Abstract
Background.
The age-specific prevalence and incidence of dementia and cognitive impairment in the United States have either remained stable or even slightly declined during the 1980s–1990s. A suggested but untested reason for this improvement in cognitive function over time is higher educational attainment among more recent cohorts.
Methods.
We used data from two large prospective population-based epidemiological dementia studies conducted in two adjacent regions during the period 1987–2012. We examined whether (i) cohort effects could be observed in age-associated trajectories of cognitive functions and (ii) the observed cohort effects could be explained by educational attainment. Trajectories of neuropsychological tests tapping three domains (psychomotor speed, executive function, and language) were compared among cohorts born between 1902 and 1911, 1912 and 1921, 1922 and 1931, and 1932 and 1943. We examined Age × Cohort interactions in mixed-effects models with/without controlling for education effects.
Results.
Cohort effects in age-associated trajectories were observed in all three domains, with consistent differences between the earliest born cohort and the most recent cohort. Executive functions showed the strongest and persistent differences between the most recent and other three cohorts. Education did not attenuate any of these associations.
Conclusions.
Cohort effects were observed in all examined cognitive domains and, surprisingly, remained significant after controlling for educational effects. Factors other than education are likely responsible for the cohort effects in cognitive decline.
Key Words: Cognitive aging, Cognitive impairment, Dementia, Epidemiology, Socioeconomic status.
It has been reported that the age-specific prevalence and incidence of dementia and cognitive impairment in the United States have either remained stable or even declined during the 1980s–1990s (1,2). A recent Dutch study also showed age-adjusted dementia incidence rates to be consistently, although nonsignificantly, lower in the subcohort assessed in 2000 than that assessed a decade earlier (3). In behavioral sciences, one of the persistent predictors of disease prevalence, incidence, and mortality is years of educational attainment. Although there is still a debate regarding potential mechanisms underlying the association between education and overall health (eg, income, accessibility to health care, lifestyle and environment, mother’s nutrition during prenatal period, nutrition during infancy, etc.), we expect that cognitive functions can be very much affected by educational attainment. Besides the above potential effects of education on overall health, test-taking skill (which influences performance on neuropsychological tests) can be also associated with educational attainment. Furthermore, where educational opportunities are uniform, higher educasstional level may reflect higher intelligence and cognitive reserve (4), and more highly educated individuals may undertake occupations, which are more cognitively stimulating and require “continuing education” throughout life. The cognitive stimulation may itself further stimulate synaptic density and dendritic branching (4). Yet, there is a paucity of studies, which examine the extent to which educational attainment explains observed cohort effects in cognitive aging, specifically age-associated cognitive decline measured longitudinally over time. Facing a rapid rate of population aging, there is growing interest in projecting future trends in dementia prevalence and incidence. These projections would be benefit from information on whether educational attainment explains cohort effects on cognitive trajectories.
In a population-based cohort of older adults, the present study assessed whether (i) cohort effects could be observed in age-associated trajectories of cognitive functions and (ii) the observed cohort effects could be explained by changes in educational attainment among cohorts. Practice or learning effects, which refer to the improvement in cognitive test scores over repeated administrations of cognitive tests, could possibly mask or distort the age-associated cognitive trajectories (5,6). Therefore, we also examined cohort differences in practice effects in the above assessments. Trajectories of neuropsychological tests tapping three cognitive domains (psychomotor speed, executive function, and language) were compared among cohorts born between 1902 and 1911, 1912 and 1921, 1922 and 1931, and 1932 and 1943.
Data
Data come from two large epidemiological studies of dementia: the Monongahela Valley Independent Elders Study (aka, MoVIES) and the Monongahela-Youghiogheny Healthy Aging Team study (aka, MYHAT). The two studies were conducted in geographically contiguous areas of southwestern Pennsylvania between 1987 and 2012. Both studies recruited age-stratified random samples of individuals aged 65 and older from the Voter Registration lists for targeted communities. Brief descriptions of each study are given below.
MoVIES.
This project recruited and assessed 1,681 individuals during the years 1987–1989 from a group of largely rural communities and followed them biennially until 2001 to investigate incidence, risk factors for cognitive impairment, and dementia. Details of sampling, recruitment, assessments, and follow-up have been reported previously (7,8). At baseline, the study response rate was approximately 60%.
MYHAT.
This project recruited and assessed 1,982 individuals during the years 2005–2007 from a group of small-town communities and followed them annually to investigate outcomes and predictors of outcomes in mild cognitive impairment. Details of sampling, recruitment, assessments, and follow-up have been reported previously (9,10). The study is currently in the sixth wave of data collection. At baseline, the study response rate was approximately 63% (11).
Pooling data from the two studies, we categorized participants into the following four 10-year birth cohorts: those born between 1902 and 1911, between 1912 and 1921, between 1922 and 1931, and between 1932 and 1943. We excluded 46 participants born before 1901 from longitudinal analyses due to small sample size.
Educational attainment.
We used three education categories: less than high school education, completed high school education but less than college education, and completed college or more education.
Neuropsychological tests.
The following four neuropsychological tests were administered in the identical manner across two studies and were, therefore, examined in this study: Trail Making Test A (attention/psychomotor speed) (12); Trail Making Test B (executive function) (12); verbal fluency for initial letters P and S, aka letter fluency (executive function); and verbal fluency for the category of animals, aka category fluency (language) (13). The Trail Making Tests are conventionally scored in the time (in seconds) to complete the test, but in our population-based cohort, this measure showed skewed distributions and ceiling effects. Hence, we calculated the number of correct connections per second (connections/s) to use in the current study. Although both studies assessed memory in detail, they used different memory tests as MoVIES addressed dementia and MYHAT was focused on mild cognitive impairment; we, therefore, did not include memory measures in the present study. For fair comparisons of magnitudes of coefficients across four cognitive tests, all tests were standardized using mean and standard deviation of each test score at baseline of the combined data set.
Statistical Analysis
Differences in educational attainment by birth cohorts were compared using Pearson chi-square statistics. We used mixed-effects models with the outcomes being standardized scores on each cognitive test to examine age-associated cognitive trajectories. We fit two models (Models 1 and 2) for each outcome. In both models, we controlled for sex, practice effects, which identify second and third assessments (two dummy variables, each indicating second or third assessment) and its interaction with a variable indicating MYHAT cohort. The interactions were included because annual assessment in MYHAT (as opposed to biannual assessment in MoVIES) could lead to larger practice effects. In Model 1, we included age at each assessment, cohort, and Cohort × Age interactions (for assessing cohort effect on age-associated cognitive trajectories) and Cohort × Practice effects (for assessing cohort differences in practice effects observed at second and third assessments). In Model 2, we added education, Age × Education, and Age × Education × Cohort interactions. In exploratory analysis, we also examined nonlinear effects (age2, age3), but these were not significant and not included in the final model; practice effects were adequate to capture nonlinearity. Age was centered at age 80 for efficient convergence. Intercept and age were treated as random effects. Model fitness was examined through visual inspection of residuals and formal statistical tests. We used SAS version 9.2 (SAS Institute, Inc.) and R version 2.11 (R Foundation) for statistical analyses.
Results
As the MYHAT project is focused on mild cognitive impairment rather than dementia, 54 participants with age-education-adjusted Mini-Mental State Examination score less than or equal to 21 (14,15) at recruitment were considered too impaired for a study of mild cognitive impairment; they were triaged out at screening and excluded from further assessment. To make the two cohorts comparable, we excluded 37 subjects from MoVIES cohort applying the same Mini-Mental State Examination criterion. The remaining 1,644 subjects in MoVIES, together with 1,982 MYHAT participants, comprised the pooled sample for the analyses reported here. Average education level increased with successive birth cohorts (Table 1); 58.7%, 61.5%, 36.7%, and 14.8% of the individuals born in 1882–1901, 1902–1911, 1912–1921, and 1922–1931 had less than high school graduate level education, respectively, whereas only 6.6% of the individuals born in 1932–1943 had less than high school graduate level education (cohort differences in educational distribution, p < .001).
Table 1.
Combined MoVIES–MYHAT Sample: Educational Level by Birth Cohort
| Birth Cohort | Education | ||||
|---|---|---|---|---|---|
| <High School Graduate | High School Graduate | >High School Graduate | Total | % From MYHAT Cohort | |
| n (row %) | n (row %) | n (row %) | |||
| 1882–1901 | 27 (58.70%) | 8 (17.39%) | 11 (23.91%) | 46 | 0 |
| 1902–1911 | 244 (61.00%) | 62 (15.50%) | 94 (23.50%) | 400 | 3.00% |
| 1912–1921 | 505 (36.41%) | 546 (39.37%) | 336 (24.22%) | 1,387 | 23.4% |
| 1922–1931 | 159 (14.79%) | 506 (47.07%) | 410 (38.14%) | 1,075 | 86.3% |
| 1932–1943 | 47 (6.55%) | 307 (42.76%) | 364 (50.70%) | 718 | 100.0% |
Notes: Birth cohort 1882–1901 was not used in the subsequent analysis due to small sample size. MoVIES = Monongahela Valley Independent Elders Study; MYHAT = Monongahela-Youghiogheny Healthy Aging Team study.
Models Unadjusted for Education Effects
In the mixed-effects models (Tables 2–5), for all four outcomes (cognitive test scores), we observed significant baseline cohort effects, as well as significant Age × Cohort interaction effects in Model 1, using the latest, most recent birth cohort (1932–1943) as the reference group. A negative coefficient of Age × Cohort interactions means that the earlier birth cohort has steeper age-associated cognitive declines compared with the latest birth cohort. The earliest birth cohort (1902–1911) had significantly steeper age-associated declines than the latest birth cohort (1932–1943) in all cognitive outcomes (coefficient = −.03, p = .003 for speed; coefficient = −.07, p < .0001 for executive function [Trail Making Test B]; coefficient = −.05, p < .0001 for executive function [letter fluency]; and coefficient = −.05, p < .0001 for language). For executive function indicated by Trail Making Test B, all three cohorts showed significantly steeper decline compared with the most recent birth cohort, with larger declines for earlier birth cohort (coefficient = −.03 for the 1922–1931 cohort, −.05 for the 1912–1921 cohort, and −.07 for the 1902–1911 cohort). Letter fluency, another measure of executive functions, showed a trend similar to Trail Making Test B, although the 1922–1931 cohort was not statistically different from the most recent cohort (p = .11). We observed significantly higher practice effects at the second and third assessments for Trail Making Test B and lower practice effects among MYHAT cohort at the third assessment for Language, compared with MoVIES cohort.
Table 2.
Results of Mixed-Effects Models Examining Differences in Age-Associated Cognitive Slope by Birth Cohorts: Psychomotor Speed (Trail Making Test A, connections/s) as Outcome
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p Value | Coefficient | SE | p Value | |
| Intercept | .02 | 0.08 | .79 | .10 | 0.08 | .19 |
| Female | .19 | 0.03 | <.0001 | .19 | 0.03 | <.0001 |
| Age | −.05 | 0.007 | <.0001 | −.04 | 0.007 | <.0001 |
| Second assessment | .05 | 0.05 | .26 | .06 | 0.05 | .21 |
| Third assessment | .02 | 0.05 | .76 | .02 | 0.05 | .72 |
| Second assessment × MYHAT | −.02 | 0.04 | .67 | −.02 | 0.04 | .56 |
| Third assessment × MYHAT | .06 | 0.04 | .14 | .06 | 0.04 | .16 |
| Cohort 1902–1911 | −.77 | 0.09 | <.0001 | −.56 | 0.09 | <.0001 |
| Cohort 1912–1921 | −.51 | 0.08 | <.0001 | −.39 | 0.08 | <.0001 |
| Cohort 1922–1931 | −.24 | 0.08 | .002 | −.21 | 0.08 | .006 |
| Age × Cohort 1902–1911 | −.03 | 0.009 | .003 | −.03 | 0.009 | .0009 |
| Age × Cohort 1912–1921 | −.002 | 0.008 | .77 | −.005 | 0.008 | .53 |
| Age × Cohort 1922–1931 | .002 | 0.008 | .82 | −.0001 | 0.008 | .99 |
| Second assessment × Cohort 1902–1911 | .007 | 0.06 | .91 | −.003 | 0.06 | .96 |
| Second assessment × Cohort 1912–1921 | .009 | 0.05 | .85 | .003 | 0.05 | .95 |
| Second assessment × Cohort 1922–1931 | −.02 | 0.04 | .56 | −.02 | 0.04 | .52 |
| Third assessment × Cohort 1902–1911 | −.05 | 0.07 | .49 | −.05 | 0.07 | .44 |
| Third assessment × Cohort 1912–1921 | −.02 | 0.05 | .68 | −.02 | 0.05 | .64 |
| Third assessment × Cohort 1922–1931 | −.05 | 0.04 | .20 | −.05 | 0.04 | .20 |
| Education | ||||||
| <High school | −.45 | 0.04 | <.0001 | |||
| =High school | −.08 | 0.03 | 0.009 | |||
| Age × Education (<high school) | .004 | 0.004 | 0.28 | |||
| Age × Education (=high school) | .0001 | 0.004 | 0.98 | |||
Notes: Reference groups: male; Cohort 1932–1943; Age × Cohort 1932–1943; Age × Education (>high school). Age variable was centered at age 80 (ie, age at each assessment −80). Age and intercept are treated as random effects. Second and third assessments refer to the practice (assessment) effects at the second and third assessments. MYHAT = Monongahela-Youghiogheny Healthy Aging Team study.
Table 5.
Results of Mixed-Effects Models Examining Differences in Age-Associated Cognitive Slope by Birth Cohorts: Language (Category Fluency Animals) as Outcome
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p Value | Coefficient | SE | p Value | |
| Intercept | .35 | 0.08 | <.0001 | .47 | 0.08 | <.0001 |
| Female | −.04 | 0.03 | .12 | −.04 | 0.03 | .14 |
| Age | −.03 | 0.008 | .0004 | −.02 | 0.008 | .001 |
| Second assessment | .01 | 0.05 | .77 | .02 | 0.05 | .69 |
| Third assessment | .07 | 0.05 | .20 | .07 | 0.05 | .18 |
| Second assessment × MYHAT | −.01 | 0.04 | .76 | −.02 | 0.04 | .68 |
| Third assessment × MYHAT | −.10 | 0.04 | .03 | −.1 | 0.04 | .02 |
| Cohort 1902–1911 | −.89 | 0.09 | <.0001 | −.69 | 0.09 | <.0001 |
| Cohort 1912–1921 | −.64 | 0.08 | <.0001 | −.52 | 0.08 | <.0001 |
| Cohort 1922–1931 | −.39 | 0.08 | <.0001 | −.35 | 0.08 | <.0001 |
| Age × Cohort 1902–1911 | −.05 | 0.01 | <.0001 | −.05 | 0.01 | <.0001 |
| Age × Cohort 1912–1921 | −.01 | 0.008 | .14 | −.01 | 0.008 | .10 |
| Age × Cohort 1922–1931 | −.006 | 0.009 | .50 | −.008 | 0.008 | .35 |
| Second assessment × Cohort 1902–1911 | .10 | 0.07 | .15 | .09 | 0.07 | .20 |
| Second assessment × Cohort 1912–1921 | .04 | 0.05 | .42 | .03 | 0.05 | .49 |
| Second assessment × Cohort 1922–1931 | .03 | 0.04 | .45 | .03 | 0.04 | .48 |
| Third assessment × Cohort 1902–1911 | −.04 | 0.07 | .56 | −.05 | 0.07 | .50 |
| Third assessment × Cohort 1912–1921 | −.01 | 0.05 | .83 | −.01 | 0.05 | .78 |
| Third assessment × Cohort 1922–1931 | .06 | 0.04 | .16 | .06 | 0.04 | .16 |
| Education | ||||||
| <High school | −.48 | 0.04 | <.0001 | |||
| =High school | −.19 | 0.04 | <.0001 | |||
| Age × Education (<high school) | −.003 | 0.004 | .56 | |||
| Age × Education (=high school) | −.001 | 0.004 | .81 | |||
Notes: Reference groups: male; Cohort 1932–1943; Age × Cohort 1932–1943; Age × Education (>high school). Age variable was centered at age 80 (ie, age at each assessment −80). Age and intercept are treated as random effects. Second and third assessments refer to the practice (assessment) effects at the second and third assessments. MYHAT = Monongahela-Youghiogheny Healthy Aging Team study.
Table 3.
Results of Mixed-Effects Models Examining Differences in Age-Associated Cognitive Slope by Birth Cohorts: Executive Functions (Trail Making Test B, connections/s) as Outcome
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p Value | Coefficient | SE | p Value | |
| Intercept | .40 | 0.08 | <.0001 | .49 | 0.08 | <.0001 |
| Female | .13 | 0.03 | <.0001 | .12 | 0.03 | <.0001 |
| Age | −.01 | 0.007 | .09 | −.01 | 0.007 | .07 |
| Second assessment | −.08 | 0.05 | .09 | −.08 | 0.05 | .11 |
| Third assessment | −.02 | 0.05 | .71 | −.02 | 0.05 | .77 |
| Second assessment × MYHAT | .09 | 0.04 | .02 | .09 | 0.04 | .03 |
| Third assessment × MYHAT | .16 | 0.04 | .0003 | .16 | 0.04 | .0004 |
| Cohort 1902–1911 | −1.24 | 0.09 | <.0001 | −.95 | 0.09 | <.0001 |
| Cohort 1912–1921 | −.95 | 0.08 | <.0001 | −.77 | 0.08 | <.0001 |
| Cohort 1922–1931 | −.54 | 0.08 | <.0001 | −.49 | 0.08 | <.0001 |
| Age × Cohort 1902–1911 | −.07 | 0.009 | <.0001 | −.08 | 0.009 | <.0001 |
| Age × Cohort 1912–1921 | −.05 | 0.008 | <.0001 | −.05 | 0.008 | <.0001 |
| Age × Cohort 1922–1931 | −.03 | 0.008 | .0001 | −.03 | 0.008 | <.0001 |
| Second assessment × Cohort 1902–1911 | .02 | 0.06 | .72 | .01 | 0.06 | .85 |
| Second assessment × Cohort 1912–1921 | .09 | 0.05 | .07 | .08 | 0.05 | .09 |
| Second assessment × Cohort 1922–1931 | −.007 | 0.04 | .84 | −.009 | 0.04 | .81 |
| Third assessment × Cohort 1902–1911 | −.06 | 0.07 | .39 | −.07 | 0.07 | .31 |
| Third assessment × Cohort 1912–1921 | .005 | 0.05 | .92 | −.0007 | 0.05 | .99 |
| Third assessment × Cohort 1922–1931 | −.05 | 0.04 | .22 | −.05 | 0.04 | .20 |
| Education | ||||||
| <High school | −.60 | 0.04 | <.0001 | |||
| = High school | −.12 | 0.03 | <.0001 | |||
| Age × Education (<high school) | .008 | 0.004 | .04 | |||
| Age × Education (=high school) | .0005 | 0.004 | .89 | |||
Notes: Reference groups: male; Cohort 1932–1943; Age × Cohort 1932–1943; Age × Education (>high school). Age variable was centered at age 80 (ie, age at each assessment −80). Age and intercept are treated as random effects. Second and third assessments refer to the practice (assessment) effects at the second and third assessments. MYHAT = Monongahela-Youghiogheny Healthy Aging Team study.
Table 4.
Results of Mixed-Effects Models Examining Differences in Age-Associated Cognitive Slope by Birth Cohorts: Executive Functions (Letter Fluency P and S) as Outcome
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p Value | Coefficient | SE | p Value | |
| Intercept | .37 | 0.08 | <.0001 | .51 | 0.08 | <.0001 |
| Female | .17 | 0.03 | <.0001 | .17 | 0.03 | <.0001 |
| Age | .003 | 0.007 | .65 | .004 | 0.007 | .54 |
| Second assessment | .02 | 0.04 | .65 | .02 | 0.04 | .57 |
| Third assessment | .08 | 0.05 | .08 | .08 | 0.05 | .07 |
| Second assessment × MYHAT | −.005 | 0.04 | .88 | −.01 | 0.04 | .78 |
| Third assessment × MYHAT | −.05 | 0.04 | .15 | −.06 | 0.04 | .12 |
| Cohort 1902–1911 | −.85 | 0.09 | <.0001 | −.57 | 0.09 | <.0001 |
| Cohort 1912–1921 | −.66 | 0.08 | <.0001 | −.48 | 0.08 | <.0001 |
| Cohort 1922–1931 | −.43 | 0.08 | <.0001 | −.38 | 0.08 | <.0001 |
| Age × Cohort 1902–1911 | −.05 | 0.009 | <.0001 | −.05 | 0.009 | <.0001 |
| Age × Cohort 1912–1921 | −.02 | 0.008 | .004 | −.02 | 0.008 | .005 |
| Age × Cohort 1922–1931 | −.01 | 0.008 | .11 | −.01 | 0.008 | .08 |
| Second assessment × Cohort 1902–1911 | .009 | 0.06 | .88 | .0005 | 0.06 | .99 |
| Second assessment × Cohort 1912–1921 | −.007 | 0.04 | .87 | −.01 | 0.04 | .77 |
| Second assessment × Cohort 1922–1931 | −.02 | 0.03 | .53 | −.02 | 0.03 | .50 |
| Third assessment × Cohort 1902–1911 | −.06 | 0.06 | .34 | −.06 | 0.06 | .29 |
| Third assessment × Cohort 1912–1921 | −.03 | 0.04 | .51 | −.03 | 0.04 | .46 |
| Third assessment × Cohort 1922–1931 | −.03 | 0.03 | .43 | −.03 | 0.03 | .42 |
| Education | ||||||
| <High school | −.64 | 0.04 | <.0001 | |||
| =High school | −.20 | 0.04 | <.0001 | |||
| Age × Education (<high school) | −.006 | 0.004 | .15 | |||
| Age × Education (=high school) | .0007 | 0.004 | .86 | |||
Notes: Reference groups: male; Cohort 1932–1943; Age × Cohort 1932–1943; Age × Education (>high school). Age variable was centered at age 80 (ie, age at each assessment −80). Age and intercept are treated as random effects. Second and third assessments refer to the practice (assessment) effects at the second and third assessments. MYHAT = Monongahela-Youghiogheny Healthy Aging Team study.
Models Adjusted for Education Effects
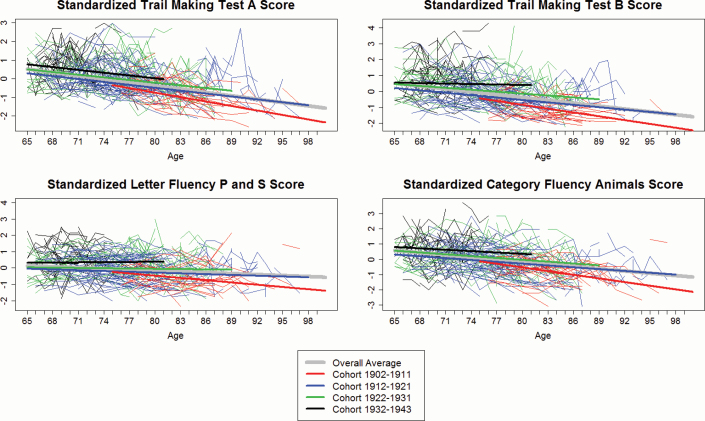
The patterns observed in cognitive test scores and cohorts, both trajectory and practice effects, unchanged after we included education, Age × Education, and Age × Education × Cohort effects in Model 2. As no three-way interactions were significant, none were included in the final model reported. Finally, we estimated marginal age-associated trajectories by cohort for each outcome (Figure 1, colored bold lines) using the coefficients estimated by the mixed-effects model after factoring/removing practice effects. In Figure 1, for illustration, we also show the observed trajectory of 300 randomly selected subjects. As expected, the age range observed during follow-up varies by birth cohort, but it is clear that the later born cohorts have much shallower age-associated declines. As a post hoc analysis, we applied the same model to men and women separately, with results remarkably similar to those using the combined data. The only difference was that Age × Cohort 1912–1921 lost significance for the outcome of executive functions (letter fluency P and S) among women while retaining its significance among men (results not shown in Tables). The levels of gains in cognitive functions in the most recently born birth cohort seem similar between genders.
Figure 1.
Estimated age-associated trajectories based on the coefficients obtained from the mixed-effects models (fully controlled models) by birth cohorts and plots of observed data for randomly selected 300 individual subjects.
Discussion
Using data from two large epidemiological cohort studies conducted in the same region, we assessed birth cohort effects in age-associated trajectories of cognitive functions. We examined three domains of cognitive functions including psychomotor speed, executive functions, and language. Four birth cohorts—those born between 1902 and 1911, 1912 and 1921, 1922 and 1931, and 1932 and 1941 (the reference group)—were compared both on their baseline cognitive test scores and also on their subsequent cognitive trajectories. Cohort effects in age-associated trajectories were observed in all examined cognitive domains, with consistent differences between the earliest born cohort and the most recent cohort across all cognitive domains. The domain of executive functions showed the strongest and persistent differences between the most recent and other three cohorts. Education did not attenuate any of these associations.
In the social science field, socioeconomic status, for which educational attainment is the major component, has been one of the robust predictors of mortality and health. One of the earliest and most comprehensive studies on the socioeconomic status and mortality in the United States was conducted by Kitagawa and Hauser (16), a few decades ago. They found a strong inverse relation between mortality and educational attainment, with the range of mortality differentials being larger among individuals 25–64 years of age than among older individuals, and greater among women than men. The mortality disadvantage of those with lower socioeconomic status has been found to be mainly due to their unhealthy behaviors including smoking, alcohol consumption, dietary patterns, and physical activity (17–25). For example, using nationwide Finnish health behavior surveys from the years 1979 to 2001, Laaksonen and colleagues (18) found that educational level showed a graded association with cardiovascular disease, coronary heart disease, stroke, and all-cause mortality. They found smoking, low vegetable consumption, and physical inactivity explained a substantial part of educational level differences in the mortality. Recent studies conducted in England using a very large cohort of civil servants also showed a strong association between socioeconomic position and mortality (26,27). This association was substantially accounted for by adjustment for health behaviors including diet, physical activities, and alcohol consumption (26), and almost half of the differences in incidence of type 2 diabetes by socioeconomic status (27) was explained by potentially modifiable risk factors such as health behaviors and obesity.
As for cognitive health and educational attainment, it has been observed that during the 20th century, subsequent cohorts always outperformed previous generations on IQ tests. This is known as the Flynn Effect (28). Schaie and colleagues (29), using the Seattle Longitudinal Study, further showed that cohort differences in intelligence occurred even before 1900s (earlier than Flynn’s studies), with a more dramatic improvement being observed between those born before 1900 (median birth year of 1896) and those born in the early 1900s (median birth year 1924), with somewhat diminished improvements among subsequent cohorts. Schaie and colleagues (29) speculated that a dramatic increase in educational attainment among those born around 1924 (mainly due to the GI Bill), compared with those born around 1896, could have contributed to marked cohort differences for both verbal ability (so-called crystallized intelligence, which is thought to be improved through schooling) and inductive reasoning (fluid intelligence). Although Flynn’s theory refers to the improvement in fluid intelligence, Schaie hypothesized that both crystallized and fluid intelligence are interrelated, and thus improvement in both intelligence could be seen as cohort gains higher educational attainment. Based on past studies, which showed links between overall health and socioeconomic status/education (16–25) as discussed earlier and between educational attainment and cognition (28,29), we hypothesized that we would see cohort effects in age-associated trajectories of both language fluency and executive function, and that the cohort differences in trajectory could be explained by educational attainment (ie, the cohort differences would be attenuated if we controlled for educational attainment in the model). Consistently with Flynn’s study results, we found strong cohort effects in executive functions (fluid ability). However, we also found cohort effects in verbal ability at least between the earliest born cohort (1902–1911) and the most recent cohorts (1932–1943). Unexpectedly, educational attainment did not attenuate any of the cohort differences, either in baseline test scores, in age-associated trajectories of cognitive scores, or in practice effects. Additionally, a post hoc analysis, where models were run separately by men and women, showed no major differences in the results between genders, suggesting that the gains in cognitive functions are similar between genders and are not limited to men (eg, through educational benefits under the GI Bill).
One potential explanation for our finding (ie, no attenuation of cohort effects on cognitive trajectories after controlling for educational attainment) includes that our study participants were aged at least 65 years. As Kitagawa and Hauser (16) have shown, the beneficial effect of education effect is stronger in younger and middle-aged adults and may diminish with advancing age. Possibly, factors other than educational attainment (eg, general improvement in nutritional intake, more cognitively stimulating environment among recent birth cohorts) could play larger roles in determining cognitive trajectory among those aged 65 and older we examined in this study. Alternatively, quality of education could differ across cohorts, a factor difficult to capture by simply using grade level. Finally selective attrition, including from mortality, could also distort the true association. However, attrition bias mostly occurs when follow-up is truncated (due to dropout), preventing observation of subsequent cognitive decline. If this was the case, earlier cohorts with higher mortality should have shown better cognitive trajectories than later cohorts, given other factors being equal (ie, trajectory is truncated before declining). This was not the case in our finding.
Cohort effects in age-associated trajectories of cognitive function could have a large impact on the incidence and prevalence of late-onset dementia. A less steep trajectory of cognitive decline among the most recently born cohort could potentially mean that onset of dementia may be delayed compared with the earliest born cohort. Although examining this impact is beyond the scope of our current study, there have been some indications (without statistical significance) that age-specific dementia prevalence has either remained stable or declined slightly between 1980s and 1990s in the United States (1,2). Incidence also showed statistically nonsignificant yet declining trends in Europe; Sacuiu and colleagues (30) showed dementia incidence between age 70 and 75 years was 5.0% in cohort born in 1901–1902 and 4.4% in cohort born in 1930 during the 5 years of follow-up in Sweden. Schrijvers and colleagues (3) also showed that dementia incidence has decreased between 1990 and 2005 using data from the Rotterdam Study. In the latter study, participants in 2005–2006 had statistically significant larger total brain volumes and less cerebral small vessel disease (although nonsignificant in men) than participants in 1995–1996. In contrast to the United States and some European countries, however, all-cause dementia prevalence seems to be increasing in some other regions including Japan, possibly due to increase in metabolic diseases (31). The large increase in obesity and metabolic diseases in the United States and other regions in the 21st century (32) could also influence future trends in dementia incidence and prevalence. Furthermore, our study showed that educational attainments per se do not explain the upward trends in cognitive test scores. Thus, it is premature to predict that future generations will have lower prevalence of dementia. It remains highly uncertain whether the compression of morbidity (33) will apply to cognitive health. Factors, which reduce or increase dementia prevalence at population levels, should be carefully monitored, for example, by estimating population attributable risk.
Strengths of our study include repeated measures of cognitive functioning being administered in the same manner between the two cohorts across the same region, with relatively large sample sizes and low levels of in- and out-migration. Given that we recruited the cohorts from a stable general population, the internal validity of our results is high. However, since both studies were limited to one geographical area in Pennsylvania, and the cohorts were both predominantly white, replication of our study in other regions and more ethnically diverse populations will help establish external validity.
Conclusions
We observed strong cohort effects with the more recent cohorts showing less age-associated cognitive declines. Surprisingly, the cohort effects remained very significant after controlling for educational effects. Factors other than education are likely responsible for the cohort effects in cognitive trajectories among those aged 65 and older. Future research should focus on identifying the factors that potentially explain or modify the observed age-associated cognitive decline.
Funding
Funding sources: R01AG023651, R01AG07562, P30AG008017, and R01AG033581 from National Institute on Aging, National Institutes of Health, and U.S. Department of Health and Human Services .
References
- 1. Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Langa KM, Larson EB, Karlawish JH, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining?: trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78:1456–1463 [DOI] [PubMed] [Google Scholar]
- 4. Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:112–117 [DOI] [PubMed] [Google Scholar]
- 5. Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodge HH, Wang CN, Chang CC, Ganguli M. Terminal decline and practice effects in older adults without dementia: the MoVIES project. Neurology. 2011;77:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurology. 2000;54:1109–1116 [DOI] [PubMed] [Google Scholar]
- 8. Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol. 1993;48:M152–M161 [DOI] [PubMed] [Google Scholar]
- 9. Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: the Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. 2010;18:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganguli M, Fu B, Snitz BE, Hughes TF, Chang CC. Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology. 2013;80:2112–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganguli M, Snitz B, Vander Bilt J, Chang CC. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. 2009;24:1277–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reitan RM. Validity of the Trail-making Tests as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276 [Google Scholar]
- 13. Lezak MD. Neuropsychological Assessment. New York, NY: Oxford University Press; 1995 [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 15. Mungas D, Marshall SC, Weldon M, Haan M, Reed BR. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology. 1996;46:700–706 [DOI] [PubMed] [Google Scholar]
- 16. Kitagawa EM, Hauser PM. Differential Mortality in the United States: A Study in Socioeconomic Epidemiology. Cambridge, MA: Harvard University Press; 1973 [Google Scholar]
- 17. Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44:809–819 [DOI] [PubMed] [Google Scholar]
- 18. Laaksonen M, Talala K, Martelin T, et al. Health behaviours as explanations for educational level differences in cardiovascular and all-cause mortality: a follow-up of 60 000 men and women over 23 years. Eur J Public Health. 2008;18:38–43 [DOI] [PubMed] [Google Scholar]
- 19. Martikainen P, Brunner E, Marmot M. Socioeconomic differences in dietary patterns among middle-aged men and women. Soc Sci Med. 2003;56:1397–1410 [DOI] [PubMed] [Google Scholar]
- 20. Mäkinen TE, Sippola R, Borodulin K, et al. Explaining educational differences in leisure-time physical activity in Europe: the contribution of work-related factors. Scand J Med Sci Sports. 2012;22:439–447 [DOI] [PubMed] [Google Scholar]
- 21. Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279:1703–1708 [DOI] [PubMed] [Google Scholar]
- 22. Schrijvers CT, Stronks K, van de Mheen HD, Mackenbach JP. Explaining educational differences in mortality: the role of behavioral and material factors. Am J Public Health. 1999;89:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strand BH, Tverdal A. Can cardiovascular risk factors and lifestyle explain the educational inequalities in mortality from ischaemic heart disease and from other heart diseases? 26 year follow up of 50,000 Norwegian men and women. J Epidemiol Community Health. 2004;58:705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Oort FV, van Lenthe FJ, Mackenbach JP. Material, psychosocial, and behavioural factors in the explanation of educational inequalities in mortality in The Netherlands. J Epidemiol Community Health. 2005;59:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woodward M, Oliphant J, Lowe G, Tunstall-Pedoe H. Contribution of contemporaneous risk factors to social inequality in coronary heart disease and all causes mortality. Prev Med. 2003;36:561–568 [DOI] [PubMed] [Google Scholar]
- 26. Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303:1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stringhini S, Tabak AG, Akbaraly TN, et al. Contribution of modifiable risk factors to social inequalities in type 2 diabetes: prospective Whitehall II cohort study. BMJ. 2012;345:e5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flynn JR. Massive IQ gains in 14 nations: what IQ tests really measure. Psychol Bull. 1987;101:71–191 [Google Scholar]
- 29. Schaie KW, Willis SL, Pennak S. An Historical Framework for Cohort Differences in Intelligence. Res Hum Dev. 2005;2:43–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sacuiu S, Gustafson D, Sjögren M, et al. Secular changes in cognitive predictors of dementia and mortality in 70-year-olds. Neurology. 2010;75:779–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dodge HH, Buracchio TJ, Fisher GG, et al. Trends in the prevalence of dementia in Japan. Int J Alzheimers Dis. 2012;2012:956354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145 [DOI] [PubMed] [Google Scholar]
- 33. Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135 [DOI] [PubMed] [Google Scholar]