Abstract
Background.
Diagnostic criteria for sarcopenia from appendicular lean mass (ALM), strength, and performance have been proposed, but little is known regarding the progression of sarcopenia.
We examined the time course of sarcopenia and determinants of transitioning toward and away from sarcopenia.
Methods.
ALM, gait speed, and grip strength were assessed seven times over 9 years in 2,928 initially well-functioning adults aged 70–79. Low ALM was defined as less than 7.95 kg/m2 (men) or less than 6.24 kg/m2 (women), low performance as gait speed less than 1.0 m/s, low strength as grip strength less than 30 kg (men) or less than 20 kg (women). Presarcopenia was defined as low ALM and sarcopenia as low ALM with low performance or low strength. Hidden Markov modeling was used to characterize states of ALM, strength, and performance and model transitions leading to sarcopenia and death. Determinants of transitioning toward and away from sarcopenia were examined with logistic regression.
Results.
Initially, 54% of participants had normal ALM, strength, and performance; 21% had presarcopenia; 5% had sarcopenia; and 20% had intermediate characteristics. Of participants with normal ALM, strength, and performance, 1% transitioned to presarcopenia and none transitioned to sarcopenia. The greatest transition to sarcopenia (7%) was in presarcopenic individuals. Low-functioning and sarcopenia states were more likely to lead to death (12% and 13%). Higher body mass index (p < .001) and pain (p = .05) predicted transition toward sarcopenia, whereas moderate activity predicted transition from presarcopenia to more normal states (p = .02).
Conclusions.
Pain, physical activity, and body mass index, potentially modifiable factors, are determinants of transitions. Promotion of health approaching old age is important as few individuals transition away from their initial state.
Key Words: Muscle, Aging, Physical function, Sarcopenia, Epidemiology.
Progressive loss of skeletal muscle is a hallmark of advancing age. Age-related loss of muscle has multiple contributing factors including bed rest, inactivity, chronic disease, pain, and some drugs (1–3). Low muscle with muscle weakness increases the risk of falls, fractures, loss of function, and disability (4). Therefore, identification of individuals with low muscle mass and/or strength is important in geriatric care.
The term sarcopenia has been defined as age-related muscle loss with low muscle strength and/or low muscle performance (5,6). To facilitate the identification of individuals with sarcopenia, several definitions and diagnostic criteria have been proposed (7–9). These definitions recognize that sarcopenia is multidimensional and occurs across a continuum, varying in severity and stage: (a) presarcopenia: low muscle mass; (b) sarcopenia: low muscle mass with low muscle strength or low performance; and (c) severe sarcopenia: low muscle mass, low muscle strength, and low performance (8). Comparatively, little is known about the progression of sarcopenia and transitions in and out of sarcopenia. Therefore, the purpose of this study was to examine the time course of sarcopenia and to explore potential determinants of transition between stages of sarcopenia using serial measures of appendicular lean mass (ALM), grip strength, and gait speed over 9 years from the Health, Aging, and Body Composition Study.
Methods
Study Population
The Health, Aging, and Body Composition Study is a prospective population-based study of 3,075, black and white men and women initially aged 70–79 in the Memphis, Tennessee, and Pittsburgh, Pennsylvania areas. All participants reported no difficulty walking one-quarter mile or climbing 10 steps without resting. Additional study details have been published (4). All participants signed informed consent forms approved by institutional review boards of the clinic sites.
Body Composition
The Supplementary Material provides details of computed tomography image analysis. Briefly, total body dual energy x-ray absorptiometry was performed (Hologic QDR4500A, Waltham, NY) annually from baseline (year 1) through year 10 with the exception of years 7 and 9 when there was no clinic visit. ALM the sum of bone-free lean tissue in the arms and legs was standardized for height (m2). Low ALM was defined as the 20th percentile of ALM: less than 7.95 kg/m2 for men and less than 6.24 kg/m2 for women (10).
Performance
Gait speed was measured annually from baseline through year 10 with the exception of years 7 and 9. Usual gait speed was determined from assessments 6 m (years 1, 4, 6, and 10), 20 m (years 3 and 5) or 2 minutes (years 2 and 8) (6). Gait speed less than 1.0 m/s was used to identify low physical performance (7,8).
Muscle Strength
Grip strength was measured using an isometric dynamometer (Jaymar, Boling-brook, IL). Grip strength was assessed as the maximum force of two trials with each hand at baseline, years 2, 4, 6, 8, and 10 and in subsets of participants at years 3 and 5. Low muscle strength was defined as grip strength less than 30 kg for men and less than 20 kg for women, which are values that discriminate early stages of reduced physical function in older adults (5).
Sarcopenia-Related Variables
Previously identified risk factors for sarcopenia (11–15) were examined to assess factors related to transitions toward and away from sarcopenia. Variables were all assessed at year 1. Body mass index (BMI) was assessed as a continuous variable as categories for underweight, normal weight, overweight, and obesity in old age are controversial (16). Physical activity was assessed as kcal/wk spent walking or exercising in the prior week (17). Self-reported health was categorized as excellent, very good to good, and fair to poor. Pain and knee pain was self-reported in the previous 30 days. Knee pain was missing in one participant. Smoking was categorized as never, former, or current. Diabetes was determined from self-report and medications; impaired fasting glucose was defined as more than or equal to 6.1 mmol/L. Details of mediator analyses are provided in Supplementary Material. Serum was collected following an overnight fast. Insulin was not measured in participants with known diabetes (N = 305). Free testosterone, interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were measured by ELISA kits. Participants with missing data were excluded only from analysis of transition predictors.
Mortality was determined from death certificates, hospital records, and interview with next of kin through August 10, 2011 representing 14 years of follow-up.
Statistical Analyses
ALM, muscle performance, and strength over 9 years were analyzed with discrete hidden Markov modeling (HMM) (18,19). HMM uses information from each participant’s history to conceptualize the course of sarcopenia as a sequence of observable indicators of sarcopenia driven by an underlying sequence of latent states. The optimal model is determined from Bayesian goodness-of-fit criterion (20) (Figure 1). The HMM produces (a) states characterized by scoring the three sarcopenia criteria at each point in time, (b) estimates for the prevalence of each state at any given time point, and (c) estimates for transition probabilities between states. Death was included as a final absorbing state (N = 840 died during follow-up).
Figure 1.
Bayesian information criteria results for Hidden Markov models with between three and eight states. Lower Bayesian information criteria values indicate better fitting models, in this case the eight latent state model.
Determinants of transitioning away from normal ALM, strength, and performance (N = 2,355) toward sarcopenia were assessed with logistic regression. Since sarcopenia was the end point, the death state was excluded, but deceased individuals remained in the analysis. Logistic regression was also used to assess determinants of remaining presarcopenic or transitioning toward sarcopenia versus transitioning to more normal states in initially presarcopenic individuals (N = 536). Statistical analyses were performed with SAS 9.2 PROC GENMOD and specialized programs (18,21) written in MATLAB (Mathworks Inc., Natick, MA).
Results
Individuals without follow-up data were excluded (N = 147) and were more likely to be black, current smokers, report low physical activity, have poor health, lower grip strength, and lower gait speed (p < .05 for all). Baseline characteristics of the 2,928 included participants are shown in Table 1. On average, men and women were overweight and more than 50% had diabetes or impaired fasting glucose. Most participants reported low levels of activity, a history of pain, and very good or good health.
Table 1.
Characteristics of Participants in the Health, Aging, and Body Composition Study at Year 1
| Men | Women | |
|---|---|---|
| N (%) | 1426 (48.7) | 1502 (51.3) |
| Black race, n (%) | 514 (36.1) | 679 (45.2) |
| Age, y; mean (SD) | 73.8±2.85 | 73.5±2.88 |
| Smoking, n (%) | ||
| Never | 425 (29.9) | 865 (57.7) |
| Current | 151 (10.6) | 142 (9.47) |
| Former | 848 (59.6) | 493 (32.9) |
| Body mass index (kg/m2), mean (SD) | 27.0±3.90 | 27.7±5.50 |
| Appendicular lean mass (kg/m2), mean (SD) | 7.95±1.01 | 6.52±1.14 |
| Gait speed, mean (SD) | 1.24±0.24 | 1.12±0.22 |
| Grip strength, mean (SD) | 40.9±8.53 | 25.1±6.37 |
| Diabetes or impaired fasting glucose, n (%) | 721 (50.6) | 798 (53.1) |
| Insulin in IU/mL, mean (SD) | 8.42±7.42 | 8.52±5.95 |
| Free testosterone in pg/mL mean (SD) | 8.43±3.79 | 3.33±2.10 |
| Physical activity, n (%) | ||
| <500 kcal/wk | 595 (41.7) | 911 (60.7) |
| 500–1,499 kcal/wk | 409 (28.7) | 401 (26.7) |
| >1,500 kcal/wk | 422 (29.6) | 190 (12.7) |
| Self-reported health, n (%) | ||
| Excellent | 210 (14.7) | 194 (12.9) |
| Very good–good | 994 (69.8) | 1074 (71.6) |
| Fair–poor | 221 (15.5) | 232 (15.5) |
| Pain in last 30 days, n (%) | 857 (60.1) | 1064 (70.8) |
| Knee pain in last 30 days, n (%) | 333 (23.4) | 415 (27.6) |
Notes: IU = international units; SD = standard deviation. Numbers may not sum to total N due to missing data as outlined in the Methods.
HMM resulted in eight state profiles of ALM, strength, and performance (Figure 2). Within a state, longer bars represent a higher probability of having normal values. States are approximately ordered in descending order. State 1 represents the most normal state: nearly all participants have ALM above the 20th percentile, grip strength more than 20 kg (women) or more than 30 kg (men), and gait speed more than 1 m/s. States 2 and 3 represent intermediate subclinical states, and state 4 is characterized by low function (low strength and performance). State 5 approximates presarcopenia, states 6 and 7 approximate sarcopenia, and state 8 is death. Participants in this population were selected to be initially well functioning. As a result, participants meeting criteria for severe sarcopenia averaged to 1.5% across all time points and was too low to constitute a state.
Figure 2.
Model of eight states of appendicular lean mass (ALM), muscle performance and strength. Bars represent the probability of observing a participant having gait speed, ALM or grip strength above their respective cut-points thus longer bars indicate higher probability of having a normal measure (ie, in state 1, the most normal state nearly all participants have gait speed >1 m/s, and all participants have ALM and grip strength above cut-points). States 2 and 3 are intermediate states, state 4 is a state characterized by low function, state 5 represents presarcopenia, states 6 and 7 represent sarcopenia, and state 8 is death. Participants can be classified as belonging to any of these eight states and may transition to any of these states during the 9-year follow-up period. ALM = appendicular lean mass.
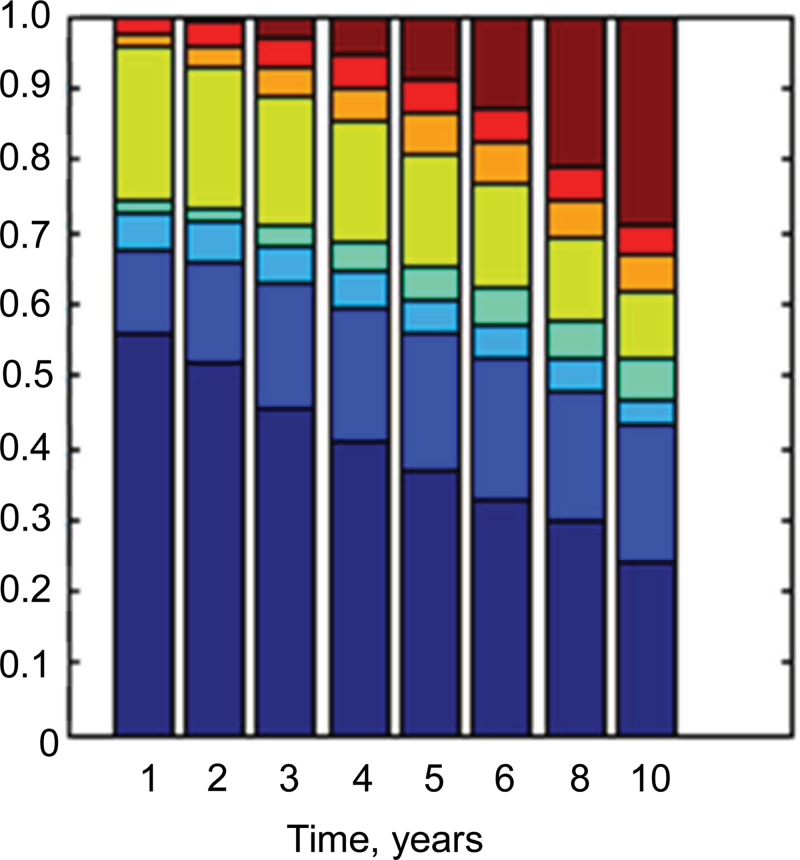
The prevalence of states at each time over the 9-year period is shown in Figure 3. The most normal state is at the bottom, and death is at the top. Over time, the prevalence of the normal state declines from 54% to less than 30%. The prevalence of the presarcopenia state declines from 21% to less than 10%. Conversely, the prevalence of sarcopenia states increases, as does the prevalence of the low function state and death.
Figure 3.
State prevalence across the 9-year study period. States of appendicular lean mass, gait speed, and grip strength are ordered from the most normal: state 1 (bottom in dark blue) to least: state 8 (top in brown). Each color represents a state.
Initial state and transition probabilities are shown in Table 2. Overall, individuals tended to remain in their current state. In the normal state, 88% of individuals remained there, 1% transitioned to presarcopenia, and 3% died. In intermediate state 2, 88% remained in that state, 3% transitioned to sarcopenia, and 6% died. In intermediate state 3, 83% of individuals remained in that state, 3% transitioned to sarcopenia, and 4% died. In the low-functioning state, 86% remained there, 2% transitioned to more normal states, and 13% died. For individuals with presarcopenia, 7% transitioned to sarcopenia, 4% transitioned to more normal states, and 4% died. Only 1% (state 6) to 2% (state 7) of sarcopenic individuals transitioned to more normal states, 12% (state 6) and 8% (state 7) died.
Table 2.
Initial (year 1) and the Probability of Transition Between States of Appendicular Lean Mass, Strength and Physical Performance Over 9 Years in the Health, Aging, and Body Composition Study
| Initial % | State 1 | State 2 | State 3 | State 4 | State 5 | State 6 | State 7 | State 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 54 | 12 | 6 | 1 | 21 | 2 | 3 | 0 | ||
| Probability of transition % | State 1 | 88 | 6 | 1 | 0 | 1 | 0 | 0 | 3 |
| State 2 | 0 | 88 | 0 | 3 | 0 | 3 | 0 | 6 | |
| State 3 | 1 | 0 | 83 | 9 | 0 | 0 | 3 | 4 | |
| State 4 | 0 | 2 | 0 | 86 | 0 | 0 | 0 | 13 | |
| State 5 | 3 | 1 | 0 | 0 | 85 | 4 | 3 | 4 | |
| State 6 | 0 | 1 | 0 | 0 | 0 | 86 | 1 | 12 | |
| State 7 | 0 | 0 | 2 | 0 | 0 | 1 | 88 | 8 | |
| State 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
Notes: State1 represents the most normal state where almost all participants have appendicular lean mass, strength and performance above cutoffs for sarcopenia. States 2 and 3 represent intermediate subclinical states, state 4 is low functioning (low strength and performance), state 5 approximates presarcopenia, states 6 and 7 approximate sarcopenia, and state 8 is death. Initial is the prevalence of each state at year 1, ie. 54% of participants are initially in state 1. The transition probability indicates the probability of transition from a row state to a column state, ie, participants who are initially in state 1 have 88% probability of staying in state 1 (row 1 column 1), 6% probability of transition to state 2 (row 1, column 2), a 3% probability of transition to state 3 (row 1, column 3), etc. Rows may not sum to 100 due to rounding.
Table 3 shows the odds ratios (OR) and confidence intervals (CI) of transitioning from the normal state toward sarcopenia (state 6 or 7) and transitioning out of presarcopenia to more normal states. Increasing age (OR 1.12, 95% CI [0.80–1.18], p < .001), a history of pain (OR 1.18, 95% CI [1.01–1.39], p = .05), and higher BMI (OR 1.30, 95% CI [1.25–1.36], p < .001) were predictive of transitioning toward sarcopenia. Individuals with more physical activity tended to be more likely to remain in the normal state (OR 0.66, 95% CI [0.60–0.97], p = .09). Fair or poor health (OR 1.39, 95% CI [0.99–1.95], p = .06) also tended to predict transition toward sarcopenia. For presarcopenic individuals, moderate physical activity between 500 and 1,499 kcal/wk (OR 2.51, 95% CI [1.27–4.96], p = .02) and lower IL-6 (OR 0.71, 95% CI [0.52–0.97], p = .03) were associated with greater odds of transitioning out of presarcopenia.
Table 3.
Determinants of Transitions Between States of Appendicular Lean Mass, Strength and Performance Over a 9-Year Period in the Health, Aging, and Body Composition Study
| Odds Ratio (95% confidence interval) | p-Value | |
|---|---|---|
| From normal state towards sarcopenia, N = 2,355 | ||
| Sex | 0.83 (0.65–1.06) | .14 |
| Race | 0.97 (0.80–1.18) | .75 |
| Age, y | 1.12 (1.08–1.15) | <.001 |
| Current smoker* | 1.07 (0.78–1.48) | .55 |
| Former smoker | 0.96 (0.79–1.15) | .45 |
| Body mass index, kg/m2 | 1.30 (1.25–1.36) | <.001 |
| Physical activity† | ||
| 500–1,499 kcal/wk | 0.87 (0.70–1.06) | .91 |
| >1,500 kcal/wk | 0.77 (0.60–0.97) | .09 |
| Self-reported health‡ | ||
| Very good-good | 1.13 (0.88–1.46) | .68 |
| Fair-poor | 1.39 (0.99–1.95) | .06 |
| Pain in last 30 days§ | 1.18 (1.01–1.39) | .05 |
| Knee pain in last 30 days§ | 1.11 (0.94–1.31) | .23 |
| Diabetes | 1.12 (0.94–1.33) | .22 |
| Insulin, IU/mL | 1.00 (0.98–1.01) | .57 |
| Free testosterone, pg/mL | 0.98 (0.95–1.01) | .18 |
| IL-6, pg/mL | 1.01 (0.96–1.05) | .82 |
| TNF-α, pg/mL | 0.96 (0.91–1.02) | .16 |
| From presarcopenia to more normal states, N = 536 | ||
| Sex | 0.50 (0.19–1.32) | .16 |
| Race | 0.78 (0.28–2.16) | .63 |
| Age, y | 0.91 (0.82–1.02) | .10 |
| Current smoker* | 0.50 (0.10–2.40) | .32 |
| Former smoker | 1.12 (0.61–2.26) | .27 |
| Body mass index, kg/m2 | 2.57 (0.49–13.5) | .29 |
| Physical activity† | ||
| 500–1,499 kcal/wk | 2.51 (1.27–4.96) | .02 |
| >1,500 kcal/wk | 1.53 (0.62–3.79) | .93 |
| Self-reported health‡ | ||
| Very good–good | 0.83 (0.38–1.84) | .45 |
| Fair–poor | 1.17 (0.35–1.84) | .64 |
| Pain in last 30 days§ | 1.39 (0.84–2.31) | .20 |
| Knee pain in last 30 days§ | 1.31 (0.82–2.10) | .26 |
| Diabetes | 1.09 (0.60–2.00) | .78 |
| Insulin, IU/mL | 1.04 (0.96–1.13) | .31 |
| Free testosterone, pg/mL | 0.97 (0.85–1.12) | .70 |
| IL-6, pg/mL | 0.71 (0.52–0.97) | .03 |
| TNF-α, pg/mL | 1.04 (0.86–1.25) | .71 |
Notes: IU = international units; IL-6 = interleukin 6; TNF-α = tumor necrosis factor-alpha. p-value from chi-square.
*Reference category never smoker.
†Reference category <500 kcal/wk.
‡Reference category excellent health.
§Reference category no pain.
Discussion
To our knowledge, this is the first study to examine the natural time course of transitions between sarcopenia stages. Our results show that few participants who entered old age in the normal state of ALM, strength, and performance developed presarcopenia, and none became sarcopenic despite an age range of 79–88 at the end of the study. Individuals who transitioned to sarcopenia did so via declines in strength and performance, emphasizing the multidimensional nature of sarcopenia. Older age, higher BMI, and pain were predictors of transition from the normal state toward sarcopenia. Conversely, moderate physical activity and lower IL-6 were predictive of transitions from presarcopenia to more normal states. These results provide a first step toward characterizing the natural time course of sarcopenia and identifying individuals who may be at risk of developing sarcopenia.
There were several factors associated with transition toward sarcopenia and out of presarcopenia to more normal states. Older participants had increased odds of transition to sarcopenia, which was expected since the prevalence of sarcopenia increases with age (22). The finding of higher BMI as a predictor of transition to sarcopenia is consistent with previous reports showing a relationship between high BMI and risk of functional impairment (23,24). These results also suggest physical activity may be protective of transition toward sarcopenia and transitioning out of presarcopenia to more normal states. Pain was also a significant predictor of transition toward sarcopenia, which may reflect avoidance of physical activity due to pain-related fear (25). Alternatively, pain may indicate inflammation that contributes to muscle loss (26) although TNF-α and IL-6 were not associated with transitions toward sarcopenia. Sex was not related to transitions possibly due to the sex-specific criteria used for ALM and strength. Race also did not predict transition to sarcopenia despite studies that show that body composition (27,28) and changes in body composition vary by race (29,30). However, it is possible that results would differ with inclusion of additional racial backgrounds.
Little is known regarding the time course of sarcopenia, but a study of bone loss that often accompanies muscle loss (29,31) mirrors our results. Gourlay et al. (32) reported a relationship between transition time to osteoporosis and initial bone mineral density such that less than 10% of women with normal bone density or mild osteopenia transitioned to osteoporosis over 15 years, whereas women with moderate or advanced osteopenia transitioned to osteoporosis in 5 years and 1 year, respectively. Although translation of these results to clinical guidelines was controversial (33,34), it supports the notion observed here that few individuals with greater initial reserve transition to worse states.
In contrast to bone mineral density, there are no guidelines for sarcopenia screening. This is due in part to inconsistent diagnostic criteria, need for imaging to quantify ALM, and the absence of effective treatments. Nevertheless, health risks attributable to sarcopenia are far reaching, including morbidity (35), disability (36), high health care costs (37), and, as observed here, mortality. Thus, identifying individuals with sarcopenia is important for clinical practice and for advancing treatment development. Our results suggest that if screening guidelines are developed, there is a need for multidimensional assessment of sarcopenia as states with low ALM in combination with low performance and/or strength were more likely to lead to death than low ALM alone. This is supported by studies that report inconsistent relationships between ALM and disability in old age (36,38,39). Additionally, our results suggest the need for early identification as once individuals are sarcopenic, they are unlikely to transition out. Inclusion of subclinical states of sarcopenia characterized here may assist in early identification. Age, self-reported physical activity, BMI, and pain history may also be useful indicators of individuals at risk of transitioning to sarcopenia.
A strength of this study was the use of HMM that is robust to missing data and resulted in the identification of intermediate subclinical states of sarcopenia that are not outlined in current sarcopenia definitions. A further strength was the rich data set of serial grip strength, gait speed, and dual energy x-ray absorptiometry measurements over 9 years and population of individuals without mobility disability at baseline, a mix of sex and two races. Those without mobility disability represent approximately 70% of the population aged 70–79 (NHANES 2009–2010). However, since our population was restricted to initially well-functioning individuals, states and transition probabilities may differ for less healthy populations or other ages. Our analysis utilized dual energy x-ray absorptiometry measurements of ALM rather than direct assessment of skeletal muscle as computed tomography was performed at only three time points in our population. Data on muscle fat infiltration, which reflects muscle quality, may also provide important information on transitions toward low muscle function beyond the functional measures assessed here.
Conclusions
Our results may have important clinical implications for the identification of sarcopenia. First, it suggests that sarcopenia is not simply the presence of low ALM, strength, and performance; there are several intermediate subclinical states that may be important in the progression to sarcopenia. Second, it identifies pain, physical activity, and BMI, three potentially modifiable factors, as determinants of transitions. Finally, since none of the individuals with normal ALM, strength, and performance transitioned to sarcopenia, this may indicate the importance of health promotion earlier in life such that individuals enter old age with a healthy reserve of ALM, muscle strength, and performance.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research was supported by National Institute on Aging (Contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106); National Institute on Aging (grant R01-AG028050); National Institute of Nursing Research (grants R01-NR-012459 and R01-AG031827A). This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. R.A.M. is supported by a Banting Postdoctoral Fellowship.
References
- 1. Thompson DD. Aging and sarcopenia. J Musculoskelet Neuronal Interact. 2007;7:344–345 [PubMed] [Google Scholar]
- 2. Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87:1562S–1566S [DOI] [PubMed] [Google Scholar]
- 4. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333 [DOI] [PubMed] [Google Scholar]
- 5. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860 [DOI] [PubMed] [Google Scholar]
- 6. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680 [DOI] [PubMed] [Google Scholar]
- 7. Morley JE, Abbatecola AM, Argiles JM, et al. Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newman AB, Kupelian V, Visser M, et al. Health ABC Study Investigators. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609 [DOI] [PubMed] [Google Scholar]
- 11. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–1774 [DOI] [PubMed] [Google Scholar]
- 12. Morley JE. Hormones and the aging process. J Am Geriatr Soc. 2003;51(suppl 7):S333–S337 [DOI] [PubMed] [Google Scholar]
- 13. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248 [DOI] [PubMed] [Google Scholar]
- 14. Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity, and inflammation–results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–434 [DOI] [PubMed] [Google Scholar]
- 15. Evans WJ, Farrell PA, Jefferson JS, Cherrington AD, eds. The aging pancreas: the effects of aging on insulin secretion and action. The Handbook of Physiology. Oxford, UK: Oxford University Press; 2001: 969–998 [Google Scholar]
- 16. Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond). 2005;29:1011–1029 [DOI] [PubMed] [Google Scholar]
- 17. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; Health, Aging and Body Composition Study Research Group. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509 [DOI] [PubMed] [Google Scholar]
- 18. Ip EH, Jones AS, Heckert DA, Zhang Q, Gondolf ED. Latent Markov model for analyzing temporal configuration for violence profiles and trajectories in a sample of batterers. Socio Meth Res. 2010; 39:222–255 [Google Scholar]
- 19. MacDonald I, Zucchini W. Hidden Markov and Other Models for Discrete-valued Time Series. London: Chapman and Hall; 1997 [Google Scholar]
- 20. Schwartz G. Estimating the dimensions of a model. Ann Stat. 1978; 6:461–464 [Google Scholar]
- 21. Zhang QA, Jones AS, Rijmen F, Ip EH. Multivariate discrete hidden Markov models for domain-based measurements and assessment of risk factors in child development. J Comput Graph Stat. 2010; 19:746–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763 [DOI] [PubMed] [Google Scholar]
- 23. Marsh AP, Rejeski WJ, Espeland MA, et al. ; LIFE Study Investigators. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P). J Gerontol A Biol Sci Med Sci. 2011;66:1376–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up study of NHANES I. JAMA. 1994;271:1093–1098 [PubMed] [Google Scholar]
- 25. Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332 [DOI] [PubMed] [Google Scholar]
- 26. Roubenoff R, Roubenoff RA, Cannon JG, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387 [DOI] [PubMed] [Google Scholar]
- 28. Perry AC, Applegate EB, Jackson ML, et al. Racial differences in visceral adipose tissue but not anthropometric markers of health-related variables. J Appl Physiol. 2000;89:636–643 [DOI] [PubMed] [Google Scholar]
- 29. Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064 [DOI] [PubMed] [Google Scholar]
- 30. Aloia JF, Vaswani A, Mikhail M, Flaster ER. Body composition by dual-energy X-ray absorptiometry in black compared with white women. Osteoporos Int. 1999;10:114–119 [DOI] [PubMed] [Google Scholar]
- 31. Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94:2368–2374 [DOI] [PubMed] [Google Scholar]
- 32. Gourlay ML, Fine JP, Preisser JS, et al. ; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lewiecki EM, Miller PD, Bilezikian JP. Bone-density testing interval and transition to osteoporosis. N Engl J Med. 2012;366:1546–1547 [DOI] [PubMed] [Google Scholar]
- 34. Cheung AM, Papaioannou A; Osteoporosis Canada Scientific Advisory Council Guidelines Committee. Bone-density testing interval and transition to osteoporosis. N Engl J Med. 2012;366:1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28:2541–2542 [DOI] [PubMed] [Google Scholar]
- 36. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896 [DOI] [PubMed] [Google Scholar]
- 37. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85 [DOI] [PubMed] [Google Scholar]
- 38. Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221 [DOI] [PubMed] [Google Scholar]
- 39. Visser M, Langlois J, Guralnik JM, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–590 [DOI] [PubMed] [Google Scholar]