude by mutating EPAS-1, the gene encoding HIF-2α. High Alt Med Biol. 2011;12(2):157–167 [DOI] [PubMed] [Google Scholar]