Abstract
Sarcopenia leads to many changes in skeletal muscle that contribute to atrophy, force deficits, and subsequent frailty. The purpose of this study was to characterize motor unit remodeling related to sarcopenia seen in extreme old age. Whole extensor digitorum longus muscle and motor unit contractile properties were measured in 19 adult (11–13 months) and 12 oldest old (36–37 months) Brown-Norway rats. Compared with adults, oldest old rats had significantly fewer motor units per muscle, smaller muscle cross-sectional area, and lower muscle specific force. However, mean motor unit force generation was similar between the two groups due to an increase in innervation ratio by the oldest old rats. These findings suggest that even in extreme old age both fast- and slow-twitch motor units maintain the ability to undergo motor unit remodeling that offsets some effects of sarcopenia.
Key Words: Motor unit, Innervation ratio, Sarcopenia, Aging.
Sarcopenia is an age-related condition typified by decreased muscle mass and weakness, which begins during the fifth decade of human life (1). As with other sequelae of aging, sarcopenia leads to increased frailty, impaired mobility, loss of independence, and general disability (2). In addition, sarcopenia confers a substantial economic burden to both the individual and society, as its health care costs have been estimated to exceed $18 billion per year in the United States (3). With greater awareness and understanding of the morbidity associated with sarcopenia, there has been considerable interest in efforts to rehabilitate aged muscle and an emphasis on intervention through exercise training to reverse the deterioration of muscle function (4).
The relentless process of aging results in a progressive degradation of the neuromuscular system, manifested by a steady decline in muscle mass and strength (5,6). In humans, it has been shown that skeletal muscle exhibits a linear decline in size after age 50 of approximately 1% per year, and this correlates with findings of reduced force production with increasing age (7–9). At the level of the single motor unit, aging is associated with muscle atrophy as well as a dynamic process of denervation and reinnervation of muscle fibers (10). It is well known that aged muscles display a decreased total number of muscle fibers as a direct consequence of loss of motor neurons (11,12). When a motor neuron undergoes cell death, there is resultant denervation of muscle fibers within its territory. Adjacent motor neurons reinnervate the denervated muscle fibers through terminal axonal sprouting, which increases the innervation ratio of these motor units (13). However, aging is associated with a decreased capacity for reinnervation and thus there is incomplete reclamation of denervated muscle fibers, leading to a reduction in the number of motor units and an increase in the number of denervated muscle fibers (10).
Over the past few decades, many studies in both animals and humans have characterized the phenomenon of age-related motor unit remodeling and preferential atrophy of Type II muscle fibers with increasing age (14). However, particular attention should be given to those in advanced age (>80 years old) as these individuals can demonstrate a greater than 50% reduction in strength, leading to the inability to perform activities of daily living (15). It remains unclear whether patients in advanced age retain the ability to undergo motor unit remodeling and what compensatory mechanisms are functioning. The purpose of this study is to quantify the normal adaptation of extremely old motor units to sarcopenia by examining whole muscle and motor unit contractile properties in oldest old rats.
Methods
The experimental group consisted of 12 oldest old (36–37 months) specific pathogen-free Brown-Norway rats (Charles River Laboratories, Wilmington, MA). Longevity curves for the Brown-Norway rat show that less than 20% remain living at 36–37 months (16). Knowing that the presence of age-related changes is more pronounced in late life, this study used rats with ages far beyond the 20–30 month age range used by other studies to represent extremely old humans (17). Nineteen adult (11–13 months old) Brown-Norway rats served as the adult control group. All animal care, housing, and operative procedures were conducted in accordance with the Guide for the Care of Laboratory Animals (National Institute of Health publication no. 85–23).
Rats were anesthetized with an initial intraperitoneal injection of pentobarbital sodium (60mg/kg for adult and 30mg/kg for oldest old rats); supplemental doses (15mg/kg for adult and 5mg/kg for oldest old rats) were administered as necessary to maintain a deep plane of anesthesia. The airway was secured via a tracheostomy and insertion of an 18-gauge flexible catheter. After an oblique skin incision was made in the left groin crease, the femoral and obturator nerves to the medial, anterior, and lateral thigh musculature were identified and divided to eliminate contractions during performance of the in situ measurements of whole muscle and single motor unit contractile properties.
Whole Muscle Contractile Properties
An incision was made on the lateral aspect of the left thigh, and dissection proceeded deep to the biceps femoris until the sciatic nerve was exposed from the sciatic notch to the anterior and lateral compartment of the lower leg. The superior gluteal, tibial, and sural nerves were divided distally and reflected proximally in the thigh. The common peroneal nerve was carefully dissected free from surrounding connective tissue; the deep branch of the peroneal nerve was then meticulously preserved, whereas the superficial branch of the peroneal nerve was divided. To minimize motion artifact from adjacent muscles during stimulation, the peroneus longus, peroneus brevis, peroneus tertias, gastrocnemius, soleus, plantaris, and tibialis anterior muscles were divided distally and reflected out of the operative field. The distal tendon of the extensor digitorum longus (EDL) was identified, divided, and folded into a tendon loop. Throughout the operation and evaluation, the EDL and peroneal nerve were regularly bathed with warm mineral oil (36°C).
In situ measurements of whole muscle force contractile properties were performed on the EDL muscle as previously described (18–20). The rat was placed supine on a platform with the femoral condyle and foot firmly secured; the EDL tendon loop was attached to a force transducer (BG-1000; Kulite Semiconductor Products, Leonia, NJ). A shielded bipolar silver wire electrode (Harvard Apparatus, Type I, South Natick, MA) was then used to deliver supramaximal stimuli (square pulses, 0.2ms pulse duration, 2–6V) generated by a Grass S88 Stimulator (Grass Instrument Co., Quincy, MA) to the proximal peroneal nerve in order to indirectly activate the EDL muscle. Twitch contractions were utilized to determine the optimal muscle length (L o) for force production, which was subsequently used for all isometric force measurements. During single twitch contractions, peak twitch force (F t), time to peak tension, and half relaxation time were recorded. To measure maximum isometric tetanic force (F o), the EDL muscle was stimulated for 250ms at increasing frequencies from 30 to 300 Hz. Two minutes were allowed between tetanic contractions to permit muscle recovery. The total muscle fiber cross-sectional area (CSA) was determined as previously described (20–22). The maximum specific isometric tetanic force (sF o) was calculated as the maximum tetanic force (F o) divided by the total muscle fiber CSA.
Single Motor Unit Contractile Properties
An incision was made along the midline back, and dissection proceeded to the spinous processes. The operative field was maintained at a temperature of 36°C by frequent application of warm mineral oil and a thermocouple-regulated heat lamp. The posterior bony elements of the thoracic and lumbar spinal column were carefully removed while preserving the integrity of the spinal cord. The spinal cord was divided at the midthoracic level, and the distal portion was reflected caudally to allow identification of the ventral roots supplying axons to the peroneal nerve (L4, L5, L6). Dissection proceeded under the operating microscope until a single motor axon was isolated. Evoked twitches were accepted as resulting from a single motor unit when the response was “all-or-none” and there was no further increase in twitch force with increases in stimulation amplitude (23). After a single motor unit had been confirmed, the contractile properties were measured using the same procedure as outlined for the whole muscle force measurements.
Classification of Physiologic Motor Unit by Contractile Properties
Motor units were classified based upon their functional response to standard sag and fatigue protocols (23,24). Sag was defined as the gradual decline in force production during an incompletely fused 700-millisecond tetanic contraction. The stimulation frequency for the determination of sag was the inverse of 1.25 times the time to peak tension (seconds). All motor units that exhibited sag were classified as fast-twitch motor units and those that did not display sag were classified as slow-twitch motor units. A standard fatigue protocol was then utilized to further classify the fast motor units based upon their oxidative and glycolytic capabilities (24). Single motor units were stimulated for 325 milliseconds at a frequency of 40 Hz every second for a 2-minute period (23,24). The fatigue index was defined as the measured isometric force at the end of the 2-minute stimulation period divided by the maximum force recorded during stimulation, which was usually at the initiation of the protocol. Fast motor units with fatigue indices of 0.75 or greater were classified as fast fatigue resistant (FR); those with indices between 0.75 and 0.25 were classified as fast fatigue intermediate (FI); those with indices less than 0.25 were classified as fast fatigable (FF) (25).
Histochemistry and Morphometry of Muscle
After completion of whole muscle and single motor unit force measurements, the EDL muscle was harvested and frozen at resting length for histochemical processing. Serial transverse 10-µm-thick cross sections were obtained through the midportion of the EDL muscle. The samples were stained with hematoxylin and eosin and myosin ATPase. Muscle fibers were subsequently typed as either slow oxidative (Type I), fast oxidative glycolytic (Type IIA), or fast glycolytic (Type IIB) based upon differential myosin ATPase staining (26). For each rat, a representative section was selected for evaluation. A semiautomatic computer-assisted planimetry program (Bioquant, R and M Biometrics, Inc., Nashville, TN) was used on two-dimensional images of EDL muscle sections to measure whole muscle CSA and muscle fiber CSA. For each EDL muscle section, the numbers of Type I, IIA, and IIB fibers (identified by myosin ATPase staining) were counted and divided by the total number of muscle fibers in the cross section to determine the proportion of each fiber type. From this information, the relative CSA and thus the proportion of surface area occupied by each fiber type were computed. This ratio was also applied to whole muscle sF o in order to yield the proportion of force generated by each muscle fiber type.
Motor Unit Numbers and Innervation Ratios
The total number of motor units was estimated for individual EDL muscles by dividing their whole muscle F o by the mean motor unit F o for that muscle. Only EDL muscles where at least six motor units were measured were included in this analysis. The numbers of motor units for each of four motor unit classifications were calculated per EDL muscle. The calculation was based on the proportion of each motor unit classification multiplied by the estimated total number of motor units for an individual whole EDL muscle. Force production values of motor units of the same classification were summed together (tF o). Then the percentage contribution of each motor unit type to whole muscle force was determined by dividing whole muscle F o by tF o. The number of muscle fibers innervated by a single axon, or innervation ratio, was then calculated for each motor unit utilizing an indirect method. This calculation was based on two assumptions: (a) classification of muscle fibers based upon contractile properties was not different from classification of muscle fibers based upon histological examination and (b) each motor unit is comprised of the same histological fiber type. The first assumption is founded on data from a number of models demonstrating correspondence between the four classifications of motor units and the three types of muscle fibers (S corresponds to Type I; FR and FI correspond to Type IIA; FF corresponds to Type IIB) (26,27). The second is based on motor unit glycogen depletion studies with concurrent histological analysis (28). Mean single fiber F o for each of the three muscle fiber types were estimated from the tF o attributed to each of the three motor unit classifications (collapsed from four) within the whole muscle. This was achieved by dividing the tF o by the counted number of fibers of that type on histological examination. To ascertain the innervation ratio for each motor unit, the measured motor unit F o was divided by the mean single fiber F o of the corresponding fiber type.
Data Analysis
For each variable measured, the data are expressed as mean ± standard deviation. Individual group means such as for body mass, whole muscle, and motor units numbers from adult and oldest old rats were compared using a t test. Data were compared with a two-way analysis of variance for (a) age group by motor unit type and (b) age group by muscle fiber types. If an overall statistical difference was found, a post hoc Tukey method was applied to obtain applicable subgroup comparisons between age groups or within each age group. Mann–Whitney U tests were used to analyze data that were not normally distributed. Statistical calculations were performed using Statistical Analysis System (SAS, Inc., Cary, NC). For this study, α was set a priori at .05.
Results
A total of 31 male Brown-Norway rats were entered into the study, 19 adult and 12 oldest old. Although both groups displayed similar mean body masses, muscle sarcopenia was evident in the oldest old group. The mean EDL muscle mass of the oldest old rats was almost 40% less than that of the adult rats (Table 1). In addition, L o and CSA were also significantly less in the oldest old rats compared with the adult rats. Oldest old EDL muscle displayed 65% less maximum tetanic isometric force (F o) compared with adult EDL muscle. When F o was normalized to whole muscle CSA to calculate specific force (sF o), oldest old EDL muscle exhibited 46% less sF o in comparison to the adult EDL muscle.
Table 1.
Data Summary Demonstrating Whole Extensor Digitorum Longus Muscle Sarcopenia in Oldest Old Rats
| Adult Rats n = 19 | Oldest Old Rats n = 12 | |
|---|---|---|
| Age (mo) | 12±1 | 36±1 |
| Body mass (g) | 444±31 | 439±47 |
| Muscle mass (mg) | 181±19 | 110±19* |
| L o (mm) | 34.3±1.2 | 32.3±1.6* |
| L f (mm) | 12.0±0.5 | 11.3±0.6 |
| CSA (mm2) | 14.3±1.6 | 9.3±1.9* |
| F o (mN) | 3920±384 | 1388±353* |
| sF o (kN/m2) | 278±29 | 150±30* |
Notes: Data are expressed as mean ± standard deviation. L o = optimal muscle length for force production; L f = calculated extensor digitorum longus muscle fiber length; CSA = cross-sectional area; F o = whole muscle maximum tetanic isometric force; sF o = whole muscle specific force.
*Denotes statistically significant difference compared with adult rats (p < .05).
Overall, 164 motor units in the adult rats and 82 motor units in the oldest old rats were identified and evaluated for their contractile properties (Table 2). There was no difference in mean motor unit force between adult and oldest old rats. However, oldest old motor units demonstrated slower contractile properties (time to peak tension and half relaxation time), and there were significantly lower (68%) mean number of motor units present within the oldest old EDL muscles compared with adult muscles.
Table 2.
Summary of Data for Single Motor Units Recorded From Extensor Digitorum Longus Muscle of Adult and Oldest Old Rats
| Adult Rats, n = 18 (of 19) | Oldest Old Rats, n = 10 (of 12) | |
|---|---|---|
| Motor units measured | 164 | 82 |
| Motor units measured per rat | 9.1±4.4 | 8.2±2.8 |
| Motor unit F o (mN) | 62.9±32.2 | 68.5±41.9 |
| Motor unit TPT (ms) | 13.9±3.6 | 15.8±4.2* |
| Motor unit HRT (ms) | 14.1±4.9 | 17.8±7.0* |
| Estimated motor units per muscle | 62.7±19.1 | 20.2±7.2* |
Notes: Data are expressed as mean ± standard deviation. Note that only muscles that had six or more motor units measured were included in the analysis. F o = motor unit maximum tetanic isometric force, TPT = time to peak tension, HRT = half relaxation time.
*Denotes statistically significant difference compared with adult rats (p < .05).
Analysis of motor unit contractile properties was performed with consideration of the four physiologic motor unit classifications (Table 3). Oldest old rats possessed approximately half the number of S, FI, and FF motor units per EDL muscle compared with adult rats; more striking was the paucity in number of FR motor units in oldest old rats. Although there was no difference in overall mean motor unit force between adult and oldest old rats, when motor units were compared by physiologic classification, slow-twitch motor unit F o of oldest old rats was nearly 60% greater than slow motor units in adult rats. In contrast, no significant differences were found between the F o of FR, FI, and FF motor units of adult versus oldest old rats.
Table 3.
Data Summary for Adult and Oldest Old Rat Extensor Digitorum Longus Muscle Motor Units Classified by Contractile Properties
| Motor Unit Classification | S | FR | FI | FF |
|---|---|---|---|---|
| Total number of motor units sampled | ||||
| Adult | 22 | 71 | 58 | 13 |
| Oldest old | 17 | 8 | 46 | 11 |
| Proportion of motor units per muscle | ||||
| Adult | 13.4% | 43.3% | 35.4% | 7.9% |
| Oldest old | 20.7% | 9.8% | 56.1% | 13.4% |
| Estimated number of motor units per muscle | ||||
| Adult | 8.4±8.6 | 27.1±18.6 | 22.2±1.9 | 5.0±7.0 |
| Oldest old | 4.2±2.5* | 2.0±3.5* | 11.3±6.8* | 2.7±2.7* |
| Motor unit F o (mN) | ||||
| Adult | 24.7±10.6 | 66.4±29.3 | 65.3±34.3 | 105.6±27.8 |
| Oldest old | 39.1±23.5* | 65.5±29.9 | 73.7±44 | 94.3±62.2 |
Notes: Data are expressed as counts, percentage, or mean ± standard deviation. F o = whole muscle maximum tetanic isometric force, S = slow oxidative, FR = fast fatigue resistant, FI = fast fatigue intermediate, FF = fast fatigable.
*Denotes statistically significant difference compared with adult rats (p < .05).
Histochemical analysis permitted comparison of muscle fiber types between adult versus oldest old rats (Table 4). There were no differences in mean CSA of Type I and IIA muscle fibers, but there was a significant decrease in CSA of Type IIB muscle fibers of the oldest old rats. Within both groups, the mean CSA and number of Type IIB fibers was greater than that of Type I and Type IIA fibers, which is consistent with the fact that the EDL muscle is composed predominately of fast-twitch motor units (29). No significant differences were found between the two groups regarding the percentage of the total EDL muscle CSA occupied by each muscle fiber type. Overall, there was no significant difference in total muscle fiber numbers between adult and oldest old groups; oldest old rats did display 21% fewer Type IIB fibers compared with adult rats although this difference was not statistically significant. All muscle fiber types of oldest old rats showed significantly lower single fiber F o compared with adult rats. Furthermore, in adult rats, the mean single fiber F o generated by Type IIB fibers was higher compared with Type I and Type IIA, whereas this was not observed with oldest old rats. Advanced age conferred a considerable effect on innervation ratio. Oldest old rats exhibited an approximately fourfold greater number of Type I and Type IIA fibers per motor unit compared with adult rats and also displayed 46% more Type IIB fibers per motor unit.
Table 4.
Summary of Adult and Oldest Old Rat Extensor Digitorum Longus Muscle Histological Data by Muscle Fiber Type
| Adult rats n = 18 (of 19) | Oldest old rats n = 10 (of 12) | |
|---|---|---|
| Single muscle fiber CSA (µm2) | ||
| Type I | 673±138 | 639±212 |
| Type IIA | 900±161 | 969±428 |
| Type IIB | 2042±402† | 1507±849*, † |
| Total fiber CSA (%) of whole muscle | ||
| Type I | 2±1 | 4±6 |
| Type IIA | 14±5 | 26±25 |
| Type IIB | 84±5 | 70±25 |
| Number of fibers per muscle | ||
| Type I | 84±34 | 158±201 |
| Type IIA | 584±169 | 632±567 |
| Type IIB | 1536±234† | 1211±481† |
| Estimated single fiber F o (µN) | ||
| Type I | 186±43 | 88±42* |
| Type IIA | 248±50 | 125±57* |
| Type IIB | 565±130† | 187±83* |
| Estimated number of muscle fibers per motor unit (innervation ratio) | ||
| Type I | 10±4.0 | 37.6±47.9* |
| Type IIA | 11.8±3.4 | 47.5±42.6* |
| Type IIB | 307.2±46.8† | 448.5±178.1*, † |
Notes: Data are expressed as mean ± standard deviation. CSA = cross-sectional area, sF o = muscle fiber specific force. Type I = slow oxidative muscle fiber, Type IIA = fast oxidative glycolytic muscle fiber, Type IIB = fast glycolytic muscle fiber.
*Denotes statistically significant difference compared with adult rats (p < .05).
†Denotes statistically significant difference compared with Type I and Type IIA fibers within same age group (p < .05).
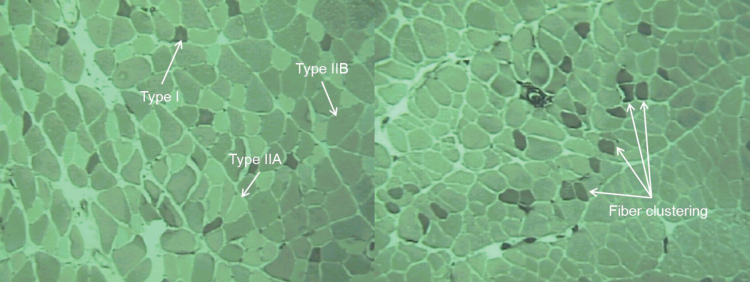
After hematoxylin and eosin staining, oldest old EDL muscles displayed subjectively greater numbers of atrophic muscle fibers and a substantial amount of connective tissue within the perimysium compared to adult muscles (Figure 1). Staining with myosin ATPase (Figure 2) showed a normal random distribution of muscle fibers from varying motor units in the adult rats but revealed clustering of muscle fibers of the same type in the oldest old specimens, which is characteristic of motor unit remodeling seen with aging (30,31).
Figure 1.
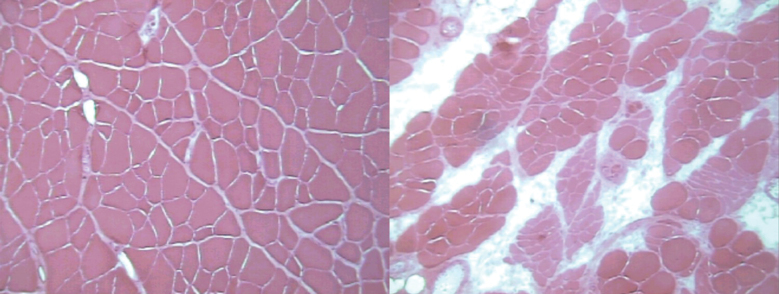
Representative hematoxylin- and eosin-stained cross sections from the extensor digitorum longus muscle from adult (left) and oldest old (right) rats. Note the numerous atrophic fibers and the considerable amount of infiltrating connective tissue in the oldest old specimen.
Figure 2.
Representative myosin ATPase stained (pH = 4.6) cross sections from the extensor digitorum longus muscle from adult (left) and oldest old (right) rats. Type I (dark color), Type IIB (intermediate), and Type IIIA (light) fibers are shown by myosin ATPase staining. Note clustering of muscle fibers of the same type in the oldest old specimen signifying the occurrence of denervation and reinnervation.
Discussion
The progressive phenomenon of sarcopenia results in significant atrophy and weakness of skeletal muscle in advanced age. Many published studies on sarcopenia using a rat model have included animals that have reached late adulthood (25–30 months) (23,25,27,32–36); however, the full impact of age-related motor unit remodeling is difficult to measure because sarcopenia is a gradual process that accelerates in extreme old age (31). Researchers have shown that in the Fisher 344 × Brown-Norway rat model, evidence of a significant decline in muscle mass, fiber number, whole muscle CSA, and muscle fiber CSA begins at around 30 months of age (37). Our study used oldest old rats (36–37 months old) to focus on the fully developed effects of sarcopenia, and this is likely the reason why the declines in muscle mass, muscle force, muscle specific force, and the number of motor units per EDL muscle were more substantial than previous studies have reported (23,25,27,35). At extreme old age the number of motor neurons has diminished to a level that requires motor units to maximally utilize their compensatory mechanisms (35,38,39). As muscle fibers are denervated during loss of motor units with age, nearby motor units will attempt to reinnervate these denervated muscle fibers through terminal axonal sprouting. However, aged subjects demonstrate decreased nerve sprouting capacity of motor neurons and therefore the course of remodeling will leave some muscle fibers permanently denervated. Interestingly, an increase in intramuscular connective tissue in advanced age, seen in this study during histological examination and confirmed by others (37), may contribute to the compromised ability for motor neurons to regenerate by serving as a barrier for sprouting axons searching for denervated muscle fibers.
This study demonstrates that oldest old rats possess substantially reduced whole muscle size and contractile force compared with adult rats. Even when the effects of atrophy are accounted for by normalizing whole muscle force to CSA to obtain sF o, there remained a 46% specific force deficit in the oldest old rats compared with the adult rats’ EDL muscles. Despite these large differences, mean motor unit maximum tetanic isometric force was similar between adult and oldest old rats. This observation also held true when contractile properties were analyzed according to physiologic motor unit type as all types of fast-twitch motor units of oldest old rats demonstrated comparable force production to respective adult fast-twitch motor units. An exception was seen with slow motor units, where oldest old rats displayed a significantly higher average motor unit force. These findings suggest an active mechanism in extremely old subjects where single motor units can increase their innervation ratios through terminal axonal sprouting and reinnervation of denenervated muscle fibers, which acts to offset the age-related decrements in motor unit numbers. Lushaj et al. (37) identified a potential age-related adaptive response whereby oldest old rats showed no further sarcopenic declines between 36–39 months of age and also demonstrated increased Type I muscle fibers in various skeletal muscles. Possibly, their observations may reflect the significance of compensatory neuromuscular remodeling shown through our investigation.
We found that the capacity of a motor unit to generate force is influenced by the innervation ratio, the average single fiber CSA, the physiologic motor unit type, whole muscle specific force, and the force of single fibers. With advanced age, sarcopenia leads to muscle fiber atrophy, decreased number of motor units, and a reduction in muscle specific force capacity. Age-related atrophy of fast-twitch muscle fibers is more pronounced compared with slow-twitch fibers (7). Additionally, selective loss of fast motor units greatly affects muscle function although this phenomenon is distinctive to aged rodents, whereas in humans fast- and slow-twitch fibers decline equally over time (40,41). Oldest old rats in this study exhibited lower whole EDL muscle mass, CSA, and specific force compared with adult rats. Both fast- and slow-twitch motor units were lost in advanced age, but there was a considerably lower number of FR motor units seen in the oldest old rats compared with adults. However, despite muscle atrophy and weaker single fiber force with advanced age, oldest old rats demonstrated innervation ratios that were higher than adult rats. We suspect that this is a compensatory process that allowed the fast-twitch motor units in oldest old rats to maintain the same force as in adult rats. Furthermore, oldest old slow-twitch motor units also displayed less single fiber force production but exhibited 60% greater motor unit force compared with adult rats. This finding may also be attributed to the higher innervation ratios found in oldest old rats. This is consistent with the findings of other authors who showed that peak force generation of slow-twitch motor units of aged animals can exceed that of young animals by increasing innervation ratio (12). We conclude that even in extreme old age, both fast- and slow-twitch motor units retain the ability to undergo motor unit remodeling by increasing innervation ratio. Nonetheless, with fewer total motor units, decreased specific force, and significant fast-twitch fiber atrophy, oldest old rats still displayed an overall decrement in whole muscle strength despite evidence of dynamic remodeling that counteracts the effects of sarcopenia.
Several limitation of this study should be noted. The proportion of each physiologic motor unit type per muscle was determined based on the number of motor neurons successfully identified from each rat during surgery and may not represent the expected proportion of slow-twitch or fast-twitch motor units for either adult or oldest old rats. Evaluation of additional motor units per rat may have reduced potential sampling error but was not possible due to the technically challenging experimental preparation. Another limitation is the high standard deviations found in some of the results, namely in the data presented in Table 4. This was due to wide variations seen in the number of muscle fibers of each physiologic type counted within each muscle. The number of Type I fibers identified in oldest old EDL muscles ranged between 3 and 682. The EDL is predominantly a fast-twitch muscle and as a result, we would predict a low percentage of Type 1 muscle fibers in all cases. It is possible that this large variability in the number of Type 1 muscle fibers in the oldest old EDL muscles was due to investigator error. However, it is also likely that these differences can be attributed to innate variation in oldest old rats. Finally, histological analysis in this study identified the three conventional muscle fiber types (I, IIA, IIB) for comparison between adult and oldest old rats but did not distinguish between the more recently described fiber subtypes (IC, IIC, IIAC, and IIAB) (42,43). Although a more complete characterization of muscle fiber types would have provided a comprehensive description of motor unit changes seen with sarcopenia, utilization of the original three fiber types still permitted demonstration of motor unit remodeling in oldest old rats.
The markedly low number of FR motor units from oldest old rats resulted in a relative increase in the proportions of S, FI, and FF motor units within oldest old EDL muscles. This degree of FR motor unit deficit within aged skeletal muscle in the rat has not been shown by others (27). The finding may be partially explained by the fact that sarcopenia is more pronounced in oldest old subjects and FR motor units in the Brown-Norway rat may be especially sensitive to age-related motor neuron loss. Alternatively, the discrepancy may also represent a selection bias during the process of dissection and isolation of individual motor neuron axons. Given that fast-twitch motor units have large-diameter motor neurons capable of innervating an extensive muscle fiber territory compared with slow-twitch motor units, large motor units may have been underrepresented during selection in an effort to ensure that evoked twitches were not artifacts. Although this possibility may also provide some explanation for the relatively low numbers of FF motor units found in both adult and oldest old rats, it would not completely account for the abatement of FR motor units with age because a proportionately similar reduction was not seen with FI or FF motor units.
Innervation ratio for each motor unit classification was calculated indirectly in this study by taking the motor unit force and dividing by muscle fiber force. In contrast to the glycogen depletion method, which may overestimate the number of fast muscle fibers due to the fact that glycogen is more readily consumed by fatigable motor units, the indirect method may overestimate the innervation ratio of slow motor units (44). This is attributed to the use of several averaged parameters that are used in the calculation, such as the proportion of CSA occupied by a particular fiber type and mean single fiber F o. Despite this limitation, the innervation ratios obtained in this study from differing fiber types can still be considered relative to each other and reveal that oldest old rats may compensate for sarcopenia by increasing innervation ratio of slow-twitch motor units more substantially than FF (Type IIB fibers) motor units.
This investigation identifies the potential role of motor unit remodeling as an adaptive response to the reduction of motor neurons during the progression of sarcopenia. However, the pathophysiology of sarcopenia remains unclear; multiple mechanisms may in fact be involved and were not addressed in this study design. These additional mechanisms involve interrelated derangements of molecular, cellular, and physiologic pathways and likely contribute to sarcopenia development in addition to motor neuron loss. For example, a growing body of evidence suggests that abnormalities in aging skeletal muscle mitochondria result in cellular dysregulation, the generation of reactive oxygen species, and myocyte apoptosis (45,46). Each of these can play an important role in muscle atrophy and weakness during the course of aging and especially at senescence. Others have examined the possibility that a decline in satellite cells in advanced age results in an impaired ability of skeletal muscles to undergo regeneration later in life (47). Abnormal molecular signaling pathways involving calcium, inflammatory cytokines, and myostatin have also been identified as potential mediators of skeletal muscle loss (48–51). Additionally, alterations in hormone levels with aging result in an increase in catabolic factors that have been shown to negatively influence muscle mass and strength (52,53). Taken together, motor unit remodeling is a compensatory process that occurs in the setting of a complex interplay of unfavorable factors present in elderly patients that act to accelerate the symptoms of sarcopenia.
Our findings are relevant to the effects of sarcopenia for humans in extremely advanced age. The proportion of elderly patients requiring nursing care and hospitalization for medical or surgical treatments has been steadily increasing (54,55). The fact that people in advanced age preserve the potential for motor unit remodeling reinforces the concept that interventions such as exercise training and physical therapy can positively influence health status and outcomes in elderly patients. This has enormous implications for all hospitalized patients and particularly postoperative patients who have undergone surgery that results in temporary or permanent immobilization. Exercise is the primary countermeasure for patients with sarcopenia, and resistance training has been shown to provide the greatest benefit (56,57). Longitudinal studies using animal models should be conducted to fully evaluate the benefits of exercise intervention on sarcopenia to better elucidate the direct effects of training on neuromuscular remodeling in oldest old subjects.
Conclusions
Skeletal muscle sarcopenia in oldest old Brown-Norway rats was confirmed by demonstration of lower EDL muscle mass, CSA, whole muscle force, and specific force compared with adults. The significant motor unit characteristics associated with these age-related changes included fewer motor units per EDL muscle and fewer numbers of motor units of each physiologic type. There were no differences between oldest old and adult rats in regards to the average force produced by fast-twitch motor units. However, oldest old slow motor units displayed higher motor unit force production compared with those in adults. Despite these findings, single muscle fiber force in oldest old rats was lower than adults among all fiber types, and innervation ratios for all fiber types were higher in the oldest old group. Therefore, the data suggest that in extreme old age, both fast- and slow-twitch motor units retain the ability to undergo motor unit remodeling even though there is still an overall decline in whole muscle strength resulting from fewer total motor units, decreased specific force, and fast-twitch fiber atrophy.
Funding
This work was supported by grants from the National Institutes of Health (PO1 AG10821) and National Institute on Aging (T32 AG00114).
Conflict of Interest
The authors have no financial disclosures.
References
- 1. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89:81–88 [DOI] [PubMed] [Google Scholar]
- 2. Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty - a mini-review. Gerontology. 2012;58:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85 [DOI] [PubMed] [Google Scholar]
- 4. Matthews GD, Huang CL, Sun L, Zaidi M. Translational musculoskeletal science: is sarcopenia the next clinical target after osteoporosis? Ann N Y Acad Sci. 2011;1237:95–105 [DOI] [PubMed] [Google Scholar]
- 5. Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824 [DOI] [PubMed] [Google Scholar]
- 6. Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217 [DOI] [PubMed] [Google Scholar]
- 7. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294 [DOI] [PubMed] [Google Scholar]
- 8. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326 [DOI] [PubMed] [Google Scholar]
- 9. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 10. Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011;4:209–220 [DOI] [PubMed] [Google Scholar]
- 11. Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–219 [DOI] [PubMed] [Google Scholar]
- 12. McComas AJ, Galea V, de Bruin H. Motor unit populations in healthy and diseased muscles. Phys Ther. 1993;73:868–877 [DOI] [PubMed] [Google Scholar]
- 13. Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95 [DOI] [PubMed] [Google Scholar]
- 14. Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol. 1993;18:331–358 [DOI] [PubMed] [Google Scholar]
- 15. Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25 [DOI] [PubMed] [Google Scholar]
- 16. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501 [DOI] [PubMed] [Google Scholar]
- 17. Hall KE. Aging and neural control of the GI tract. II. Neural control of the aging gut: can an old dog learn new tricks? Am J Physiol Gastrointest Liver Physiol. 2002;283:G827–G832 [DOI] [PubMed] [Google Scholar]
- 18. Cederna PS, Asato H, Gu X, et al. Motor unit properties of nerve-intact extensor digitorum longus muscle grafts in young and old rats. J Gerontol A Biol Sci Med Sci. 2001;56:B254–B258 [DOI] [PubMed] [Google Scholar]
- 19. Cederna PS, Youssef MK, Asato H, Urbanchek MG, Kuzon WM., Jr Skeletal muscle reinnervation by reduced axonal numbers results in whole muscle force deficits. Plast Reconstr Surg. 2000;105:2003–9; discussion 2010. [DOI] [PubMed] [Google Scholar]
- 20. Yoshimura K, Asato H, Cederna PS, Urbanchek MG, Kuzon WM. The effect of reinnervation on force production and power output in skeletal muscle. J Surg Res. 1999;81:201–208 [DOI] [PubMed] [Google Scholar]
- 21. Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188 [Google Scholar]
- 22. Gans C. Fiber architecture and muscle function. Exerc Sport Sci Rev. 1982;10:160–207 [PubMed] [Google Scholar]
- 23. Kadhiresan VA, Hassett CA, Faulkner JA. Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol. 1996;493(Pt 2):543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol. 1989;61:737–746 [DOI] [PubMed] [Google Scholar]
- 26. Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379 [DOI] [PubMed] [Google Scholar]
- 27. Einsiedel LJ, Luff AR. Alterations in the contractile properties of motor units within the ageing rat medial gastrocnemius. J Neurol Sci. 1992;112:170–177 [DOI] [PubMed] [Google Scholar]
- 28. Tötösy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Motor-unit categorization based on contractile and histochemical properties: a glycogen depletion analysis of normal and reinnervated rat tibialis anterior muscle. J Neurophysiol. 1992;67:1404–1415 [DOI] [PubMed] [Google Scholar]
- 29. Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967;193:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roth SM, Ferrell RF, Hurley BF. Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging. 2000;4:143–155 [PubMed] [Google Scholar]
- 31. Waters DL, Baumgartner RN, Garry PJ. Sarcopenia: current perspectives. J Nutr Health Aging. 2000;4:133–139 [PubMed] [Google Scholar]
- 32. Schertzer JD, Plant DR, Ryall JG, Beitzel F, Stupka N, Lynch GS. Beta2-agonist administration increases sarcoplasmic reticulum Ca2+-ATPase activity in aged rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E526–E533 [DOI] [PubMed] [Google Scholar]
- 33. Ansved T, Larsson L. Effects of ageing on enzyme-histochemical, morphometrical and contractile properties of the soleus muscle in the rat. J Neurol Sci. 1989;93:105–124 [DOI] [PubMed] [Google Scholar]
- 34. Altun M, Besche HC, Overkleeft HS, et al. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem. 2010;285:39597–39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Urbanchek MG, Picken EB, Kalliainen LK, Kuzon WM., Jr Specific force deficit in skeletal muscles of old rats is partially explained by the existence of denervated muscle fibers. J Gerontol A Biol Sci Med Sci. 2001;56:B191–B197 [DOI] [PubMed] [Google Scholar]
- 36. Ryall JG, Schertzer JD, Lynch GS. Attenuation of age-related muscle wasting and weakness in rats after formoterol treatment: therapeutic implications for sarcopenia. J Gerontol A Biol Sci Med Sci. 2007;62:813–823 [DOI] [PubMed] [Google Scholar]
- 37. Lushaj EB, Johnson JK, McKenzie D, Aiken JM. Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A Biol Sci Med Sci. 2008;63:921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacob JM, Robbins N. Differential effects of age on neuromuscular transmission in partially denervated mouse muscle. J Neurosci. 1990;10:1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robbins N. Compensatory plasticity of aging at the neuromuscular junction. Exp Gerontol. 1992;27:75–81 [DOI] [PubMed] [Google Scholar]
- 40. Ishihara A, Naitoh H, Katsuta S. Effects of ageing on the total number of muscle fibers and motoneurons of the tibialis anterior and soleus muscles in the rat. Brain Res. 1987;435:355–358 [DOI] [PubMed] [Google Scholar]
- 41. Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6: 588–595 [DOI] [PubMed] [Google Scholar]
- 42. Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81:1810–1816 [PubMed] [Google Scholar]
- 43. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531 [DOI] [PubMed] [Google Scholar]
- 44. Tötösy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. J Neurophysiol. 1992;67:1385–1403 [DOI] [PubMed] [Google Scholar]
- 45. Leeuwenburgh C. Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci. 2003;58:999–1001 [DOI] [PubMed] [Google Scholar]
- 46. Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kadi F, Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: effects of aging and physical activity. Scand J Med Sci Sports. 2010;20:39–48 [DOI] [PubMed] [Google Scholar]
- 48. Andersson DC, Betzenhauser MJ, Reiken S, et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12–22 [DOI] [PubMed] [Google Scholar]
- 50. Schaap LA, Pluijm SM, Deeg DJ, et al. Health ABC Study. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Siriett V, Platt L, Salerno MS, Ling N, Kambadur R, Sharma M. Prolonged absence of myostatin reduces sarcopenia. J Cell Physiol. 2006;209:866–873 [DOI] [PubMed] [Google Scholar]
- 52. Maggio M, Lauretani F, Ceda GP. Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr Metab Care. 2013;16:3–13 [DOI] [PubMed] [Google Scholar]
- 53. Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67:1140–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67:48–55 [DOI] [PubMed] [Google Scholar]
- 55. Kozak LJ, Owings MF, Hall MJ. National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2005;158:1–199 [PubMed] [Google Scholar]
- 56. Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12:388–396 [DOI] [PubMed] [Google Scholar]
- 57. Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–M916 [DOI] [PubMed] [Google Scholar]