Abstract
Background.
Loss of muscle mass and strength with ageing is a major cause for falls, disability, and morbidity in older people. Previous studies have found that angiotensin-converting enzyme inhibitors (ACEi) may improve physical function in older people. It is unclear whether ACEi provide additional benefit when added to a standard exercise training program. We examined the effects of ACEi therapy on physical function in older people undergoing exercise training.
Methods.
Community-dwelling people aged ≥65 years with functional impairment were recruited through general (family) practices. All participants received progressive exercise training. Participants were randomized to receive either 4 mg perindopril or matching placebo daily for 20 weeks. The primary outcome was between-group change in 6-minute walk distance from baseline to 20 weeks. Secondary outcomes included changes in Short Physical Performance Battery, handgrip and quadriceps strength, self-reported quality of life using the EQ-5D, and functional impairment measured using the Functional Limitations Profile.
Results.
A total of 170 participants (n = 86 perindopril, n = 84 placebo) were randomized. Mean age was 75.7 (standard deviation [SD] 6.8) years. Baseline 6-minute walk distance was 306 m (SD 99). Both groups increased their walk distance (by 29.6 m perindopril, 36.4 m placebo group) at 20 weeks, but there was no statistically significant treatment effect between groups (−8.6m [95% confidence interval: −30.1, 12.9], p = .43). No statistically significant treatment effects were observed between groups for the secondary outcomes. Adverse events leading to withdrawal were few (n = 0 perindopril, n = 4 placebo).
Interpretation.
ACE inhibitors did not enhance the effect of exercise training on physical function in functionally impaired older people.
Key Words: Physical function, ACE inhibitors, Exercise.
The age-related loss of skeletal muscle mass, quality, and strength (sarcopenia) is a major contributor to loss of mobility and independence among older people (1). To date the mainstay in attempting to counter the effects of sarcopenia has been progressive exercise training. However, older people have a finite capacity for exercise participation and are more likely to need to interrupt an exercise program because of ill health (2). Although older exercisers experience a markedly slower rate of physical functional decline than their less active counterparts, year-on-year functional decline occurs even in older people who meet target exercise recommendations (3). Identifying interventions that improve physical function will help maximize healthy ageing in our growing older population, a global public health imperative (4).
Angiotensin-converting enzyme inhibitors (ACEi) may have the potential to improve physical function and improve the response to exercise training. Younger people with the II genotype of the ACE gene have low serum ACE levels. This genotype displays not only better endurance performance but also greater improvements in endurance following training (5). It is therefore possible that pharmacologically reducing serum ACE levels with ACEi and combining this with exercise might enhance the beneficial effects of exercise training. We have previously shown that ACEi produced a significant improvement in 6-minute walk distance (6MWD) in functionally impaired older people (mean age 79 years) who did not have heart failure (6). The magnitude of improvement in 6MWD as a test of physical function was comparable with that reported after 6 months of exercise training (7). More recently, Buford et al. (8) found that older people who were taking ACEi for clinical indications had a greater functional response to exercise than those not taking ACEi. No randomized studies have been conducted to examine the effect of adding ACEi to exercise training on physical function in humans. Observational studies have shown that the use of ACE inhibitors is associated with a slower decline in muscle mass, muscle strength, and walking speed in older people suggesting that there may be a direct effect on skeletal muscle (9,10). It is possible that exercise training and ACEi target different components of muscle function producing an additive effect. This trial aimed to establish whether the known beneficial effect of exercise training could be amplified by concomitant ACE inhibition in older people with functional impairment.
Methods
This was a double-blind randomized controlled parallel group trial approved by the East of Scotland Research Ethics Committee (09/S0501/48). It conformed to the principles of the Declaration of Helsinki. The trial was registered at www.controlled-trials.com (ISRCTN67166885; full study protocol can be accessed as an online Supplementary file). Between March 2010 and February 2012, community-dwelling people aged ≥65 years were recruited on a rolling basis. Following a preliminary general (family) practitioner database search through the Eastern Node of the Scottish Primary Care Research Network, letters of invitation were sent to potential participants. Potential participants attending secondary care medicine for the elderly services in Tayside and Fife were also sent letters of invitation. The research team then contacted those who expressed an interest in taking part for a screening visit if they reported mobility impairment requiring the use of a walking aid and/or dependence in functional activities of daily living (transfers, stairs, washing, or dressing). Following written informed consent, people aged ≥ 65 years with a Short Physical Performance Battery (SPPB) score ≤ 10 were included. We excluded those already receiving ACEi or angiotensin receptor blocker; those with contraindications to ACEi (significant aortic outflow obstruction with pressure gradient > 30 mmHg, estimated glomerular filtration rate < 30 mL/min/1.73 m2 by MDRD4 equation (11), systolic blood pressure < 90 mmHg); those with a clinical diagnosis of heart failure according to the European Society of Cardiology guidelines (12) or left ventricular systolic dysfunction on echocardiography; those who regularly participated in exercise training; those with moderate to severe cognitive impairment (Mini Mental State Examination < 20/30); and those who were wheelchair bound. A diagnosis of hypertension or use of antihypertensive medications was not an exclusion criterion.
Interventions
Participants were randomized (1:1 allocation ratio, no stratification) to receive either perindopril or matching placebo for 20 weeks. Computer-generated randomization was performed by Tayside Pharmaceuticals who dispensed the trial medication in identical, sequentially numbered bottles. Treatment allocation was concealed to all others involved in the study. A total of 86 participants were randomized to the perindopril group and 84 to the placebo group. The starting dose of perindopril 2 mg daily was commenced simultaneously with exercise training. This was uptitrated to 4 mg after 2 weeks if tolerated. The placebo group also underwent a “mock” uptitration. Although we anticipate the effect is generic to ACEi, perindopril was chosen as it is well tolerated in older people and because of existing evidence that 20 weeks of perindopril therapy improved 6-minute walking distance in older people (6).
All participants received a 20 week duration progressive exercise training program previously shown to be acceptable to very old people with multimorbidity (13). This comprised 10 weeks of supervised hospital-based training followed by 10 weeks of unsupervised home-based training. This strategy was adopted because group exercise therapy is the standard approach in clinical practice, but evidence suggests that adherence to exercise in older people is higher with home-based training (14). We used a combination of exercise strategies for both efficiency and to achieve maximal benefit.
Phase I: Supervised hospital-based training twice per week for 10 weeks.
Individually tailored, twice-weekly, hospital-based group outpatient progressive exercise training sessions were held (group sizes of 4–10). Exercises comprised a mixture of intermittent functional exercise (marching, stepping on stairs, shuttle walking, sit-to-stands, wall presses), and strength and balance (heel and toe touch, heel and toe raise, dynamic leg swing, tandem walk) training, with resistance provided by the use of elasticated resistance bands (elbow flexion and extension; shoulder flexion, extension, and abduction; punching; hip extension and abduction; knee flexion). Baseline exercise level was assessed on information gained during the first-group session and from participants’ personal exercise logbook. The duration and intensity of exercise was tailored accordingly. Participants were commenced on the highest band resistance level that allowed them to complete at least eight repetitions. For the functional and balance exercises, the number of repetitions they could achieve at their fastest pace was taken as their baseline, aiming for a minimum of 10 repetitions. Group-guided discussion sessions based on cognitive and behavioral techniques were incorporated into this phase to achieve behavior change. Participants were given a structured educational package reinforcing the topics covered (available on request from authors), and they were encouraged to set realistic goals for their exercise program. The duration and intensity of the exercise was gradually increased, and participants were encouraged to increase these by 10% from baseline each week. Resistance exercise was commenced at 8–10 repetitions gradually increasing to two sets of 10 repetitions at the end of 10 weeks. Individual ability was assessed on an ongoing basis, and participants who achieved their targets early were commenced on a higher level of band resistance. Balance exercises were carried out at between 10 and 20 repetitions with the main focus being on the quality of movement. Participants were encouraged to record the number of repetitions of exercise in their logbook for personal reference only. Participants were also encouraged to continue their exercises at home in between the exercise classes.
Phase II: Unsupervised home-based exercise training for 10 weeks.
Participants used the daily-activity self-monitoring log to record goals, strategies for dealing with setbacks, and estimates of time spent engaging in exercise. The physiotherapist telephoned participants weekly for 4 weeks, and then fortnightly. Such a strategy is an effective alternative to face-to-face contact with older people, giving equally good adherence for endurance exercise over extended periods (15). All information on progression or maintenance of the exercises was reported subjectively via telephone consultation. For those who had plateaued during the first 10 weeks, maintenance of their exercise levels was encouraged, and for those who had not achieved their personal goals, further guidance on progression was given, and resistance levels were increased as they progressed This period of telephone review was mainly motivational, encouraging regular exercise through home exercise and seeking out local exercise programs that would be suitable to maintain good exercise adherence for the future. Participants were asked to follow the exercise strategy on at least 2 days per week with encouragement to increase the number of days they performed these.
Outcomes
Outcomes were assessed at baseline and again at 10 and 20 weeks after randomization by a single researcher blind to treatment allocation.
Primary outcome.
The primary outcome was the change from baseline in 6-minute walking distance at 20 weeks. This is a validated, safe, and reliable measure of physical functional status and exercise capacity in older people (16,17).
Secondary outcomes.
Secondary outcomes include the following:
1. Physical performance
SPPB: This assesses lower extremity function and has been shown to predict subsequent disability, institutionalization, and mortality (18) and is responsive to the effects of exercise in older people (19).
Quadriceps strength: This was measured using a handheld dynamometer (model 01163 Lafayette Instrument Company, Lafayette, Indiana). The device has good correlation with the gold standard of Biodex dynamometry in older people (20).
Grip strength: Upper limb muscle strength was measured using a handheld dynamometer (T.K.K 5401 Grip D). This measure is sensitive to change in older people.
2. Health-related quality of life and self-reported function—EQ-5D and Functional Limitation Profile (FLP):
The EQ-5D provides a brief measure of health status in a single index score (21). The FLP (22), a modified version of the Sickness Impact Profile, is a generic health status measure of change in behavior as a consequence of illness.
Baseline demographics were collected. The Scottish Index of Multiple Deprivation was used to derive quintiles of deprivation based on post code (23). Serum urea, creatinine, and potassium were measured, and blood pressure was recorded at each visit. Adverse events were recorded at each visit.
Sample Size Calculation and Power
Our previous data in a similar population showed a mean improvement in 6MWD of 31 m with a standard deviation of change of 50 m (6). We powered our trial around an improvement in 6MWD of 22.5m in the ACEi group (over and above the anticipated increase with exercise training). This magnitude of improvement is of clinical significance (24), is realistic, and if observed would be sufficiently compelling to alter clinical practice. To detect this between-group difference with 80% power at alpha = 0.05 using a two-sample t-test of the change over baseline requires 158 participants (79 per group). Anticipating a 15% dropout rate, we planned to recruit 186 participants. However, as dropout rate was lower but recruitment was slower than anticipated, a total of 170 participants were randomized.
Statistical Analysis
Statistical analyses were preplanned and performed using SAS v9.2. The analysis plan was finalized, and analysis was performed prior to breaking the treatment code. All outcomes were summarized at baseline, 10, and 20 weeks, and changes over baseline were estimated and tested using paired t-tests. Between-group differences in outcomes were analyzed using analysis of covariance, with the change over baseline as the response variable, and with treatment group, the baseline value of the outcome and sex as predictor variables. Sex was included as a predictor in all regression models at the request of the Data Monitoring Committee due to an imbalance between groups.
Analyses were conducted using the intention to treat principle, with individuals analyzed according to randomized group, regardless of compliance with study medication or procedures. Per-protocol analyses were also carried out based on a population who took at least 80% of expected study medication, attended all study visits, and participated in at least 80% of exercise classes (supervised hospital-based training only). To assess the impact of missing data, the primary analysis was repeated using multiple imputations. Ten imputations were run and combined for missing 6MWD at 20 weeks. Baseline 6MWD, treatment group, age, sex, and deprivation quintile were used to generate imputed values. Repeated measures analyses of all outcomes at 10 and 20 weeks were also performed as secondary analyses. A two-sided p value of <.05 was taken as significant for all analyses; correction for multiple analyses was not performed. Prespecified subgroup analysis was performed for change in 6MWD at 20 weeks for subgroups defined at baseline by the presence/absence of hypertension. In addition, a further subgroup analysis for sex was carried out to investigate whether the intervention effects were different between the males and females in terms of the primary outcome.
Results
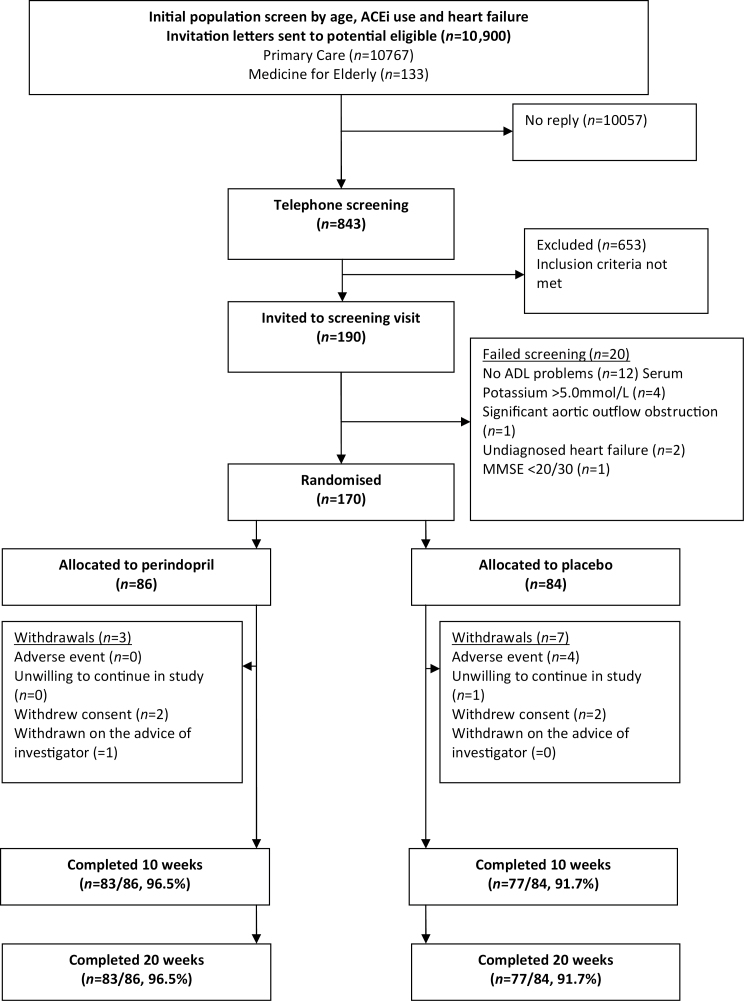
A total of 10,900 potential participants were identified based on a preliminary screen excluding those less than 65 years, those already taking ACE inhibitors, and those with a diagnosis of heart failure. These potential participants were contacted by letter, and 92% (10,057/10,900) failed to reply. Following a brief telephone screen, 190/843 of responders attended a screening visit. One hundred and seventy participants were randomized, and 160/170 (94%) participants completed the study. Figure 1 shows participant flow through the trial. Table 1 shows the baseline characteristics of the two groups. Table 2 displays summaries of changes from baseline at 10 and 20 weeks, and estimated treatment effect based on analysis of covariance.
Figure 1.
CONSORT diagram—participant flow through the study.
Table 1.
Summaries of Baseline Characteristics by Treatment Group
| Perindopril (N = 86) | Placebo (N = 84) | |
|---|---|---|
| Age (years) | 76.3 (7.3) | 75.1 (6.2) |
| MMSE | 27.8 (1.9) | 27.7 (2.0) |
| Male | 32 (37%) | 39 (46%) |
| Use of walking aids | 35 (41%) | 35 (42%) |
| Deprivation quintile | ||
| 1 (most deprived) | 25 (29%) | 22 (26%) |
| 2 | 18 (21%) | 12 (14%) |
| 3 | 9 (10%) | 15 (18%) |
| 4 | 22 (26%) | 20 (24%) |
| 5 (least deprived) | 12 (14%) | 15 (18%) |
| Medical history | ||
| Osteoarthritis | 45 (52%) | 38 (45%) |
| Chronic obstructive pulmonary disease | 14 (16%) | 8 (10%) |
| Hypertension | 31 (36%) | 21 (25%) |
| Angina | 8 (9%) | 6 (7%) |
| Diabetes mellitus | 6 (7%) | 6 (7%) |
| Peripheral arterial disease | 1 (1%) | 1 (1%) |
| Atrial fibrillation | 3 (3%) | 7 (8%) |
| Medications | ||
| Medications total number | 5.2 (3.1) | 5.1 (3.3) |
| Beta blockers | 14 (16%) | 17 (20%) |
| Calcium channel blockers | 16 (19%) | 15 (18%) |
| Diuretics | 23 (27%) | 17 (20%) |
| Analgesics | 37 (43%) | 41 (49%) |
| Measurements | ||
| BMI (kg/m2) | 28.0 (5.3) | 29.2 (5.5) |
| SBP (mmHg) | 146.6 (18.8) | 148.7 (19.6) |
| DBP (mmHg) | 79.8 (13.9) | 79.7 (11.0) |
| Creatinine (μmol/L) | 75.2 (14.9) | 79.4 (19.4) |
| 6MWD (m) | 300.4 (101.1) | 312.2 (97.5) |
| Quadriceps strength (kg) | 17.3 (10.7) | 19.5 (11.6) |
| Handgrip strength (kg) | 19.5 (7.5) | 20.6 (8.8) |
| EQ-5D | 0.68 (0.23) | 0.68 (0.23) |
| EQ-VAS | 70.9 (16.2) | 72.3 (15.7) |
| SPPB | 7.5 (1.7) | 7.7 (2.0) |
| FLP | 851.5 (205.9) | 867.5 (207.4) |
Notes: 6MWD = 6-minute walk distance; BMI = body mass index; DBP = diastolic blood pressure; EQ-5D = EuroQol 5D; EQ-VAS = EuroQol Visual Analogue Scale; FLP = Functional Limitation Profile; MMSE = Mini Mental State Examination; SBP = systolic blood pressure; SPPB = Short Physical Performance Battery.
Data are expressed as mean (SD) except where number of participants (%) are given.
Table 2.
Change in Outcomes From Baseline by Treatment Group
| Outcome | Time Point | Perindopril | Placebo | Treatment Effect (Perindopril − Placebo) | p |
|---|---|---|---|---|---|
| Change From Baseline | Change From Baseline | ||||
| Mean (SD) | Mean (SD) | Estimate (95% Confidence Interval) | |||
| 6MWD (m) | 10w | 36.7 (63.8) | 23.9 (55.4) | 10.8 (−8.2, 29.9) | 27 |
| 20w | 29.6 (74.9) | 36.4 (59.2) | −8.6 (−30.1, 12.9) | .43 | |
| Quadriceps strength (kg) | 10w | 2.7 (12.5) | 3.9 (14.0) | −1.7 (−5.2, 1.9) | .35 |
| 20w | 0.1 (12.5) | 2.0 (14.8) | −2.1 (−5.5, 1.2) | .21 | |
| Grip strength (kg) | 10w | 0.6 (3.3) | 1.1 (4.6) | −0.5 (−1.7, 0.6) | .34 |
| 20w | 1.0 (3.5) | 1.0 (5.2) | 0.1 (−1.3, 1.4) | .94 | |
| EQ-5D | 10w | 0.07 (0.23) | 0.06 (0.20) | 0.01 (−0.05, 0.07) | .69 |
| 20w | 0.05 (0.25) | 0.05 (0.22) | 0.00 (−0.06, 0.06) | .96 | |
| EQ-5D VAS | 10w | 5.5 (13.5) | 1.9 (14.4) | 3.1 (−0.8, 7.1) | .12 |
| 20w | 1.2 (14.7) | 1.9 (14.8) | −1.3 (−5.7, 3.1) | .56 | |
| SPPB total score | 10w | 1.4 (1.6) | 1.2 (1.7) | 0.1 (−0.4, 0.7) | .58 |
| 20w | 1.0 (2.2) | 1.1 (2.0) | −0.1 (−0.7, 0.6) | .79 | |
| FLP score* | 10w | −80.4 (130.1) | −84.6 (144.2) | 1.8 (−41.1, 44.8) | .93 |
| 20w | −65.7 (157.6) | −68.2 (149.3) | 3.2 (−45.3, 51.6) | .90 |
Notes: 6MWD = 6-minute walk distance); EQ-5D = EuroQol 5D; EQ-VAS = EuroQol Visual Analogue Scale; FLP = Functional Limitation Profile; SPPB = Short Physical Performance Battery.
*Lower values indicate improvement.
Primary Outcome
Mean baseline 6MWD was 306 m and was significantly increased from baseline by 30.5 m (95% confidence interval [95% CI]: 20.9, 40.1; p < .001) at 10 weeks and by 32.9 m (95% CI: 22.1, 43.7; p < .001) at 20 weeks in the overall population recruited. However, there was no evidence of a treatment effect between the perindopril and placebo groups at 20 weeks (estimated difference, −8.6m [95% CI: −30.1, 12.9], p = .43; Table 2). Additional analyses of the treatment effect estimated using repeated measures analyses (−8.4 [95% CI: −28.7, 11.9], p = .42), per protocol analysis (−5.3 [95% CI: −32.3, 21.7], p = .70), and the primary analysis with multiple imputation for missing data at 20 weeks (−10.3 [95% CI: – 31.0, 10.4], p = .33) comparing the perindopril and placebo groups were similar to the main analyses. Subgroup analysis showed no difference in treatment effect between those and without hypertension (−17.6 m vs −4.2 m; p = .58). A subgroup analysis by sex suggests no difference in terms of the primary outcome between males and females (males: −11.1 [95% CI: −44.7, 22.5], p = .52; females: −6.9 [95% CI: 35.1, 21.4], p = .63; p for interaction = 0.85).
Secondary Outcomes
No statistically significant treatment effect differences were observed between perindopril and placebo groups for quadriceps or grip strength, EQ-5D score or Visual Analogue Scale, SPPB score, or FLP score at 10 or 20 weeks (Table 2).
Other Measures
As expected, the mean blood pressure at 20 weeks in the perindopril group was significantly lower by 6.5 mmHg, (95%CI: 1.6, 11.5; p = .01) systolic and by 3.9 mmHg (95% CI: 1.0, 6.8; p = .009) diastolic compared with the placebo group. Two participants had antihypertensive medication commenced during the study, two had an additional antihypertensive agent added, and two had one antihypertensive medication stopped. No participant was commenced on an ACEi out with the study. Mean creatinine and potassium levels were unchanged throughout the study, with no evidence of any differences between perindopril or placebo groups.
Adverse Events
There were no suspected unexpected serious adverse reactions, and no participant died during the trial. As expected in this older population with multimorbidity, adverse event rates were high, with 88% of participants in the safety population (randomized subjects who took at least one dose of study medication) reporting at least one adverse event (Table 3). Four adverse events led to withdrawal in the placebo group (Figure 1; n = 1 worsening of Parkinson’s disease, n = 1 exacerbation of chronic obstructive pulmonary disease, n = 1 cerebral hemorrhage, n = 1 stroke). One participant was withdrawn on the advice of the investigator (Figure 1) as the researcher felt that the participant had not retained information about the study and therefore was unable to continue in the study with full informed consent.
Table 3.
Adverse Events in Randomized Subjects Who Took At Least One Dose of Study Medication
| Perindopril | Placebo | |
|---|---|---|
| Safety population, N | 85 | 79 |
| Number of adverse events, N | 239 | 184 |
| Subjects with at least one adverse event | ||
| Any event, N (%) | 77 (91%) | 68 (86%) |
| Cardiovascular, N (%) | 3 (4%) | 1 (1%) |
| Cough, N (%) | 8 (9%) | 1 (1%) |
| Dizziness, N (%) | 10 (12%) | 9 (11%) |
| Falls, N (%) | 22 (26%) | 17 (22%) |
| Fractures, N (%) | 3 (4%) | 1 (1%) |
| Gastrointestinal, N (%) | 23 (27%) | 21 (27%) |
| Infections, N (%) | 33 (39%) | 26 (33%) |
| Musculoskeletal, N (%) | 33 (39%) | 31 (39%) |
| Syncope, N (%) | 2 (2%) | 0 (0%) |
Serious adverse events resulting in admission to hospital were seen in 7 participants in the perindopril group (2 collapse; 1 vasovagal syncope following diarrhea and vomiting; 2 falls of which one had a fracture; 1 exacerbation of chronic obstructive pulmonary disease; 1 bleeding following elective surgery) and 10 participants in the placebo group (1 fall; 1 participant had back pain, a fall with a fracture and a deep vein thrombus; 2 cerebral vascular accident of whom one was also admitted for abdominal pain; 1 hernia and abdominal pain; 1 spinal nerve compression; 1 corneal ulcer; 1 chest pain; 1 acute pulmonary edema; 1 exacerbation of cryptogenic organizing pneumonia)
Adherence With Interventions
Six participants declined to take study medication following randomization, and 45/164 (27%) participants discontinued study medication (33% and 22% in the perindopril and placebo groups, respectively) after commencing. The median [interquartile range] adherence estimated by tablet counting in people continuing medication was 99% [95%, 101%] for the perindopril group and 99% [98%, 101%] for the placebo group. Attendance at supervised exercise sessions was good, with a median [interquartile range] attendance of 18 [16, 20] for both groups out of 20 possible sessions. Adherence to exercise during 11–20 weeks were not recorded as the only basis for this would have been self-report from participants, which is known to be inaccurate.
Discussion
We found no evidence that the perindopril enhanced the effect of exercise training on physical function in a group of functionally impaired older people without heart failure. The effect of exercise was apparent as both groups increased their mean 6MWD by around 30 m from baseline, similar to that reported in other studies of exercise training in older people (7,25). This was achieved by 10 weeks with little further improvement. Similarly, there were no statistically significant differences between groups in the secondary outcome measures and there were modest improvements from baseline in both groups that can be attributable to the exercise training.
It is possible that ACEi have no effect on physical function in older people without heart failure, but the evidence is contradictory. The Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study compared 6 months of fosinopril versus placebo in a crossover study and found no change in SPPB (mean age 66 years) (26). In another study comparing ACEi with nifedipine (mean age 75 years), no significant differences in physical performance was seen between the two groups (27). However, in a small study of 36 older men with hypertension, endurance increased in those receiving ACEi (28), and our previous randomized controlled trial in functionally impaired people showed that 20 weeks of ACEi treatment significantly improved 6MWD by 31.6 m compared with placebo and resulted in a clinically significant improvement in quality of life (6). In rodents, improvements in performance with ACEi in combination with exercise but not with ACEi alone have been reported (29), but the evidence is not consistent (30,31).
Although it is possible that our previous study was spuriously positive and the current study reflects the actual effect of ACEi on physical function in older people, other explanations are possible. ACEi therapy may have very similar biological effects on physical function to 20 weeks of exercise training, and therefore, there is no added benefit of using these together. However, the wider benefits of exercise training, not achieved by ACEi therapy, should not be overlooked. An adequate period of preconditioning with ACEi may be required to enhance the benefits of exercise. In the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study, older people who were already taking ACEi for clinical reasons had a greater functional response to exercise compared with those not taking ACEi (8). It is also possible that the degree of improvement possible in functionally impaired older people in only 20 weeks is fixed. Therefore, a longer study duration may be required to allow potential additive effects of ACEi to emerge. This, however, is unlikely given that most of the improvement we found with exercise was in the first 10 weeks. Finally, differences in the study population between the current study and our previous study may explain the different results. Although the mean age of participants was similar and the mean 6MWD was similar to our previous study, the current study may have attracted people who were interested in participating in exercise, whereas our previous study could have attracted more sedentary people interested in an exercise mimic.
The strengths of our study are the double-blind randomized controlled study design, the population of older people with functional impairment, and the range of outcomes measuring different aspects of physical function. However, we did not measure body composition to characterize participant phenotype in detail. A limitation is that the study included only two treatment arms. Inclusion of a control group (receiving no exercise or ACEi) and an ACEi-only group would have allowed direct comparison of effects of ACEi and exercise training. However, potential bias due to difficulties of blinding exercise interventions would limit the value of such comparisons. A further limitation is that almost 27% discontinued study medication. This figure is similar to our previous study with ACEi where 27% dropped out suggesting that this is a real-life intention to treat scenario in older people. Supervised progressive exercise training was only provided for 10 weeks. The lack of improvement in physical function measures beyond 10 weeks may be the result of poor adherence to the regimen during the second 10 weeks of unsupervised home-based exercise. However, the exercise ability of many participants had started to plateau by 10 weeks, and this phase of intervention for them was mostly targeted to maintaining improvements achieved.
In conclusion, concurrent ACEi therapy for 20 weeks did not enhance the response to progressive exercise training in older people with functional limitation. The body of evidence regarding the effect of ACEi on physical function in older people is contradictory and requires clarification. Participant differences in physical activity/ability levels may influence the results of studies. Given the individual and societal impact of functional impairment, it is vital to identify the phenotype of the older person most likely to benefit from ACEi therapy. A systematic review to synthesize the current evidence should guide further research strategies.
Supplementary Data
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by Chief Scientist Office, Scottish Government Heath Directorate (CBZ/4/708) and National Health Service Support for Science (2008GR03).
Acknowledgments
We acknowledge the Scottish Primary Care Research Network, the staff and participants of NHS Tayside, and NHS Fife who supported this study.
References
- 1. Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–M724 [DOI] [PubMed] [Google Scholar]
- 2. Rhodes RE, Martin AD, Taunton JE, Rhodes EC, Donnelly M, Elliot J. Factors associated with exercise adherence among older adults. An individual perspective. Sports Med. 1999;28:397–411 [DOI] [PubMed] [Google Scholar]
- 3. Visser M, Simonsick EM, Colbert LH, et al. Health ABC Study. Type and intensity of activity and risk of mobility limitation: the mediating role of muscle parameters. J Am Geriatr Soc. 2005;53:762–770 [DOI] [PubMed] [Google Scholar]
- 4. UNFPA and Help Age International. Ageing in the Twenty- First Century: A Celebration and A Challenge. 2012. New York: United Nations Population Fund (UNFPA) and London: Help Age International; ISBN: 978-0-89714-981-5 [Google Scholar]
- 5. Woods DR, Humphries SE, Montgomery HE. The ACE I/D polymorphism and human physical performance. Trends Endocrinol Metab. 2000;11:416–420 [DOI] [PubMed] [Google Scholar]
- 6. Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177:867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gudlaugsson J, Gudnason V, Aspelund T, et al. Effects of a 6-month multimodal training intervention on retention of functional fitness in older adults: a randomized-controlled cross-over design. Int J Behav Nutr Phys Act. 2012;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buford TW, Manini TM, Hsu FC, et al. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60:1244–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Bari M, van de Poll-Franse LV, Onder G, et al. Health, Aging and Body Composition Study. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:961–966 [DOI] [PubMed] [Google Scholar]
- 10. Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930 [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 12. Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29:2388–2442 [DOI] [PubMed] [Google Scholar]
- 13. Witham MD, Daykin AR, McMurdo ME. Pilot study of an exercise intervention suitable for older heart failure patients with left ventricular systolic dysfunction. Eur J Cardiovasc Nurs. 2008;7:303–306 [DOI] [PubMed] [Google Scholar]
- 14. Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst.Rev. 2005;CD004017 doi:10.1002/14651858.CD004017.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. King AC, Haskell WL, Taylor CB, Kraemer HC, DeBusk RF. Group- vs home-based exercise training in healthy older men and women. A community-based clinical trial. JAMA. 1991;266:1535–1542 [PubMed] [Google Scholar]
- 16. O’Keeffe ST, Lye M, Donnellan C, Carmichael DN. Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart. 1998;80:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–398 [DOI] [PubMed] [Google Scholar]
- 18. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bean JF, Herman S, Kiely DK, et al. Increased Velocity Exercise Specific to Task (InVEST) training: a pilot study exploring effects on leg power, balance, and mobility in community-dwelling older women. J Am Geriatr Soc. 2004;52:799–804 [DOI] [PubMed] [Google Scholar]
- 20. Martin HJ, Yule V, Syddall HE, Dennison EM, Cooper C, Aihie Sayer A. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard Bodex dynamometry. Gerontology. 2006;52:154–159 [DOI] [PubMed] [Google Scholar]
- 21. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343 [DOI] [PubMed] [Google Scholar]
- 22. Pollard B, Johnston M. Problems with the sickness impact profile: a theoretically based analysis and a proposal for a new method of implementation and scoring. Soc Sci Med. 2001;52:921–934 [DOI] [PubMed] [Google Scholar]
- 23. Scottish Index of Multiple Deprivation. A National Statistics Publication for Scotland. 2012. Edinburgh: The Scottish Government [Google Scholar]
- 24. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 25. Mangione KK, Craik RL, Palombaro KM, Tomlinson SS, Hofmann MT. Home-based leg-strengthening exercise improves function 1 year after hip fracture: a randomized controlled study. J Am Geriatr Soc. 2010;58:1911–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc. 2010;11:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bunout D, Barrera G, de la Maza MP, Leiva L, Backhouse C, Hirsch S. Effects of enalapril or nifedipine on muscle strength or functional capacity in elderly subjects. A double blind trial. J Renin Angiotensin Aldosterone Syst. 2009;10:77–84 [DOI] [PubMed] [Google Scholar]
- 28. Leonetti G, Mazzola C, Pasotti C, et al. Treatment of hypertension in the elderly: effects on blood pressure, heart rate, and physical fitness. Am J Med. 1991;90(3A):12S–13S [DOI] [PubMed] [Google Scholar]
- 29. Habouzit E, Richard H, Sanchez H, et al. Decreased muscle ACE activity enhances functional response to endurance training in rats, without change in muscle oxidative capacity or contractile phenotype. J Appl Physiol. 2009;107:346–353 [DOI] [PubMed] [Google Scholar]
- 30. Guo Q, Minami N, Mori N, et al. Effects of estradiol, angiotensin-converting enzyme inhibitor and exercise training on exercise capacity and skeletal muscle in old female rats. Clin Exp Hypertens. 2010;32:76–83 [DOI] [PubMed] [Google Scholar]
- 31. Minami N, Li Y, Guo Q, et al. Effects of angiotensin-converting enzyme inhibitor and exercise training on exercise capacity and skeletal muscle. J Hypertens. 2007;25:1241–1248 [DOI] [PubMed] [Google Scholar]