Abstract
Background.
On average, as people age, they accumulate more health deficits and have an increased risk of death. The deficit accumulation–based frailty index (FI) can quantify health and its outcomes in aging. Previous studies have suggested that women show higher FI values than men and that the highest FI score (the “limit to frailty”) occurs at a value of FI ~ 0.7. Even so, gender differences in the limit to frailty have not been reported.
Methods.
Data for this analysis were obtained from the Beijing Longitudinal Study of Aging that involved 3,257 community-dwelling Chinese people, aged 55+ years at baseline. The main outcome measure was 5-year mortality. An FI consisting of 35 health-related variables was constructed. The absolute and 99% FI limits were calculated for different age groups and analyzed by sex.
Results.
The mean level of the FI increased with age and was lower in men than in women (F = 67.87, p < .001). The 99% FI limit leveled off slightly earlier with a relatively lower value in men (60 years; 0.44 ± 0.02) compared with that in women (65 years; 0.52 ± 0.04). The highest absolute FI value was 0.61 in men and 0.69 in women. In both groups, people with an FI greater than or equal to the 99% limit showed close to 100% mortality by 5 years.
Conclusion.
Compared with men, women appeared to better tolerate deficits in health, yielding both relatively lower mortality and higher limit values to the FI. Even so, the FI did not exceed 0.7 in any individual.
Key Words: Aging, Deficit accumulation, Frailty limit, Gender difference, Mortality.
Frailty represents an increased level of vulnerability compared with other people of the same age and is variously viewed as an at-risk state (1) or as a specific syndrome (2). People with a higher level of frailty are more inclined to have adverse health outcomes, leading to a higher risk of institutionalization and death (1,2). Several approaches have been proposed to operationalize frailty (3–6). Among these, the frailty index (FI) based on the accumulation of deficits has been validated using multiple data sets and has shown several interesting characteristics (5,7–14). The mean value of the FI increases with age and is closely related to the risk of death. Women accumulate more deficits at any given age (ie, higher FI) on average but show a lower mortality than men for any level of deficit accumulation (11,15–17).
One intriguing finding from work with the FI is that there appears to be an empirical limit to the accumulation of deficits in older adults, as suggested by the reliability theory of aging (7,15,18,19). According to this theory, humans represent redundant systems, with certain numbers of irreplaceable elements that deteriorate over time. When the redundancy is exhausted, the body system reaches its limit of “physiological reserve” so that it can accumulate no more deficits (20,21). By this view, the usual tendency to redundancy exhaustion accounts for the limit to life expectancy; that is, the deficit accumulation in this person tends to decelerate along with decreased mortality, in accord with the “compensation law of mortality” (22). When the body system reaches its limit of tolerance, a single extra deficit can cause the system to fail, leading to little chance of survival. Quantification of a limit to frailty is important, with implications especially for public health planning and better patient care: for example, whether an older patient can tolerate surgery and/or a given procedure and at what risk of adverse outcomes.
Earlier work using Western data sets has demonstrated repeatedly that redundancy exhaustion may appear at FI values close to 0.7 (18). Similar findings have been shown by a recent study on Chinese sample aged 80+ years (19). Even so, previous studies have not explored the possible gender differences in the FI limits and thus not taken into account the difference in mortality and healthy life expectancy between men and women (7,15,18,19, 23–25). Using data from the Beijing Longitudinal Study of Aging, we have evaluated aging by applying the FI approach in China (14, 26). In this study, we extend the analysis to investigate the relationship between frailty limit and the short-term (5-year) mortality in men and women. Given the extent of health changes in the middle-aged people, we compared subjects aged 55–64 years with those aged 65+ years.
Methods
Participants and Data
The Beijing Longitudinal Study of Aging is a prospective cohort study of 3,257 community-dwelling Chinese population aged 55 years and older at baseline. The geographic distribution, economic status, age, and education of the sample represent the older population of Beijing, as obtained from the Fourth National Census Data (27). As described elsewhere (27,28), the cohort was assembled in 1992; the response rate was 91.2%; participants were followed every 2–3 years. At the time of the 1997 survey, 784 subjects (24.1%) had died, and 430 were missing at follow-up. The survey was based on self-reporting information that covered demographic characteristics, socioeconomic status, activities of daily living, lifestyle, physical health, psychological and self-rated health, medical conditions, cognitive status, and the use of health care services. Trained interviewers, mostly nurses or physicians, administered a standard questionnaire at the respondent’s home; where available, medical records were used to verify the presence of disease. Depressive symptoms were evaluated using the Center for Epidemiologic Studies—Depression scale, and cognitive function was assessed using the Mini-Mental State Examination (MMSE) scale.
For this secondary analysis, variables from the baseline data set were retrieved and used to construct the FI. Five-year survival outcomes were evaluated. Survival status was determined through interviews with surviving household members and/or neighbors and verified by death certificates and/or local police register records. Vital status was known for 91.6% of the participants, with censoring for dates of death or dropout. Data of the subjects (8.4%) with missing survival information were excluded from survival-related analysis only.
Frailty Index
An FI was constructed using the baseline survey data (1992) for each participant (n = 3,257) as described in the previous reports (14, 26). Specifically, each variable used in the FI satisfied the criteria of being associated with health status, accumulating with age, not saturating (ie, not becoming present in >80% of people), having more than 1% prevalence and less than 5% missing, and covering several systems (29). As a result, 35 variables were included, containing diseases (n = 8), symptoms (n = 7), psychological problems (n = 5), basic and Instrumental Activities of Daily Living disabilities (n = 14), the MMSE total score (Table1). Fifteen of these variables were binary, in which “0” was used to indicate the absence of the deficit and “1” to indicate its presence. For the remaining 20, three level variables, an additional value of 0.5 represented the intermediate status such as “sometimes”. The MMSE was coded as 0 (MMSE ≥ 24), 0.5 (MMSE = 15–23), and 1 (MMSE ≤ 14). Next, all the variables were summed and divided by 35 to yield a FI ranging from a theoretical minimum of 0 (no deficits present) to a possible maximum of 1.0 (all deficits present), with higher FI values representing a greater level of frailty and thus worse health and greater vulnerability to adverse outcomes. For individuals in whom a given variable was missing, the FI was calculated based on the items present; variables with missing values were excluded from both the numerator and the denominator. In this study, the maximum number of missing values in any individual FI was never greater than 1.
Statistical Analysis
Sample characteristics were described using means and standard deviations for interval variables and percentages for the categorical variables, with differences tested using analysis of variance and chi square (χ2), respectively. The attributable risk was calculated for each variable as the fraction referring to the proportion of risk among the exposed population that could be attributed to the exposure (30). The FI values were calculated separately for men and women and for different age groups. The 95% and 99% submaximal FIs (ie, the 95% and 99% FI limits) for a given age group were calculated as the mean FI values of the 5% and the 1% people with the greatest FI at the ages. Multivariable regression analysis was used to examine the relationship between the FI (mean and limit values) with age and mortality. Five-year mortality rates were compared between men and women using Student’s t tests, whereas the survival probability was evaluated using the Kaplan–Meier analysis. Data analyses were performed using SPSS v19.0 and codes developed using Matlab 2008.
Results
Women were slightly older and had less education than men (Table 1). Women were also more likely to have problems with function, cognition, and depression and to exercise less, especially after age 65. In the 55- to 64-year-old group, the Activities of Daily Living performance was marginally higher in men than in women. However, women aged 65+ years showed better performance with items of both Activities of Daily Living and Instrumental Activities of Daily Living compared with men of the same age. Of note, women who were between 55–64 years appeared to take more medication compared with older women or men. Considering the individual deficits that make up the FI, women were more likely than men to report most deficits, except for stroke and several function items (Table 2). The baseline demographic and health conditions did not differ significantly between the participants (n = 275, 8.4%) who missed survival information and the rest of the sample regarding mean age (70.9 ± 9.2 vs 70.1 ± 9.0, F = 2.19, p = .14), % female (53.5% vs.50.9%, χ2 = 0.67, p = .41), % married (62.9% vs 65.7%, χ2 = 0.850, p = .36), mean MMSE (23.6 ± 4.1 vs 23.1 ± 4.3, F = 1.88, p = .17), or the mean level of FI (0.12 ± 0.10 vs 0.13 ± 0.10, F = 0.14, p = .71); however, the latter were more likely to be better educated and live in an urban dwelling (9+ y education = 22.9% vs 11.5%, χ2=30.26, p < .001 and rural dwelling: 12.0% vs 36.5%, χ2 = 66.98, p < .001). This subsample was excluded from the mortality analysis only.
Table 1.
Demographics and Characteristics of Middle-Aged and Older Adult Men and Women in the Sample of the Beijing Longitudinal Study of Aging
| 55- to 64-y Old | 65+ y Old | |||||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 482) | Women (n = 557) | F/χ2 | p | Men (n = 1111) | Women (n = 1107) | F/χ2 | p | |
| Age | 59.9±4.2 | 59.8±2.8 | 0.22 | .641 | 74.6±6.3 | 75.3±6.7 | 4.87 | .027 |
| 9+ y education (%) | 23.2 | 12.6 | 20.36 | < .001 | 15.0 | 5.1 | 60.98 | < .001 |
| 5-y death rate (%) | 9.5 | 6.1 | 4.30 | .038 | 33.8 | 30.7 | 2.35 | .126 |
| Being married (%) | 92.7 | 84.2 | 18.05 | <.001 | 69.5 | 40.1 | 193.23 | <.001 |
| Rural dwellers (%) | 35.9 | 33.6 | 0.61 | .433 | 33.8 | 34.8 | 0.22 | .643 |
| Total ADL score (/18) | 6.1±0.9 | 6.0±0.2 | 6.68 | .010 | 6.3±1.4 | 6.5±1. | 8.79 | .003 |
| Total IADL score (/18) | 6.4±1.7 | 6.3±1.3 | 0.49 | .485 | 7.6±3.2 | 8.7±3.9 | 58.06 | <.001 |
| MMSE (/30) | 25.7±2.8 | 23.7±3.6 | 63.55 | <.001 | 24.1±3.8 | 20.7±4.4 | 231.02 | <.001 |
| CES-D (/60) | 6.3±7.6 | 8.2±7.3 | 11.44 | .001 | 5.6±6.7 | 8.4±7.9 | 52.11 | <.001 |
| # of medication taken | 1.1±1.2 | 1.4±1.4 | 12.97 | <.001 | 1.2±1.4 | 1.2±1.3 | 0.42 | .518 |
| Physical exercise score | 2.6±2.6 | 2.9±3.0 | 3.05 | .081 | 2.8±2.5 | 1.9±2.2 | 85.65 | <.001 |
| Frailty index | 0.08±0.07 | 0.09±0.07 | 12.91 | <.001 | 0.12±0.10 | 0.16±0.12 | 71.20 | <.001 |
Note: Data are presented as mean ± standard deviation, unless specified otherwise. ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living; MMSE = Mini-Mental State Estimation; CES-D = Center for Epidemiologic Studies—Depression. Group differences were examined using analysis of variance (F) and χ2 test, respectively, for interval and categorical variables. The level of significance (p) was set at .05.
Table 2.
Percentage Present of the Health Deficits Used in Constructing the Frailty Index, and Their Attributable Risks for 5-Year Mortality, in Middle-Aged and Older Adult Men and Women
| Variables Description | 55- to 64-y Old | 65+ y Old | ||||||
|---|---|---|---|---|---|---|---|---|
| Men (482) | Women (557) | χ2 | p | Men (1111) | Women (1107) | χ2 | p | |
| % Present (AR) | % Present (AR) | % Present (AR) | % Present (AR) | |||||
| Do not have much energy | 42.4 (0.26) | 55.2 (0.61) | 17.23 | <.001 | 59.2 (0.45) | 69.6 (0.30) | 28.78 | <.001 |
| Fell less useful | 41.1 (0.34) | 58.5 (0.33) | 31.28 | <.001 | 61.2 (0.48) | 76.7 (0.34) | 79.34 | <.001 |
| Do not feel a lot of fun in life | 36.7 (0.18) | 37.3 (0.41) | 0.05 | .976 | 37.7 (0.22) | 39.3 (0.12) | 1.04 | .594 |
| Do not feel very happy | 35.2 (0.06) | 43.5 (0.23) | 7.89 | .019 | 29.8 (0.09) | 36.7 (0.18) | 17.15 | <.001 |
| Feel nothing to do | 16.5 (0.48) | 20.6 (0.52) | 4.47 | .107 | 20.0 (0.35) | 27.2 (0.21) | 16.01 | <.001 |
| Hypertension | 19.1 (0.46) | 26.0 (0.26) | 7.08 | .008 | 18.8 (0.17) | 19.2 (−0.23) | 0.04 | .839 |
| Coronary heart disease | 13.1 (−0.58) | 18.1 (−0.03) | 4.98 | .026 | 15.4 (0.03) | 15.4 (−0.13) | 0.00 | .982 |
| Stroke | 5.6 (0.67) | 2.5 (0.81) | 6.50 | .011 | 7.7 (0.22) | 5.1 (0.48) | 5.79 | .016 |
| TIA/small stroke | 2.1 (0) | 1.3 (0.58) | 1.07 | .300 | 1.9 (−0.18) | 1.4 (−0.15) | 1.00 | .319 |
| Arthritis | 5.2 (0.22) | 11.1 (0.59) | 11.90 | .001 | 5.6 (−0.42) | 6.0 (−0.90) | 0.15 | .700 |
| Thyroid disease | 0.8 (0.62) | 2.0 (0.34) | 2.38 | .123 | 0.5 (−0.01) | 1.5 (−0.75) | 5.36 | .021 |
| Glaucoma | 1.0 (0) | 2.5 (0) | 3.14 | .077 | 2.2 (0.19) | 3.1 (0.13) | 1.81 | .179 |
| Cataract | 4.1 (0) | 7.5 (0.53) | 5.30 | .021 | 14.6 (−0.32) | 14.7 (−0.08) | 0.01 | .924 |
| Urinary incontinence | 6.0 (0.57) | 24.4 (0.22) | 65.49 | <.001 | 12.9 (0.33) | 29.5 (0.25) | 92.25 | <.001 |
| Falls | 5.0 (0.57) | 8.3 (0.07) | 4.42 | .035 | 9.8 (0.34) | 16.4 (0.27) | 20.87 | <.001 |
| Fracture | 3.7 (0.15) | 5.7 (0.02) | 2.28 | .131 | 6.5 (0.33) | 10.5 (0.17) | 11.43 | .001 |
| Tremor | 5.8 (0.35) | 4.7 (0.63) | 0.68 | .409 | 8.0 (0.34) | 8.3 (−0.14) | 0.07 | .796 |
| Do not hear clearly | 5.8 (0.35) | 4.7 (0.22) | 0.78 | .678 | 28.9 (0.42) | 24.1 (0.49) | 6.52 | .038 |
| Wear a hearing aid | 0.4 (0) | 0.5 (0) | 0.08 | .774 | 2.7 (−0.46) | 1.3 (−0.08) | 5.88 | .015 |
| Use a walking stick | 0.4 (0) | 1.3 (0) | 2.13 | .144 | 5.5 (0.31) | 7.1 (0.26) | 2.54 | .111 |
| Need help with eating | 0.8 (0.82) | 0.2 (0.94) | 2.48 | .289 | 1.9 (0.62) | 3.1 (0.67) | 3.71 | .157 |
| Need help with grooming | 1.0 (0.77) | 0.0 (0) | 5.81 | .016 | 1.2 (0.52) | 2.3 (0.65) | 4.46 | .035 |
| Need help with dressing | 1.2 (0.82) | 0.0 (0) | 6.97 | .031 | 2.8 (0.58) | 4.0 (0.68) | 2.38 | .304 |
| Need help with getting on/off bed | 1.5 (0.85) | 0.0 (0) | 8.14 | .017 | 2.4 (0.60) | 4.7 (0.68) | 6.87 | .032 |
| Need help with bathing | 2.5 (0.83) | 1.3 (0.87) | 4.46 | .108 | 8.3 (0.60) | 14.1 (0.62) | 19.29 | <.001 |
| Need help with moving in house | 1.7 (0.86) | 0.2 (0.94) | 6.72 | .035 | 3.2 (0.60) | 5.6 (0.66) | 8.19 | .017 |
| Need help with cooking meals | 6.4 (0.78) | 2.7 (0.87) | 11.39 | .003 | 23.9 (0.63) | 24.2 (0.65) | 0.57 | .754 |
| Need help with managing money | 2.7 (0.82) | 2.9 (0.86) | 0.41 | .813 | 13.9 (0.60) | 23.2 (0.54) | 32.29 | <.001 |
| Need help with taking a bus | 5.0 (0.75) | 9.5 (0.59) | 14.52 | .001 | 24.0 (0.64) | 49.9 (0.59) | 158.89 | <.001 |
| Need help with shopping | 3.5 (0.76) | 3.2 (0.75) | 0.80 | .672 | 15.9 (0.63) | 28.9 (0.62) | 54.51 | <.001 |
| Need help with walking | 2.9 (0.75) | 2.7 (0.79) | 2.83 | .243 | 10.7 (0.60) | 22.8 (0.62) | 57.89 | <.001 |
| Need help with up/down stairs | 3.1 (0.74) | 2.5 (0.85) | 0.38 | .825 | 14.3 (0.62) | 28.0 (0.64) | 62.35 | <.001 |
| Need help in running housework | 44.4 (0.39) | 24.1 (0.34) | 48.00 | <.001 | 53.8 (0.47) | 46.8 (0.56) | 10.97 | .001 |
| Need any other personal care | 1.9 (0.84) | 0.5 (0.91) | 4.00 | .046 | 5.4 (0.58) | 9.3 (0.60) | 12.41 | <.001 |
| MMSE < 24 | 18.7 (0.50) | 39.1 (0.50) | 48.44 | <.001 | 30.5 (0.47) | 47.1 (0.58) | 162.90 | <.001 |
Note: AR = attributable risk; TIA = transient ischemic attack; MMSE = Mini-Mental State Estimation. Group differences were examined using χ2 test. The level of significance (p) was set at .05.
In both men and women, most deficits were associated with an increased risk of mortality by 5 years (Table 2). For the 55- to 64-year-old group, many deficits appeared to be more lethal for women than for men. This was in contrast to those aged 65+ years: the deficits often showed a higher risk for death in men than in women. Notably, several items, when considered individually, seemed to have a protective effect regarding the 5-year mortality in the 65+ years group (ie, negative attributable risk). This was especially true regarding women (Table 2). Such variables included using hearing aids, having arthritis, transient ischemic attack, and thyroid disease, which most likely were treated.
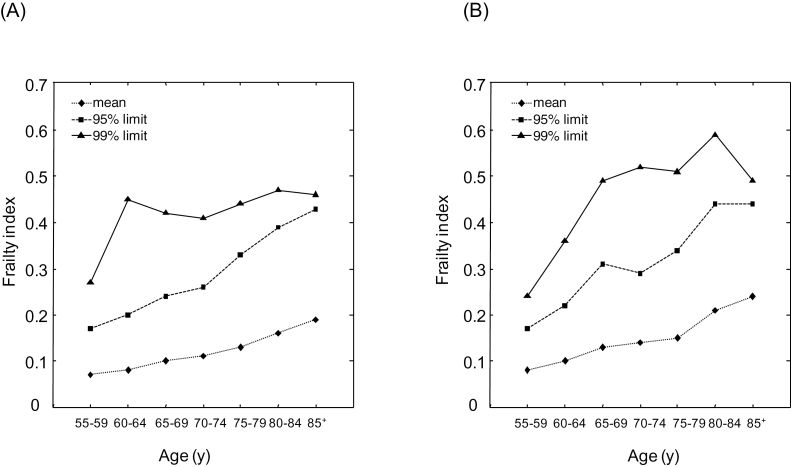
Considering deficits collectively, the mean FI increased with age for both men and women (Figure 1A and B). The minimum FI remained to be 0 at almost all ages, except for ages above 80 years (minimum FI = 0.003). For any age group, the mean FI was greater in women than in men (F = 67.87, p < .001 for the difference in the means; Figure 1A and B). The 95% limit values of the FI also increased with age, similarly in men and women. The 99% submaximal limit to the FI showed no correlation with age above the age of 60 years in men, and above the age of 65 years in women (Figure 1A and B). The 99% limit FI value was significantly higher in women (0.52 ± 0.04) than in men (0.44 ± 0.02).The highest absolute FI value was 0.61in men and 0.69 in women (ie, not exceeded 0.7).
Figure 1.
Frailty index (FI) as a function of age in men (Panel A) and in women (Panel B). Data are presented for individuals by 5-year-age groups. The level of FI was calculated for each individual as the sum of deficits present in the individual divided by 35—the total number of variables considered. Mean FIs (diamonds and dotted lines) were calculated for each age group. The 95% and 99% frailty limits for a given age group were calculated as the mean FI values of the 5% (squares and dashed lines) and the 1% (triangles and solid lines) of people with the highest FIs, respectively.
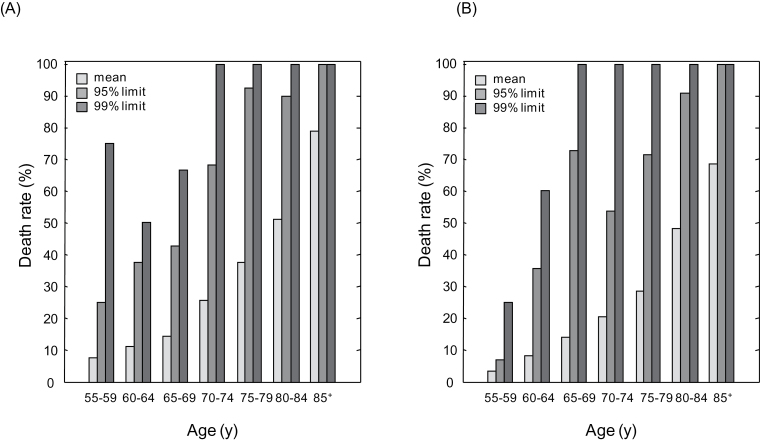
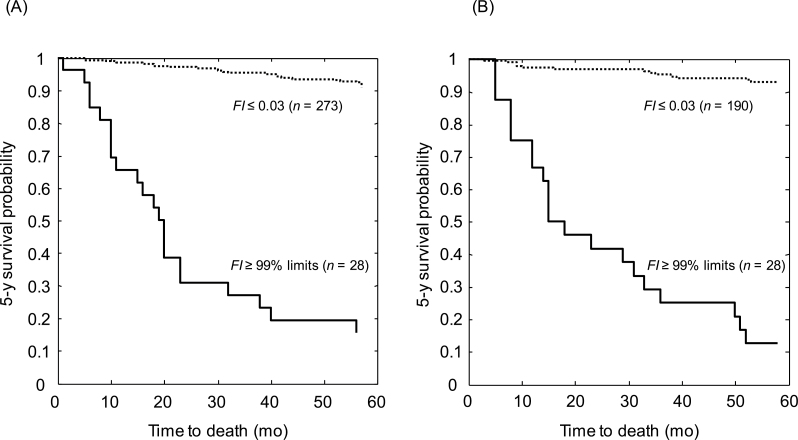
The mean death rate was higher in men than in women (t = 3.74, p = .010) and increased with age for both men and women (Figure 2A and B). For the people in whom the FI was higher than the 95% limit, the death rates were high and did not differ between men and women (n = 94 vs n = 91; t = 0.54, p = .610); this was also the case regarding the 99% limits (n = 28 men and 28 women; t = 0.10, p = .922; Figure 3A and B). All but one person aged 65+ years who had an FI at or above the 99% limit died in 5 years (ie, 15/16 men with a mean FI = 0.46 ± 0.04 and 14/14 women with the mean FI = 0.54 ± 0.05). In the 17 people younger than 65 years who had very high FIs, the 5-year mortality rates were closer to 50% and none survived to age 79.
Figure 2.
Death rates in relation to the frailty index (FI) in men (Panel A) and in women (Panel B). Data are presented for individuals by 5-year-age groups. The level of FI was calculated for each individual as the sum of deficits present in the individual divided by 35—the total number of variables considered. Mean FIs (light-gray bars) were calculated as the average of the FI values of all people within the given age groups. The 95% and 99% frailty limits for a given age group were calculated as the mean FI values of the 5% (medium-gray bars) and the 1% (dark-gray bars) people with the highest FIs, respectively.
Figure 3.
Five-year survival probabilities for men (Panel A) and women (Panel B) calculated using the Kaplan–Meier Survival function. Data are presented for people with a frailty index (FI) ≤ 0.03 (ie, the most fit people; dashed lines) and those with a FI above the 99% limit of the FI (ie, the least fit people in various age groups; solid lines). For men, the 99% limit was 0.61; for women, it was 0.69.
Discussion
In this study, we evaluated the limit of frailty in Chinese men and women by applying the deficit accumulation approach and linked it to short-term mortality. An FI was constructed using 35 variables assessing a wide range of health measures including medical history and disease conditions, signs, symptoms, psychological and cognitive health, and functions of daily living. The performance of the FI with age and the increased mortality with the FI were consistent with previous reports using the same and many other data sets (11,15–17). Our study confirmed that limits to frailty were present in this Chinese older adult sample, and further extended the FI limit analysis to include people of late middle ages. This is the first study to identify a gender difference in frailty limit in relation to healthy life expectancy and mortality. Our data suggest that the gender difference regarding the limit of how many deficits one can have has impact on the gender difference in mortality.
Consistent with other studies (17,18,31), here women showed more deficit accumulation than men. Meanwhile, women, especially those with more advanced age, were more likely to have depression and lower cognitive performance. Here, men aged 55–64 reported greater Activities of Daily Living performance than women; the situation was reversed above age 65. It is not clear how robust this conclusion is, as an age–gender interaction in how deficits affect disability has not been well studied and warrants further research using different data sets.
As expected, having many deficits increased the risk of death (Table 2). Even so, and curiously, some apparent deficits (eg, using hearing aids, having arthritis, transient ischemic attack, and thyroid disease) were associated with better 5-year survival. These findings might be attributed to several reasons. First, certain mild deficits (eg, transient ischemic attack) are indicators of subsequent severe damage (eg, stroke), and their early treatment might prevent more severe problems that otherwise would lead to death. Second, for some chronic diseases, such as arthritis and thyroid illness, medication intake (especially, nonsteroidal anti-inflammatory drugs (32) and perhaps also allopurinol) (33,34) might help to prevent the occurrence to other deficits such as inflammation and metabolic syndromes. Third, intervention strategies such as hearing aids and dentures prevent declines in health, as have better social conditions and better nutrition. On the other hand, the use of dentures and hearing aids might be surrogates for better overall access to health care services. Some factors, such as an apparently protective effect of arthritis, have been seen in other studies (35,36), including more vulnerable population of disabled individuals (37). Likewise, the impact of positive self-rated health is widely known although correcting for frailty status seems to allow additional insight—that is, self-rated health is more explanatory in relatively fit than in relatively frail people (38). Even so, commenting on specific factors that might be associated with better conditions is problematic due to the effect of multiple comparisons and confounding. Such observations must be seen as hypothesis generating only.
Our data suggest that in general, women tolerate more health problems than men do and some of the items showed an apparent protective effect only in women. The advantage in health expectancy in women was clearer in those aged 65 years or older, even though women enjoyed a lower mortality rate than men at all ages (Figure 2). This reflected in the different FI limit values between men and women. An empirical 99% FI limit of approximately 0.70 was seen in women, as reported in previous analyses that do not separate limit by sex (18,19). In fact, as shown here, the empirical 99% FI limit of men was notably lower than 0.7. In consequence, at the 99% limit, women tended to be frailer only at more advanced ages, while more men with a similar level of frailty (eg, FI
 0.45) would have already died. The argument seems to be supported by the data showing the near-zero slope of deficit accumulation at the submaximal limit in relation to age in the older men, indicating an earlier exhaustion of redundancy in men than in women in this Chinese sample. At the 99% frailty limit, little difference in mortality rates were found between men and women at this limit while the maximum (100%) death rate was approached. This result is consistent with the theory of reliability, stating that at the frailty limit, a system can age no more and little chance of survival can be expected (20). Here, when approaching redundancy exhaustion, men were more susceptible to death than women, with highest mortality reached independent of age. At the point when the redundancy exhaustion was reached in women, neither differential gender nor age effect on mortality was observed, as nearly all at the frailty limit were subjected to death.
0.45) would have already died. The argument seems to be supported by the data showing the near-zero slope of deficit accumulation at the submaximal limit in relation to age in the older men, indicating an earlier exhaustion of redundancy in men than in women in this Chinese sample. At the 99% frailty limit, little difference in mortality rates were found between men and women at this limit while the maximum (100%) death rate was approached. This result is consistent with the theory of reliability, stating that at the frailty limit, a system can age no more and little chance of survival can be expected (20). Here, when approaching redundancy exhaustion, men were more susceptible to death than women, with highest mortality reached independent of age. At the point when the redundancy exhaustion was reached in women, neither differential gender nor age effect on mortality was observed, as nearly all at the frailty limit were subjected to death.
Taken together, the question as to why frailer women live longer than men even in the poorest countries in the world (25) can be partially revealed by our data. Even though women at any given age tended to have more deficits than men, their level of tolerance of these deficits, as measured by the limit to the FI, was greater than men. In consequence, the average death rate of women was significantly lower than that of men at any given age. The maximum death rate (100%) was seen in men with much lower FI values than those in women of the same age. All these results suggested that women have a higher capability of “compensation for mortality” than men, providing a likely explanation for the worldwide observation that frailer women outlive healthier men (31).
Our data must be interpreted with caution. All the variables used to construct the FI were based on self-reported data, the accuracy of which might not be the same as a clinical assessment. Interindividual variability in respect to health care access may be reflected in population survey data, which may be especially true in China where significant regional socioeconomic and health care differences have been reported (39). Future work on studying frailty limits considering gender disparity in clinical settings will be of particular interest. Also, our data set was based on the health assessments in 1992, which likely bears variations to the current status in China. Unlike the West, fast economic growth in China has brought dramatic changes in education, income, lifestyle, culture, and health care in the past two decades, which likely has influenced the level of health measures and thus the FI (40). Recent interventions, such as protein-energy supplementation to prevent functional decline in frail older adults, would not be reflected in these data (41). Despite high demand for health care improvement, the system may have been weakened recently (42). Even so, consistent results regarding the FI values, relationships of FI with age, and gender disparity in the mean FI values were suggested by our study. These results are consistent with previous publications (11,15–19) and in support of the findings on frailty limit (14,19,26,43).
In conclusion, this study suggests a gender difference in frailty limits and its relationship with short-term mortality in older adults. The highest FI values were significantly lower and emerged earlier in men than in women; in both, the highest mortality resulted. These results have important implications for understanding health expectancy and risk of death in the older population, as well as the evolution of the gender differences in frailty, which might start in midlife (44). Quantification of frailty limits by taking into account gender differences can benefit public health planning and clinical decision making, for example, implementing frailty into clinical practice to achieve modifiable intervention outcomes (45), for better care of older patients.
Funding
The research was supported by a Canadian Institute of Health Research grant under the China-Canada Joint Health Research Initiative Program (CCI-92216). Z.Y. received faculty academic development funding support from Crandall University. K.R. accepted support from Dalhousie University Medical Health Foundation as the Kathryn Walden Alzheimer’s Research Chair.
Acknowledgments
We thank Miss Sarah Kehoe for proofreading the manuscript.
References
- 1. Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ. 1994;150:489–495 [PMC free article] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 3. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266 [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 5. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53:S9–16 [DOI] [PubMed] [Google Scholar]
- 7. Armstrong JJ, Stolee P, Hirdes JP, Poss JW. Examining three frailty conceptualizations in their ability to predict negative outcomes for home-care clients. Age Ageing. 2010;39:755–758 [DOI] [PubMed] [Google Scholar]
- 8. Dupre ME, Gu D, Warner DF, Yi Z. Frailty and type of death among older adults in China: prospective cohort study. BMJ. 2009;338:b1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci. 2005;60:1046–1051 [DOI] [PubMed] [Google Scholar]
- 10. Kulminski AM, Ukraintseva SV, Akushevich IV, Arbeev KG, Yashin AI. Cumulative index of health deficiencies as a characteristic of long life. J Am Geriatr Soc. 2007;55:935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189 [DOI] [PubMed] [Google Scholar]
- 12. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727 [DOI] [PubMed] [Google Scholar]
- 13. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743 [DOI] [PubMed] [Google Scholar]
- 14. Shi J, Song X, Yu P, et al. Analysis of frailty and survival from late middle age in the Beijing Longitudinal Study of Aging. BMC Geriatr. 2011;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitnitski A, Song X, Rockwood K. Improvement and decline in health status from late middle age: modeling age-related changes in deficit accumulation. Exp Gerontol. 2007;42:1109–1115 [DOI] [PubMed] [Google Scholar]
- 17. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687 [DOI] [PubMed] [Google Scholar]
- 18. Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127:494–496 [DOI] [PubMed] [Google Scholar]
- 19. Bennett S, Song X, Mitnitski A, Rockwood K. A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing. 2013;42:372–377 [DOI] [PubMed] [Google Scholar]
- 20. Gavrilov LA, Gavrilova NS. The reliability theory of aging and longevity. J Theor Biol. 2001;213:527–545 [DOI] [PubMed] [Google Scholar]
- 21. Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010;58:318–323 [DOI] [PubMed] [Google Scholar]
- 22. Strehler BL, Mildvan AS. General theory of mortality and aging. Science. 1960;132:14–21 [DOI] [PubMed] [Google Scholar]
- 23. Arber S, Cooper H. Gender differences in health in later life: the new paradox? Soc Sci Med. 1999;48:61–76 [DOI] [PubMed] [Google Scholar]
- 24. Jeune B, Skytthe A, Cournil A, et al. Handgrip strength among nonagenarians and centenarians in three European regions. J Gerontol A Biol Sci Med Sci. 2006;61:707–712 [DOI] [PubMed] [Google Scholar]
- 25. Barford A, Dorling D, Davey Smith G, Shaw M. Life expectancy: women now on top everywhere. BMJ. 2006;332:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu P, Song X, Shi J, et al. Frailty and survival of older Chinese adults in urban and rural areas: results from the Beijing Longitudinal Study of Aging. Arch Gerontol Geriatr. 2012;54:3–8 [DOI] [PubMed] [Google Scholar]
- 27. Jiang J, Tang Z, Meng XJ, Futatsuka M. Demographic determinants for change in activities of daily living: a cohort study of the elderly people in Beijing. J Epidemiol. 2002;12:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang Z, Wang HX, Meng C, et al. The prevalence of functional disability in activities of daily living and instrumental activities of daily living among elderly Beijing Chinese. Arch Gerontol Geriatr. 1999;29:115–125 [DOI] [PubMed] [Google Scholar]
- 29. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelsey JL, Whittemore AS, Evans AS, Thompson DW. Methods in observational epidemiology. 2nd ed. Oxford: Oxford University Press; 1996 [Google Scholar]
- 31. Hubbard RE, Rockwood K. Frailty in older women. Maturitas. 2011;69:203–207 [DOI] [PubMed] [Google Scholar]
- 32. Gilgun-Sherki Y, Melamed E, Offen D. Anti-inflammatory drugs in the treatment of neurodegenerative diseases: current state. Curr Pharm Des. 2006;12:3509–3519 [DOI] [PubMed] [Google Scholar]
- 33. Kelkar A, Kuo A, Frishman WH. Allopurinol as a cardiovascular drug. Cardiol Rev. 2011;19:265–271 [DOI] [PubMed] [Google Scholar]
- 34. Beveridge LA, Ramage L, McMurdo ME, George J, Witham MD. Allopurinol use is associated with greater functional gains in older rehabilitation patients. Age Ageing. 2013;42:400–404 [DOI] [PubMed] [Google Scholar]
- 35. Breitner JC, Gau BA, Welsh KA, et al. Inverse association of anti-inflammatory treatments and Alzheimer’s disease: initial results of a co-twin control study. Neurology. 1994;44:227–232 [DOI] [PubMed] [Google Scholar]
- 36. McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432 [DOI] [PubMed] [Google Scholar]
- 37. Kulminski AM, Kulminskaya IV, Ukraintseva SV, Land K, Yashin AI. An inverse association between self-reported arthritis and mortality in the elderly: findings from the national long-term care survey. Rejuvenation Res. 2008;11:251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lucicesare A, Hubbard RE, Searle SD, Rockwood K. An index of self-rated health deficits in relation to frailty and adverse outcomes in older adults. Aging Clin Exp Res. 2010;22:255–260 [DOI] [PubMed] [Google Scholar]
- 39. Gu D, Dupre ME, Sautter J, Zhu H, Liu Y, Yi Z. Frailty and mortality among Chinese at advanced ages. J Gerontol B Psychol Sci Soc Sci. 2009;64:279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morley JE, Perry HM, 3rd, Miller DK. Editorial: Something about frailty. J Gerontol A Biol Sci Med Sci. 2002;57:M698–M704 [DOI] [PubMed] [Google Scholar]
- 41. Kim CO, Lee KR. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: a community-based randomized controlled study. J Gerontol A Biol Sci Med Sci. 2013;68:309–316 [DOI] [PubMed] [Google Scholar]
- 42. Yip W, Hsiao WC. The Chinese health system at a crossroads. Health Aff (Millwood). 2008;27:460–468 [DOI] [PubMed] [Google Scholar]
- 43. Wang C, Song X, Mitnitski A, et al. Gender differences in the relationship between smoking and frailty: results from the Beijing Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:338–346 [DOI] [PubMed] [Google Scholar]
- 44. Stenholm S, Strandberg TE, Pitkälä K, Sainio P, Heliövaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-Year follow-up in men and women: The Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci. 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45. Sourial N, Bergman H, Karunananthan S, et al. Implementing frailty into clinical practice: A cautionary tale. J Gerontol A Biol Sci Med Sci. 2013. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]