Abstract
Previously, we showed that FI34, a frailty index based on 34 health and function ability variables, is heritable and a reliable phenotypic indicator of healthy aging. We have now examined the relationship between major components of energy expenditure and the FI34 in participants of the Louisiana Healthy Aging Study. Resting metabolic rate was associated with FI34, even after adjustment for fat-free mass, fat mass, age, sex, thyroid hormones, and insulin-like growth factor 1 levels, in multiple regression analyses. In contrast, there was no association between total daily energy expenditure and FI34. Circulating creatine phosphokinase, a clinical marker of muscle damage, was also significantly associated with FI34. However, these associations of resting metabolic rate with FI34 were restricted to the oldest old (≥90 years) and absent in younger age groups. In oldest old men, the association of FI34 with creatine phosphokinase persisted, whereas in the oldest old women, only the association with resting metabolic rate pertained with the appearance of an effect of body size and composition. These results point toward an increasing metabolic burden for the maintenance of homeodynamics as health declines in nonagenarians, and this has implications for contraction of metabolic reserve that may potentially accelerate the path to disability.
Key Words: Frailty, Energy metabolism, Age, Aging.
Energy metabolism is essential to life. Total daily energy expenditure (TDEE) in homeothermic organisms is usually partitioned into resting metabolic rate (RMR), energy cost of physical activity (PA), and diet-induced thermogenesis (1–3). RMR is the rate of energy expenditure in the postabsorptive state under resting conditions and accounts for the bulk (60%–70%) of TDEE. It refers to the amount of energy used “to preserve the integrity and functionality of the ‘body machinery’” (3). The basal metabolic rate is measured under more defined conditions, but RMR and basal metabolic rate are closely related and often used interchangeably. PA and diet-induced thermogenesis contribute to TDEE approximately 20%–30% and 10%, respectively.
Energy metabolism and body composition are interrelated (4). For example, activity energy expenditure (AEE) is closely correlated with body weight, whereas RMR is related to fat-free mass (FFM), fat mass (FM), age, and sex. For that reason, RMR is usually normalized for FFM at the least. FFM can be further divided into different components with different metabolic rates, such as organ tissues and skeletal muscle. In a study of individuals ranging in age from 18 to 50 years who were within the normal body mass index range (18–27kg/m2), 58% of the RMR was accounted for by the brain, liver, heart, and kidneys, whereas only 22% was accounted for by skeletal muscle (5). In these individuals, the organ mass contributed only 7% of FFM, whereas the skeletal muscle occupied 50% of FFM. These observations highlight a significant contribution of active tissue/organ mass to the RMR. In addition to body composition, other factors influencing RMR include age, sex, nutritional state, and thyroid function. RMR is also known to have a substantial genetic basis (6,7).
RMR tends to decline with age along with AEE, resulting in overall decrease in TDEE (2). One account for the decline in RMR with age is loss of FFM (8,9), especially reduction in the mass of the major organs and tissues (4). However, the loss of FFM may not be the only cause for the decline in RMR in the elderly persons because measured RMR is significantly lower than predicted RMR calculated from body composition (9,10). In contrast, some studies report that RMR may not decrease and may even increase with older age. A study comparing a sample of 91- to 96-year olds with a control group of 73-year olds found virtually no difference in RMR (11). Studies that report an increase in basal metabolic rate with age typically involve participants with chronic diseases or inflammatory conditions (12). In a report of the Baltimore Longitudinal Study of Aging, higher basal metabolic rate was associated with increased risk of mortality (13). Thus, it is possible that energy metabolism is altered by some chronic conditions in elderly people, and consequently the age-associated profile of RMR in frail individuals with chronic conditions may differ from that of RMR in nonfrail individuals.
Few studies are available examining the relationship between energy metabolism and frailty. Schrack and coworkers and Weiss and coworkers (14,15) used walking speed as an indicator of frailty in elderly people and found a potential association between frailty and RMR. Recently, we developed a measure of relative health, the frailty index FI34, composed of 34 common health and function variables, and showed that it increases exponentially with age, indicating declining health and function ability (16). The rate of increase accelerates 2.0% annually for offspring of long-lived parents (≥90 years old) and 2.7% annually for offspring of short-lived parents (<76 years old at death). The patterns of aging of these groups of offspring are distinct, as suggested by the hierarchical clustering of the 34 variables, and, thus perhaps not surprisingly, the heritability (narrow sense) of FI34 is relatively high (0.39). Our purpose here was to examine phenotypic factors (independent variables) that may contribute to FI34 (dependent variable) by testing parameters related to energy metabolism.
Methods
Participants
The Louisiana Healthy Aging Study has been described elsewhere (17). Its participants (N = 869) were residents of the southeastern part of Louisiana, around Baton Rouge. They were unrelated individuals aged from 20 to 100 years and older at the time of data collection (2002–2008). Their ethnic origins were inferred from structure analysis of 100 Alu genotypes with .8 assignment probability (17,18). Ages of participants were based on documentary evidence and demographic questionnaires. All participants provided informed consent according to protocols approved by the respective Institutional Review Boards. The summary statistics for the 109 Caucasian participants for whom all variables were collected are shown in Table 1. These participants were recruited in age groups of 60–74 and 90 years and older. An additional group of 20- to 34-year-old Caucasians for whom these data were collected is not included in the analyses.
Table 1.
Basic Characteristics of the Subjects (mean ± SD)
| Variable | All (n = 109) | Men (n = 51) | Women (n = 58) |
|---|---|---|---|
| Age (y) | 83±12 | 83±12 | 83±11 |
| FI34 | 0.190±0.072 | 0.174±0.066 | 0.204±0.075 |
| TDEE (kcal/d) | 2097.5±611.5 | 2439.6±631.1 | 1796.7±402.9 |
| AEE (kcal/d) | 628.5±352.5 | 760.7±393.9 | 494.6±245.7 |
| RMR (kcal/d) | 1259.2±263.8 | 1414.9±242.2 | 1122.4±199.2 |
| FM (kg) | 23.4±8.9 | 22.3±7.9 | 24.3±9.7 |
| FFM (kg) | 48.0±11.5 | 57.1±8.4 | 39.9±6.9 |
| CPK (IU/L) | 89.9±70.7 | 105.8±85.8 | 76.0±50.8 |
| IGF1 (ng/mL) | 158.2±79.4 | 177.3±80.6 | 141.5±75.1 |
| T3 (ng/mL) | 133.4±32.8 | 132.0±34.6 | 134.6±31.5 |
| T4 (ng/mL) | 7.8±1.4 | 7.5±1.2 | 8.0±1.5 |
Notes: AEE = activity energy expenditure; CPK = creatine phosphokinase; FM = fat mass; FFM = fat-free mass; IGF1 = insulin-like growth factor 1; RMR = resting metabolic rate; TDEE = total daily energy expenditure; T3 = triiodothyronine; T4 = thyroxine. Participants were recruited in three age groups 20–34, 60–74, and ≥90 y, of which the latter two groups were considered in this study.
Data Management
Collection of the data used in this study was described by Frisard coworkers (10,19) and Kim and coworkers (16). Only Caucasian participants were included in the analyses to avoid confounding by population admixture. The variables used to construct FI34 are 34 items covering various diseases, symptoms, conditions, and functional abilities (see Supplementary Appendix). They are adrenal disease, anemia, angina, asthma, bathing, body mass index, bronchitis, cataracts, chair stand, congestive heart failure, chronic obstructive pulmonary disease, diabetes, dressing, emphysema, feeding, family history of cancer, Geriatric Depression Scale, heart attack, high blood pressure (at the test), high cholesterol, history of high blood pressure, heart murmur, heart problem, kidney disease, liver disease, Mini-Mental State Exam, osteoporosis, seizure, self-rated health, semitandem balance, stroke, thyroid disease, transient ischemic attack, and urinary infection. The data were collected from medical history questionnaires, and they were either quantitative or categorical. Binary categorical responses were numerically coded 0 for the absence of the deficit and 1 for the presence of the deficit. Quantitative data and multicategorical responses were recoded essentially in the same way as reported previously (20) or with modifications as described (16).
Body Composition and Measures of Metabolism
Measurement of all the metabolic parameters used in this study was described previously (10,19). Briefly, body composition was measured using dual-emission x-ray absorptiometry. FFM is the total body weight multiplied by the proportion of non-FM. TDEE was calculated by the doubly labeled water method using 2H and 18O. AEE is TDEE − (RMR + 0.1 × TDEE), which corresponds to the energy expenditure associated with physical activities after exclusion of the thermic effect of food (10% of TDEE). RMR was measured by indirect calorimetry (oxygen consumption and CO2 production) during rest (reclining position and keeping still) using a Deltatrac II metabolic cart (Sensormedics, Yorba Linda, CA), and the measurement of the last 20 minutes was averaged to calculate RMR expressed in kilocalories per 24 hours. The Deltatrac II is considered the “gold standard” in the field (21). However, deviations of cart readout from the true value can exist, resulting in variability that may level out as the sample size increases.
Levels of fasting serum total thyroxine (T4) and total triiodothyronine (T3) were measured using immunoassays (DPC 2000; Diagnostic Product Corporation, Los Angeles, CA). Creatine phosphokinase (CPK) levels were measured using an enzymatic assay (Beckman Coulter DXC 600 Pro System; Beckman Coulter, Fullerton, CA). The serum levels of insulin-like growth factor 1 (IGF1) were measured using ELISA (Diagnostic System Laboratories, Inc. Webster, TX).
Statistical Analysis
All the statistical analyses were performed using R (22). The lm function in the base installation was used for multiple linear regression tests. The test used for data normality was the ad.test (Anderson–Darling test in nortest). Body mass and composition are the major contributors to resting energy expenditure, so RMR will vary greatly depending on FFM and FM. Therefore, we included FFM and FM as covariates. Sex and age were also included as covariates in the multiple linear regression, as RMR is known to vary as a function of these two variables. FI34 was normally distributed across the entire sample included here, according to the normality test.
Results
RMR, But Not TDEE, Is Associated With FI34
Our goal in this study was to evaluate the relationship between energy expenditure and FI34 accurately, after adjusting for known covariates. RMR was significantly associated with FI34 (b = 1.60 × 10− 4, p = 3.4 × 10− 3; Table 2). This means that it is associated with higher RMR, even after adjustment for body mass and composition (FFM and FM). As expected, FI34 was highly correlated with age, and men were healthier than women on average in our study population. Neither FFM nor FM was associated with FI34. In contrast to RMR, there was no association between TDEE and FI34 (Table 2). Indeed, the presence of TDEE in the regression did not affect the association of RMR with FI34. AEE was not included in this analysis because it is calculated from TDEE and RMR, and statistical tests show that it is not orthogonal to those variables. An additional interaction term between age and RMR (age × RMR) was not significant, and it did not alter the model, indicating that these two variables affect FI34 independently (data not shown).
Table 2.
Association of RMR With FI34
| Variable | b | SE (b) | p Value |
|---|---|---|---|
| Age | 4.30 × 10− 3 | 7.45·× 10− 4 | 8.6 × 10− 8 |
| Sex (male) | −5.58·× 10− 2 | 2.34·× 10− 2 | .019 |
| FM | 1.88·× 10− 5 | 8.95·× 10− 4 | .98 |
| FFM | −4.73·× 10− 4 | 1.56·× 10− 3 | .76 |
| TDEE | −1.50·× 10− 5 | 2.22·× 10− 5 | .50 |
| RMR | 1.60·× 10− 4 | 5.35·× 10− 5 | 3.4 × 10− 3 |
Notes: FM = fat mass; FFM = fat-free mass; RMR = resting metabolic rate; TDEE = total daily energy expenditure. For the model FI34 = b 0 + b 1·age + b 2·sex + b 3·FM + b 4·FFM + b 5·TDEE + b 6·RMR, adjusted R 2 = .327 (p = 1.78 × 10− 8) with 102 df. b is the regression coefficient and SE (b) is the standard error of the coefficient.
Association of RMR With FI34 Does Not Depend on Thyroid Hormones or IGF1
Thyroid hormones are known to affect energy expenditure, especially RMR, and levels of T3 and T4 are lower in elderly individuals compared with the levels in a younger group (10,23,24). IGF1 induces skeletal muscle growth and regeneration by activating the Akt-mammalian target of rapamycin pathway (25,26). IGF1, thus, has the potential to affect RMR by modulating the lean body mass. We tested for any impact of thyroid hormones and IGF1 on the association of RMR with FI34. As shown in Table 3, none of these altered the association of RMR with FI34 in this study, suggesting that endocrine control does not play a role. Although not directly related to metabolism, we probed inflammatory status by examining circulating interleukin-6 levels and found no significant association with FI34 (data not shown).
Table 3.
No Association of IGF1 and Thyroid Hormone Levels With FI34
| Variable | b | SE (b) | p Value |
|---|---|---|---|
| Age | 4.43 × 10− 3 | 7.56 × 10− 4 | 6.2 × 10− 8 |
| Sex (male) | −5.01 × 10− 2 | 2.34 × 10− 2 | .035 |
| FM | 8.32 × 10− 5 | 8.88 × 10− 4 | .93 |
| FFM | −1.19 × 10− 3 | 1.59 × 10− 3 | .46 |
| TDEE | −1.26 × 10− 5 | 2.21 × 10− 5 | .57 |
| RMR | 1.66 × 10− 4 | 5.41 × 10− 5 | 2.8 × 10− 3 |
| T3 | −2.88 × 10− 4 | 1.84 × 10− 4 | .12 |
| T4 | 3.19 × 10− 3 | 4.35 × 10− 3 | .46 |
| IGF1 | 1.23 × 10− 4 | 8.28 × 10− 5 | .14 |
Notes: FM = fat mass; FFM = fat-free mass; IGF1 = insulin-like growth factor 1; RMR = resting metabolic rate; TDEE = total daily energy expenditure; T3 = triiodothyronine; T4 = thyroxine. For the model FI34 = b 0 + b 1·age + b 2·sex + b 3·FM + b 4·FFM + b 5·TDEE + b 6·RMR + b 7·T3 + b 8·T4 + b 9·IGF1, adjusted R 2 = .339 (p = 6.27 × 10− 8) with 99 df. b is the regression coefficient and SE (b) is the standard error of the coefficient.
Circulating CPK Levels Are Associated With FI34 Alongside RMR
Skeletal muscle is considered the largest reservoir of CPK, and damage to muscle of various etiologies can give rise to elevation of its levels in serum (27). CPK is clinically used as an indicator of muscle damage and a diagnostic of such conditions as myocardial infarction and severe muscle breakdown (28,29). Loss of muscle function during aging might be expected to increase the metabolic cost (RMR) of tissue maintenance and of the organism’s homeodynamics in general. Therefore, we tested the association of CPK with FI34. As shown in Table 4, CPK levels were significantly associated with FI34 (b = 2.60 × 10− 4, p = 2.0 × 10− 3) alongside RMR (b = 1.64 × 10− 4, p = 1.8 × 10− 3). The notion that the association of CPK with FI34 is a reflection of the effect of muscle dysfunction on RMR was bolstered by the lack of the main effect of CPK on FI34 when its interaction with RMR was included in the model (Table 5), supporting the conclusion that muscle metabolism is an important contributor to FI34.
Table 4.
Circulating Creatine Phosphokinase Levels Are Associated With FI34 Alongside RMR
| Variable | b | SE (b) | p Value |
|---|---|---|---|
| Age | 4.21 × 10− 3 | 7.14 × 10− 4 | 5.0 × 10− 8 |
| Sex (male) | −4.68 × 10− 2 | 2.26 × 10− 2 | .041 |
| FM | 4.80 × 10− 4 | 8.69 × 10− 4 | .58 |
| FFM | −1.62 × 10− 3 | 1.54 × 10− 3 | .29 |
| TDEE | −1.07 × 10− 5 | 2.13 × 10− 5 | .62 |
| RMR | 1.64 × 10− 4 | 5.12 × 10− 5 | 1.8 × 10− 3 |
| CPK | 2.60 × 10− 4 | 8.18 × 10− 5 | 2.0 × 10− 3 |
Notes: CPK = creatine phosphokinase; FM = fat mass; FFM = fat-free mass; RMR = resting metabolic rate; TDEE = total daily energy expenditure. For the model FI34 = b 0 + b 1·age + b 2·sex + b 3·FM + b 4·FFM + b 5·TDEE + b 6·RMR + b 7·CPK, adjusted R 2 = .382 (p = 6.77 × 10−10) with 101 df. b is the regression coefficient and SE (b) is the standard error of the coefficient.
Table 5.
No Statistical Interaction Between RMR and CPK
| Variable | b | SE (b) | p Value |
|---|---|---|---|
| Age | 4.24 × 10− 3 | 7.16 × 10− 4 | 4.3 × 10−10 |
| Sex (male) | −4.52 × 10− 2 | 2.27 × 10− 2 | .050 |
| FM | 5.80 × 10− 4 | 8.77 × 10− 4 | .51 |
| FFM | −1.58 × 10− 3 | 1.54 × 10− 3 | .31 |
| TDEE | −1.11 × 10− 5 | 2.13 × 10− 5 | .60 |
| RMR | 1.30 × 10− 4 | 6.31 × 10− 5 | .042 |
| CPK | −2.07 × 10− 4 | 5.11 × 10− 4 | .69 |
| RMR·CPK | 3.54 × 10− 7 | 3.82 × 10− 7 | .36 |
Notes: CPK = creatine phosphokinase; FM = fat mass; FFM = fat-free mass; RMR = resting metabolic rate; TDEE = total daily energy expenditure. For the model FI34 = b 0 + b 1·age + b 2·sex + b 3·FM + b 4·FFM + b 5·TDEE + b 6·RMR+ b 7·CPK + b 8·RMR·CPK, adjusted R 2 = .381 (p = 1.56 × 10− 9) with 100 df. b is the regression coefficient and SE (b) is the standard error of the coefficient.
Association of RMR With FI34 in the Older Age Group
If resting energy expenditure increases as health declines during aging, we would expect the association of FI34 with RMR to be more evident among older individuals, who are likely to have more health deficiencies. The participants were divided into two age groups: the “young” age group consisting of individuals who were aged 60–74 years and the “old” group of those who were aged 90–98 years. No significant association of RMR or any of the covariates with FI34 was seen in either men or women of the “young” group (data not shown). As shown in Table 6, the association of RMR with FI34 was significant in the nonagenarian group, whether male (b = 2.05 × 10− 4, p = .040) or female (b = 3.84 × 10− 4, p = .0034), even after adjusting for TDEE. However, the association of CPK with FI34 was evident only in men (p = .0012) and not in women (p = .82). In contrast, body mass and composition became significant determinants in women (FM: p = .0072, FFM: p = .012). This suggests that the mechanisms underlying the association of RMR with decreased health differ markedly in men and women, with muscle damage playing a more important role in men and muscle mass perhaps more relevant in women.
Table 6.
Association of RMR and CPK With FI34 in “Old” Men and Women
| Variable | “Old” Male Group (90–97 y) | “Old” Female Group (90–98 y) | ||
|---|---|---|---|---|
| b | p Value | b | p Value | |
| Age | 5.05 × 10− 3 | .44 | −3.67 × 10− 3 | .48 |
| FM | 2.86 × 10− 3 | .28 | 5.00 × 10− 3 | 7.2 × 10− 3 |
| FFM | −3.06 × 10− 3 | .35 | −9.28 × 10− 3 | .012 |
| TDEE | −4.20 × 10− 6 | .91 | −1.94 × 10− 5 | .66 |
| RMR | 2.05 × 10− 4 | .040 | 3.84 × 10− 4 | 3.4 × 10− 3 |
| CPK | 4.50 × 10− 4 | 1.2 × 10− 3 | −6.31 × 10− 5 | .82 |
| IGF1 | −1.22 × 10− 5 | .95 | −5.66 × 10− 5 | .74 |
| T3 | −1.69 × 10− 4 | .60 | −2.29 × 10− 4 | .53 |
| T4 | −6.20 × 10− 5 | 1.0 | 1.01 × 10− 2 | .18 |
Notes: CPK = creatine phosphokinase; FM = fat mass; FFM = fat-free mass; IGF1 = insulin-like growth factor 1; RMR = resting metabolic rate; TDEE = total daily energy expenditure; T3 = triiodothyronine; T4 = thyroxine. For the model FI34 = b 0 + b 1·age + b 2·FM + b 3·FFM + b 4·TDEE+ b 5·RMR+ b 6·CPK + b 7·IGF1 + b 8·T3 + b 9·T4, adjusted R 2 = .325 (p = .015) for the female group and .338 (p = .033) for the male group. b is the regression coefficient, and the sample size is 30 for “old” men and 37 for “old” women.
Discussion
The goal of this study was to examine the components of energy metabolism that may contribute to decline in health and function ability during aging. Studies have shown that resting energy expenditure and activity-related energy expenditure take up 60%–70% and 20%–30% of the TDEE, respectively, thus accounting for most of the energy expenditure. We found a significant association between RMR and FI34. This association is based on multiple linear regression tests adjusted for known covariates, such as age, sex, FM, and FFM. Therefore, we conclude that RMR is an important phenotypic factor that is involved in healthy aging. This raises the question of the mediators or endophenotypes associated with this effect.
The FI34, which measures “unhealthy aging,” increases with age, and it is higher in women than in men, at any given age (16). Thus, our analyses were adjusted for age and sex. The FI34 was higher in those subjects whose RMR was higher. This finding was unexpected because RMR declines with age in most studies (1,2). In our current analyses, both RMR and TDEE, adjusted for FM, FFM, and sex, decreased with age (data not shown) and as shown previously in our population sample (30), as expected, necessitating adjustment for age to examine the association with FI34. We anticipated a significant negative association of FI34 with AEE, as PA would be expected to be higher in those who are healthier. We did not measure AEE directly in our study, so our conclusions are based on the direct measurement of RMR and TDEE. The lack of association of FI34 and TDEE implies a decrease in AEE as FI34 increases (Table 2). AEE and RMR both decrease with age after adjustment for FFM (10,19), although adjustment for FM and sex in addition makes the decrease in RMR not significant at least in some studies (30). However, this potential effect of body composition on age-related decline in RMR has not been found in all studies (9). Precise assessment of the effect of changes in organ mass on energy expenditure parameters requires careful magnetic resonance imaging during aging, which was not carried out in this study.
The association of FI34 with RMR was not related to thyroid function, as measured by circulating T3 and T4 (Table 3). Strikingly, circulating CPK levels were a predictor of FI34 (Table 4). Higher enzyme levels were associated with higher FI34 (unhealthy aging). The strong direct association of FI34 with RMR remained. The fact that the association of CPK levels with FI34 disappeared when an interaction between RMR and CPK was introduced in the model, while RMR remained a predictor, suggests that muscle functional status may underlie the contribution of RMR to FI34 (Table 5).
There exists a mutual relationship between aerobic capacity and physical function ability. Maximal energy expenditure is the upper limit of energy availability. As measured by VO2 max, it provides an estimate of the capacity for work over a given period of time. VO2 max declines with age (31), and it has been associated with walking speed (32), which in turn also declines with age (33). Not surprisingly, aerobic capacity (34) and walking speed (35) are predictors of longevity and mortality, respectively. PA, measured as TDEE adjusted for RMR, PA level or directly by accelerometry, decreases with age, and it is associated with loss of physical function ability (19). Reciprocally, maintenance of elevated levels of daily PA preserves functional capacity (30,36).
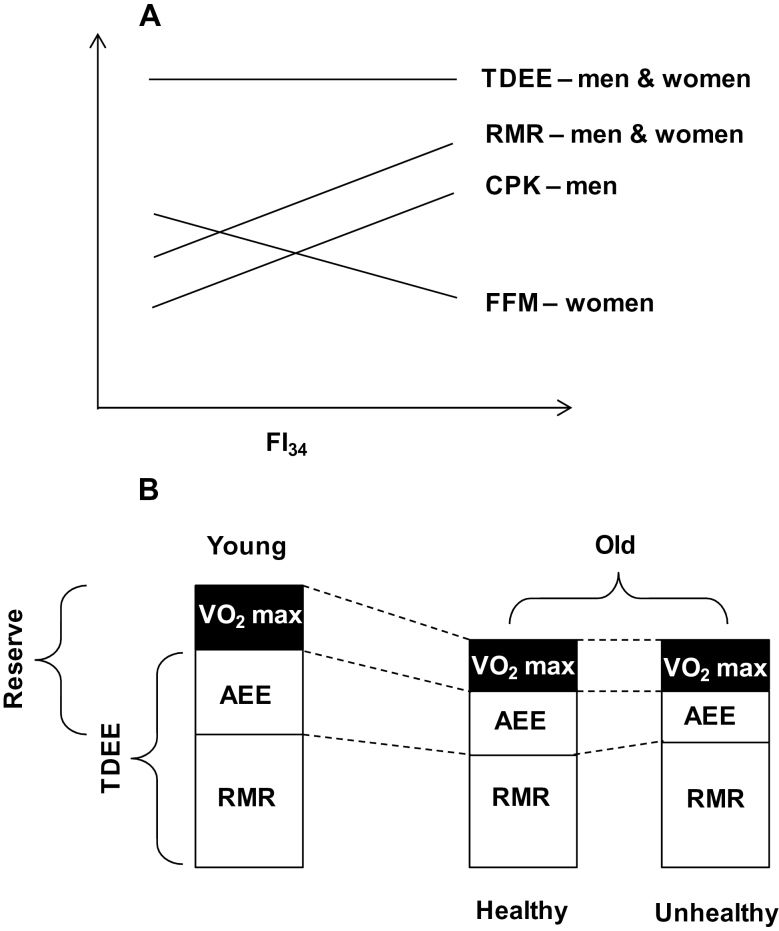
The considerations above lead to the mechanistic hypothesis that a decline in maximum energy expenditure with age and a greater requirement for RMR in unhealthy elderly individuals significantly decreases energy reserves for PA in those individuals as compared to healthier ones (Figure 1). This decrease in energy reserve could in part contribute to reduced activity tolerance and early onset of fatigue. The range of energy available for PA narrows to a greater extent in those who are less healthy and for whom the demands on RMR are higher, due to increased energy requirements for maintenance, homeodynamics, and integrated function. This study extends preliminary attempts made to address energetic needs during aging made earlier (14) by examining RMR alone in addition to simply alluding to the importance of muscle metabolism in addition to mass for RMR (19,37,38). Interestingly, individuals exhibiting no longitudinal decline in RMR (after accounting for FFM) have higher mortality than those who show the usual decline (13). Coincidentally, those individuals who have a higher FI34 display lower survival and a higher hazard rate for mortality (16) and, as shown here, a higher RMR. Validation of the model shown in Figure 1 will require additional studies of energy expenditure and metabolic capacity in the oldest old, combined with measurements of actual PA, VO2 max, and fatigue. Such studies should incorporate direct assessments of muscle metabolism and structure to probe the functional status of muscle and not only muscle mass. This research agenda will be necessary to better understand the underlying cause of the association of increase in RMR with FI34, which is distinct in men and women.
Figure 1.
Model of the impact of changes in energy metabolism during healthy and unhealthy aging. (A) Relationship between total daily energy expenditure (TDEE), resting metabolic rate (RMR), circulating creatine phosphokinase (CPK), and fat-free mass (FFM) with frailty index 34 (FI34) in male and female nonagenarians. This panel summarizes this study emphasizing the results for clarity. (B) The model derived from the results postulates that the decrease in maximum energy expenditure (VO2 max) with age, coupled with an increase in RMR for maintenance and homeodynamics during unhealthy aging, results in a contraction of the energy reserve available for physical activity, compared to what is available during healthy aging. This occurs on the background of the same decrease in TDEE in healthy and unhealthy aging, with a decline in activity energy expenditure (AEE) with age that is exaggerated in the latter.
Our study supports the notion that the decrease in RMR with age may not continue in all elderly individuals. It explains why most studies show a decline in RMR with age, while some do not. The explanation is based on health status, which declines overall in a population with age but not in all individuals to the same extent. Indeed, the positive association of RMR with FI34 was seen only in the oldest old group (Table 6) that has a higher prevalence of less healthy individuals. This distinction did not appear to be due to a floor effect or less variance in the younger group. Furthermore, the association of FI34 and age was no longer significant when the two age groups were considered individually (Table 6, data not shown for younger group), which suggests that these age groups are well separated by health status. In men, it appears that the functional status of muscle plays a predominant role during aging, based on the association of CPK with FI34, whereas in women, it is body mass and/or composition (Table 6). However, it is a muscle deficit in both oldest old men and women that is associated with an increase in FI34 and thus declining health during aging. Altogether, these results point to an increasing metabolic burden for the maintenance of homeodynamics as health decreases. A good part of this may be related to mass and functional status of muscle, but other body systems may also be involved. Muscle metabolism constitutes the tip of the iceberg, as skeletal muscle accounts for only about 25% of resting energy expenditure (5), and we suggest that our analysis of CPK may extrapolate to dysfunction in other organ systems. Support for declining metabolism in various organ systems has been provided before (39).
Lower energetic capacity leads to declining PA with age, as mentioned earlier. This results in a vicious cycle, because PA is needed to preserve functional capacity. The role of energy intake will be necessary to address in the future, to provide a more complete picture of the dynamics of the relationships we have examined here.
In sum, we have shown that declining health in the oldest old is associated with increased energy demands in the form of higher RMR. This increased RMR is likely the derivative of greater demands on maintenance of an aging organism whose tissue and integrated functions are degrading. In individuals whose integrated functions are more or less intact, as measured indirectly by a lower FI34, RMR is lower. This explains the discrepancies in the relationship of RMR with age found in various studies. It also shifts the level of analysis to intermediate phenotypes that mediate these effects, some of which may differ between men and women.
Supplementary Material
Supplementary material (references 40–45 are cited in the supplementary material) can be found at: http://biomedgerontology. oxfordjournals.org/
Funding
This research was supported in part by grants from the National Institute of General Medical Sciences of the National Institutes of Health (P20 GM103629) to S.M.J. and S.K. and by the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund [HEF (2001–2006)-02], and by the National Institute on Aging (P01 AG022064) to S.M.J.
Acknowledgments
We thank the people of Louisiana for participation in our studies. The corresponding authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Henry CJ. Mechanisms of changes in basal metabolism during ageing. Eur J Clin Nutr. 2000;54(suppl 3):S77–S91 [DOI] [PubMed] [Google Scholar]
- 2. Wilson MM, Morley JE. Invited review: aging and energy balance. J Appl Physiol. 2003;95:1728–1736 [DOI] [PubMed] [Google Scholar]
- 3. Ruggiero C, Ferrucci L. The endeavor of high maintenance homeostasis: resting metabolic rate and the legacy of longevity. J Gerontol A Biol Sci Med Sci. 2006;61:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275(2 Pt 1):E249–E258 [DOI] [PubMed] [Google Scholar]
- 6. Bogardus C, Taskinen MR, Zawadzki J, Lillioja S, Mott D, Howard BV. Increased resting metabolic rates in obese subjects with non-insulin-dependent diabetes mellitus and the effect of sulfonylurea therapy. Diabetes. 1986;35:1–5 [DOI] [PubMed] [Google Scholar]
- 7. Bouchard C, Tremblay A, Nadeau A, et al. Genetic effect in resting and exercise metabolic rates. Metabolism. 1989;38:364–370 [DOI] [PubMed] [Google Scholar]
- 8. Weinsier RL, Schutz Y, Bracco D. Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am J Clin Nutr. 1992;55:790–794 [DOI] [PubMed] [Google Scholar]
- 9. Krems C, Lührmann PM, Strassburg A, Hartmann B, Neuhäuser-Berthold M. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr. 2005;59:255–262 [DOI] [PubMed] [Google Scholar]
- 10. Frisard MI, Broussard A, Davies SS, et al. Louisiana Healthy Aging Study. Aging, resting metabolic rate, and oxidative damage: results from the Louisiana Healthy Aging Study. J Gerontol A Biol Sci Med Sci. 2007;62:752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothenberg EM, Bosaeus IG, Westerterp KR, Steen BC. Resting energy expenditure, activity energy expenditure and total energy expenditure at age 91-96 years. Br J Nutr. 2000;84:319–324 [PubMed] [Google Scholar]
- 12. Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr. 2000;54(suppl 3):S92–103 [DOI] [PubMed] [Google Scholar]
- 13. Ruggiero C, Metter EJ, Melenovsky V, et al. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2008;63:698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(suppl 2):S329–S336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiss CO, Cappola AR, Varadhan R, Fried LP. Resting metabolic rate in old-old women with and without frailty: variability and estimation of energy requirements. J Am Geriatr Soc. 2012;60:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S, Welsh DA, Cherry KE, Myers L, Jazwinski SM. Association of healthy aging with parental longevity. Age. 2013;35:1975–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jazwinski SM, Kim S, Dai J, et al. Georgia Centenarian Study and the Louisiana Healthy Aging Study. HRAS1 and LASS1 with APOE are associated with human longevity and healthy aging. Aging Cell. 2010;9:698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frisard MI, Fabre JM, Russell RD, et al. Louisiana Healthy Aging Study. Physical activity level and physical functionality in nonagenarians compared to individuals aged 60-74 years. J Gerontol A Biol Sci Med Sci. 2007;62:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schadewaldt P, Nowotny B, Strassburger K, Kotzka J, Roden M. Indirect calorimetry in humans: a postcalorimetric evaluation procedure for correction of metabolic monitor variability. Am J Clin Nutr. 2013;97:763–773 [DOI] [PubMed] [Google Scholar]
- 22. R. Version R 2.11.1; R Development Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Roundation for Statistical Computing; 2008 [Google Scholar]
- 23. Danforth E, Jr, Burger A. The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab. 1984;13:581–595 [DOI] [PubMed] [Google Scholar]
- 24. Klieverik LP, Coomans CP, Endert E, et al. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 2009;150:5639–5648 [DOI] [PubMed] [Google Scholar]
- 25. Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126 [DOI] [PubMed] [Google Scholar]
- 26. Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle. 2011;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lehmann P, Hartung W, Fleck M. Rhabdomyolysis and creatine kinase elevation. Z Rheumatol. 2013;72:236–241 [DOI] [PubMed] [Google Scholar]
- 28. Guzy PM. Creatine phosphokinase-MB (CPK-MB) and the diagnosis of myocardial infarction. West J Med. 1977;127:455–460 [PMC free article] [PubMed] [Google Scholar]
- 29. Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore). 2005;84:377–385 [DOI] [PubMed] [Google Scholar]
- 30. Johannsen DL, DeLany JP, Frisard MI, et al. Louisiana Healthy Aging Study. Physical activity in aging: comparison among young, aged, and nonagenarian individuals. J Appl Physiol. 2008;105:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Astrand I, Astrand PO, Hallbäck I, Kilbom A. Reduction in maximal oxygen uptake with age. J Appl Physiol. 1973;35:649–654 [DOI] [PubMed] [Google Scholar]
- 32. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 33. Cunningham DA, Rechnitzer PA, Pearce ME, Donner AP. Determinants of self-selected walking pace across ages 19 to 66. J Gerontol. 1982;37:560–564 [DOI] [PubMed] [Google Scholar]
- 34. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801 [DOI] [PubMed] [Google Scholar]
- 35. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026 [DOI] [PubMed] [Google Scholar]
- 36. Leveille SG, Guralnik JM, Ferrucci L, Langlois JA. Aging successfully until death in old age: opportunities for increasing active life expectancy. Am J Epidemiol. 1999;149:654–664 [DOI] [PubMed] [Google Scholar]
- 37. Edwards JD, Redmond AD, Nightingale P, Wilkins RG. Oxygen consumption following trauma: a reappraisal in severely injured patients requiring mechanical ventilation. Br J Surg. 1988;75:690–692 [DOI] [PubMed] [Google Scholar]
- 38. Kotler DP. Cachexia. Ann Intern Med. 2000;133:622–634 [DOI] [PubMed] [Google Scholar]
- 39. Gallagher D, Allen A, Wang Z, Heymsfield SB, Krasnow N. Smaller organ tissue mass in the elderly fails to explain lower resting metabolic rate. Ann N Y Acad Sci. 2000;904:449–455 [DOI] [PubMed] [Google Scholar]
- 40. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30 [DOI] [PubMed] [Google Scholar]
- 41. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919 [DOI] [PubMed] [Google Scholar]
- 42. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49 [DOI] [PubMed] [Google Scholar]
- 43. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 44. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483 [PubMed] [Google Scholar]
- 45. NHLBI NHLBI. Categories for Blood Pressure Levels in Adults (measured in millimeters of mercury, or mmHg). U.S. Department of Health & Human Services/NIH: National Heart Lung & Blood Institute. 2012. What is High Blood Pressure? http://www.nhlbi.nih.gov/health/health-topics/topics/hbp Accessed June 7, 2013