Abstract
We previously quantified frailty in aged mice with frailty index (FI) that used specialized equipment to measure health parameters. Here we developed a simplified, noninvasive method to quantify frailty through clinical assessment of C57BL/6J mice (5–28 months) and compared the relationship between FI scores and age in mice and humans. FIs calculated with the original performance-based eight-item FI increased from 0.06±0.01 at 5 months to 0.36±0.06 at 19 months and 0.38±0.04 at 28 months (n = 14). By contrast, the increase was graded with a 31-item clinical FI (0.02±0.005 at 5 months; 0.12±0.008 at 19 months; 0.33±0.02 at 28 months; n = 14). FI scores calculated from 70 self-report items from the first wave of the Survey of Health, Ageing and Retirement in Europe were plotted as function of age (n = 30,025 people). The exponential relationship between FI scores and age (normalized to 90% mortality) was similar in mice and humans for the clinical FI but not the eight-item FI. This noninvasive FI based on clinical measures can be used in longitudinal studies to quantify frailty in mice. Unlike the performance-based eight-item mouse FI, the clinical FI exhibits key features of the FI established for use in humans.
Key Words: Frailty index, Deficit index, Deficit accumulation, Senescence.
Experience tells us that people age at different rates. Because chronological age does not necessarily reflect biologic age, the health status of older adults varies from fit to frail (1,2). The concept of frailty, which is a state of increased vulnerability to adverse health outcomes for people of the same age, was developed to explain the heterogeneity in clinical outcomes for older patients (3). Frailty is a major challenge in health care as frail individuals have higher mortality and use more health care services than do fit people (4). Still, little is known about the biology of frailty, in part because until recently frailty had not been quantified in animal models of aging. Indeed, the need to develop measures of frailty in aging animal models has been identified as a key step to translate basic mechanisms of cellular dysfunction in aging into meaningful treatments (5).
How best to measure frailty in people is controversial (6). In fact, more than 20 different instruments have been used to measure frailty clinically (7). One approach to quantify frailty in older people is to construct a “frailty index,” in which an individual’s potential health deficits (eg, clinical signs, diseases, laboratory abnormalities) are counted and divided by the total number of items measured (1,8,9). Recently, we modified this approach to develop a new method to quantify frailty with a frailty index based on deficit accumulation in a mouse model of aging (10). We measured 31 health-related variables that provided information about activity levels, hemodynamic status, body composition, and metabolism. We found that aged mice (30 months) had substantially higher frailty index scores than younger (eg, 12-month-old) animals (10). We also found that deleterious changes in individual cardiac myocytes, including hypertrophy and contractile dysfunction, occurred only in cells from 30-month-old mice with the highest frailty index scores (10). These results demonstrate that a frailty index based on the concept of deficit accumulation can be used to quantify frailty in aging mice and suggest that a high frailty index predicts adverse outcomes in these animals. They also provide a link between frailty measured at the level of the whole organism and structural and functional changes at the level of the cell (2).
In our earlier study, the parameters used to construct the frailty index were measured with specialized equipment and required invasive techniques. For example, activity was measured with a video tracking system, hemodynamic parameters with a tail cuff, body composition with a dual energy x-ray absorptiometry scanner and metabolism with a blood sample in an i-STAT portable clinical blood analyzer (10). This requirement for specialized equipment limits the routine measurement of a frailty index in many laboratory settings. Furthermore, this frailty index cannot be used in longitudinal studies of frailty in mice due to the invasive nature of some of the techniques employed (eg, volume of blood required, repeated exposure to x-rays). We did report that a noninvasive performance-based eight-item frailty index based on fewer measures (eg, activity levels and weight) could be used to create a frailty index in mice although the magnitude and variance of the frailty index increased when fewer items were measured (10). This is not surprising. When frailty indices are constructed with fewer than 30 variables in humans, commonly a ceiling effect occurs where people can have all possible deficits and graded levels of frailty cannot be distinguished (11,12). The contrasting case also holds; if a frailty index is made up of only low-prevalence items, the mean value of the frailty index is low (13).
To facilitate the use of the frailty index to quantify frailty in acute and longitudinal studies of aging animals, there is a need to develop a procedure that is noninvasive, simple to implement, and based on a sufficient number of parameters (eg, >30) to provide a robust estimate of frailty. To advance the use of the frailty index in translational studies, it is also important to compare features of a frailty index developed for use in experimental animals with the frailty index in humans. The objectives of this study were (a) to develop a simple, noninvasive frailty index based on the clinical assessment of more than 30 potential deficits in aging mice and compare it with the basic eight-item frailty index based on activity levels and weight as previously characterized by our group and (b) to compare the relationship between the frailty index and age in mice and humans. The murine clinical frailty index was developed in consultation with a veterinarian, based on readily apparent signs of clinical deterioration in mice that have previously been described in the literature. These data were then compared with human frailty index scores calculated from the Survey of Health, Ageing and Retirement in Europe (SHARE).
Methods
Animals
Young female C57BL/6J mice were purchased from Charles River (St. Constant, Quebec) and aged in groups in microisolator cages in the Carlton Animal Care Facility (Dalhousie University). Mice were maintained on a 12-hour light/dark cycle, with free access to food and water. Three groups of mice were used: young adults (161 days old or ~5 months), older adults (566 days old or ~19 months), and aged mice (839 days old or ~28 months). In a few experiments, young adult and aged male C57BL/6J mice also were used. All experiments followed the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals (14). Animal protocols were approved by the Dalhousie University Committee on Laboratory Animals.
Quantification of Frailty With a Performance-Based Eight-Item Frailty Index
We quantified frailty in each mouse with a performance-based frailty index based on eight items that reflected overall activity levels plus weight as previously described by our group (10). Open-field assessments were performed between 10 am and noon each day. Two separate trials were conducted 10 days apart. Mice were weighed and activity was recorded with automated Ethovision video tracking software for 10 minutes in an open-field arena (43.5 × 21.5cm). Videos were later digitized with an analog-to-digital converter (DVDXpress DX2; ADS Tech Inc.) and analyzed with Ethovision analysis software to obtain values for the parameters used to create the eight-item frailty index. This software detects the center point of the animal and tracks its movements, activity, and behavior in the open-field arena over a specified time frame.
Videotapes of open-field behavior were analyzed for the following parameters: (a) total distance moved in 10 minutes (cm); (b) maximal distance moved between bouts of inactivity (cm); (c) total duration of movement (seconds); (d) percent of total time spent moving; (e) the change in direction per unit distance moved, called meander (degrees/cm; from 0° to 180°); (f) the average velocity of movement over 10 minutes (cm/s); and (g) rearing frequency (number of occurrences/10 min). The final item in the eight-item frailty index was weight. Mean ± SD values for each of these eight parameters were calculated for two separate trials, as shown in Supplementary Table 1. These data were homogenous (low variance) and were used as reference values to allow calculation of a unique eight-item frailty index score for each mouse. Individual values for each parameter measured in each mouse were compared with reference values for the appropriate trial (Supplementary Table 1) and a frailty index score was calculated as described previously (10). Briefly, values that differed from the reference values by less than 1 SD were given a score of 0. Values that were ±1 SD with respect to the mean reference value were given a frailty value of 0.25. Those that differed by ±2 SD were scored as 0.5, values that differed by ±3 SD were given a value of 0.75, and values that were more than 3 SD above or below the mean received the maximal frailty value of 1. These values were summed, and the total was divided by the number of parameters measured, in this case eight, to provide a frailty index score between 0 and 1 for each animal.
Quantification of Frailty With a 31-Item Clinical Frailty Index
A novel 31-item frailty index based on established clinical signs of deterioration in mice was developed in consultation with a veterinarian (B.A.H.). Clinical assessment included evaluation of the integument, the musculoskeletal system, the vestibulocochlear/auditory systems, the ocular and nasal systems, the digestive system, the urogenital system, the respiratory system, signs of discomfort, the body weight (g), and body surface temperature (°C). Table 1 lists clinical signs of deterioration/deficits evaluated in this study. To establish baseline clinical assessment techniques, we first observed and evaluated young adult mice that had few signs of clinical deterioration. With training, an experienced investigator could perform clinical frailty assessments in 3.8±0.1 minutes per animal (n = 15 young adult animals).
Table 1.
Clinical Signs of Deterioration in Aging C57BL/6J Mice
| System and Parameter | Potential Deficits | References |
|---|---|---|
| Integument | ||
| Alopecia | Hair loss due to age-related balding and/or barbering (fur trimming) | (15) |
| Loss of fur colour | Change in fur colour from black to grey or brown | (15) |
| Dermatitis | Inflammation, overgrooming, barbering or scratching causing skin erosion. Can result in open sores anywhere on the body | (17,18) |
| Loss of whiskers | Loss of vibrissae (whiskers) due to aging and/or whisker trimming | (16,19) |
| Coat condition | Ruffled fur and/or matted fur. Ungroomed appearance. Coat does not look smooth, sleek, and shiny | (17,20) |
| Physical/musculoskeletal | ||
| Tumors | Development of tumors or masses anywhere on the body | (17,18,21) |
| Distended abdomen | Enlarged abdomen. May be due to tumor growth, organ enlargement, or intraperitoneal fluid accumulation | (20) |
| Kyphosis | Exaggerated outward curvature of the lower cervical/thoracic vertebral column. Hunched back or posture | (15,17,20) |
| Tail stiffening | Tail appears stiff, even when animal is moving in the cage. Tail does not wrap freely when stroked | (15) |
| Gait disorders | Lack of coordination in movement including hopping, wobbling, or uncoordinated gait. Wide stance. Circling or weakness | (17) |
| Tremor | Involuntary shaking at rest or during movement | (17) |
| Forelimb grip strength | A decline in forelimb grip strength | (15) |
| Body condition score | Visual signs of muscle wasting or obesity based on the amount of flesh covering bony protuberances | (15,17,20) |
| Vestibulocochlear/auditory | ||
| Vestibular disturbance | Disruption in the ability to perceive motion and gravity. Reflected in problems with balance, orientation, and acceleration | (22,23) |
| Hearing loss | Failure to respond to sudden sound (eg, clicker) indicative of hearing loss or impairment | (18,23) |
| Ocular/nasal | ||
| Cataracts | Clouding of the lens of the eye. An opaque spot in the center of the eye | (18,24,25) |
| Corneal opacity | Development of white spots on the cornea. Cloudy cornea | (17,26) |
| Eye discharge/swelling | Eyes are swollen or bulging (exopthalmia). They may exhibit abnormal secretions and/or crusting | (16,17,20) |
| Microphthalmia | Eyes are small and/or sunken. May involve one or both eyes | (17,18) |
| Vision loss | Vision loss, indicated by failure to reach toward the ground when lowered by the tail | (18,27) |
| Menace reflex | Rapid eye blink and closure of the palpebral fissure in response to a nontactile visual threat to the eye. Measures the integrity of the entire visual pathway including cortical components | (28) |
| Nasal discharge | Signs of abnormal discharge from the nares | (20) |
| Digestive/urogenital | ||
| Malocclusions | Incisor teeth are uneven or overgrown. Top teeth grow back into the roof of the mouth or bottom teeth are long and easily seen | (17) |
| Rectal prolapse | Protrusion of the rectum just below the tail | (17,29) |
| Vaginal/uterine/penile prolapse | Vagina or uterus protrudes through the vagina and vulva. Penis cannot reenter the penile sheath. | (17,29–31) |
| Diarrhea | Feces on the walls of the home cage. Bedding adheres to feces in cage. Feces, blood, or bedding around the rectum | (17) |
| Respiratory | ||
| Breathing rate/depth | Difficulty breathing (dyspnea), pulmonary congestion (rales), and/or rapid breathing (tachypnea) | (17,32) |
| Discomfort | ||
| Mouse Grimace Scale | Measure of pain/discomfort based on facial expression. Assessment of five facial features: orbital tightening, nose bulge, cheek bulge, ear position (drawn back), or whisker change (either backward or forward) | (33,34) |
| Piloerection | Involuntary bristling of the fur due to sympathetic nervous system activation | (16) |
| Other | ||
| Temperature | Increase or decrease in body temperature | (16,35) |
| Weight | Increase or decrease in body weight | (15,16,35,36) |
Clinical examinations were performed at approximately the same time every day. Mice were briefly observed in their home cage and then taken to an assessment room. Each mouse was weighed, and body surface temperature was measured with an infrared temperature probe (Infrascan; La Crosse Technology) directed at the abdomen (average of three readings was used). Then the mice were assessed with a brief clinical exam to evaluate parameters described in Table 1. Details of the methods used for clinical assessment are outlined in Supplementary Table 2. The severity of each deficit was rated with a simple scale. A score of 0 was given if there was no sign of a deficit, a score of 0.5 indicated a mild deficit, and a score of 1 was given for a severe deficit (Supplementary Table 2). Deficits in body weight (g) and body surface temperature (°C) were scored based on deviation from reference values in young adult animals, as described for the eight-item frailty index. Besides the infrared temperature probe, the only additional equipment required was a clicker of the type used to train dogs (used to evaluate hearing). Table 2 shows the Mouse Frailty Assessment Form we developed for use in these studies.
Table 2.
Mouse Frailty Assessment Form©

Human Frailty Index Data
Frailty index data for humans was taken from the first wave of SHARE (release 2.5.0 of May 24, 2011), which began in 2004. There were 37,546 people (16,590 men; 20,956 women) from probability samples in 15 countries who participated in baseline interviews (wave 1: Austria, Belgium, Denmark, France, Germany, Greece, Italy, Netherlands, Spain, Sweden, Switzerland [2004–05], Israel [2005–06]; wave 2: Czech Republic, Poland [2006–07], Ireland [2007]). Frailty index scores were calculated from 70 self-report items in the survey, including measures from the physical health, behavioral risks, cognitive function, and mental health sections of the SHARE database, as reported previously by our group (37). Frailty index scores were obtained by dividing the number of deficits by the total number of measures (eg, 70). Frailty index scores were obtained for 30,025 people (55% female) aged 25 and older and plotted as a function of age. Values shown represent frailty averages at each age.
Statistics
Data are presented as the mean ± the SEM (or the SD where indicated). The relative heterogeneity of frailty index scores was calculated as the coefficient of variation (standard deviation/mean). Differences in frailty index scores between groups were evaluated with one-way analysis of variance. To determine whether a linear relationship existed between two variables, data were fit with a simple linear regression and square of the correlation coefficient (r 2) was calculated. To evaluate the relationship between age and the frailty index, data from mice and humans were normalized to 90% mortality values and fit with an exponential function. Differences between groups were considered statistically significant for p < .05. Statistical analyses were performed with MATLAB r2009b, SPSS 20 and Sigma Plot 11.0 software (Systat Software, Inc., Point Richmond, CA). Graphs were created with Sigma Plot 11.0.
Results
Relationship Between Age and Mortality
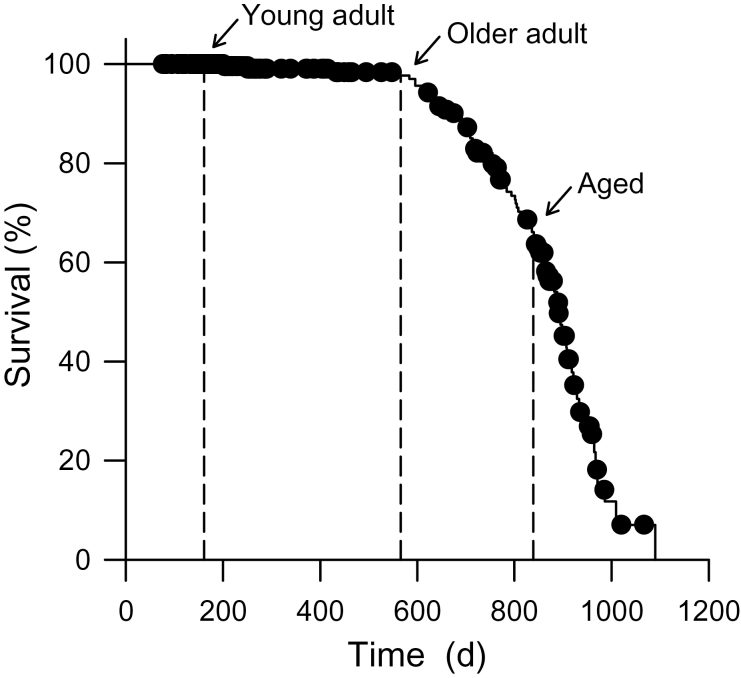
To illustrate the relationship between age and mortality in our colony of C57BL/6J female mice, a Kaplan–Meier survival curve was constructed (Figure 1). Mortality occurred when animals either died unexpectedly or were euthanized due to illness. At the start of this investigation, the young adult mice used in this study were 161 days old (~5 months; n = 5), the older adult group was 566 days old (~19 months; n = 4), and the aged group was 839 days old (~28 months; n = 5). The ages of the mice used at the start of this study are indicated on the survival curve (Figure 1; n = 293 female mice).
Figure 1.
Kaplan–Meier survival curve for mortality in C57BL/6J female mice. Mice were aged in the Carlton Animal Care Facility at Dalhousie University. Mortality occurred when mice died unexpectedly or were euthanized due to illness. The ages of mice used in the present study are indicated (n = 293 female mice).
Quantification of Murine Frailty With a Performance-Based Eight-Item Frailty Index
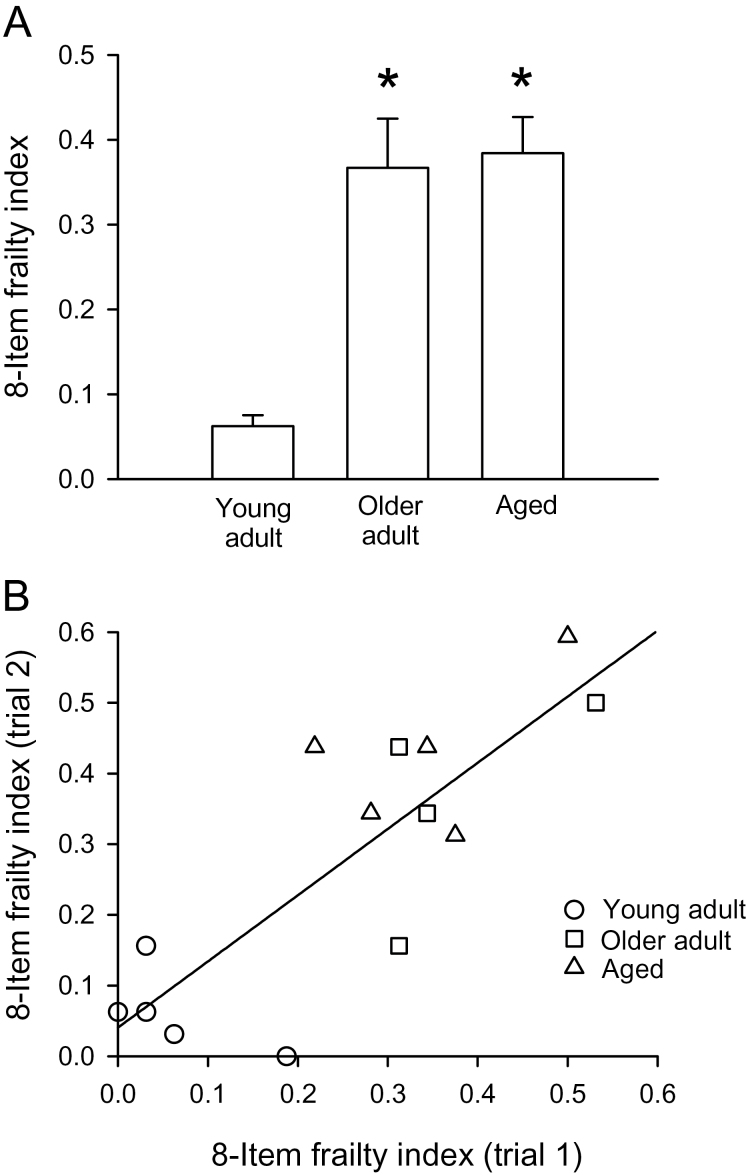
The relationship between the eight-item frailty index and age was calculated as described in the Methods. Supplementary Table 3 shows the number of mice that received frailty scores 0, 0.25, 0.5, 0.75, or 1 for each parameter in all three age cohorts. These data show that the number of mice with higher frailty scores increased markedly in the older age groups (Supplementary Table 3). Figure 2A shows the mean (± SEM) eight-item frailty index scores in young adults, older adults, and aged mice. The mean was calculated as the average eight-item frailty index score for trials #1 and #2 for each mouse. Results showed that the frailty index scores increased with age although the increase was not graded and scores were similar in the two older age groups (Figure 2A). Figure 2A shows that the standard error of the mean increased with age. The standard deviation also increased with age (values of mean ± SD were 0.062±0.029, 0.367±0.116, and 0.384±0.095 for young adult, older adult, and aged mice, respectively). This shows that the absolute heterogeneity of the frailty index is higher at older ages. By contrast, the coefficient of variation (standard deviation/mean) declined with age (from 0.47 in young adults to 0.32 and 0.25 in older adults and aged mice, respectively), which indicates that the relative heterogeneity of the frailty index scores declined with age.
Figure 2.
Scores obtained with the eight-item frailty index. (A) Mean (± SEM) eight-item frailty index scores were higher in both older age groups compared with young adults. (B) Scores from trial #1 were plotted against scores from trial #2. These data were a good fit to a straight line (r 2 = .67; p = .0003; n = 5 young adult mice, 4 older adult mice, and 5 aged mice). *Indicates significantly different from young adults (p < .05).
To evaluate the reproducibility of the frailty index scores obtained with this approach, scores from trial #1 were plotted against scores from trial #2, conducted 10–14 days after the first trial (Figure 2B). A regression line fitted through these data had an r 2 value of .68 (p = .0003). These data demonstrate that the eight-item frailty index data were reproducible from trial to trial.
Quantification of Murine Frailty With a Clinical Frailty Index
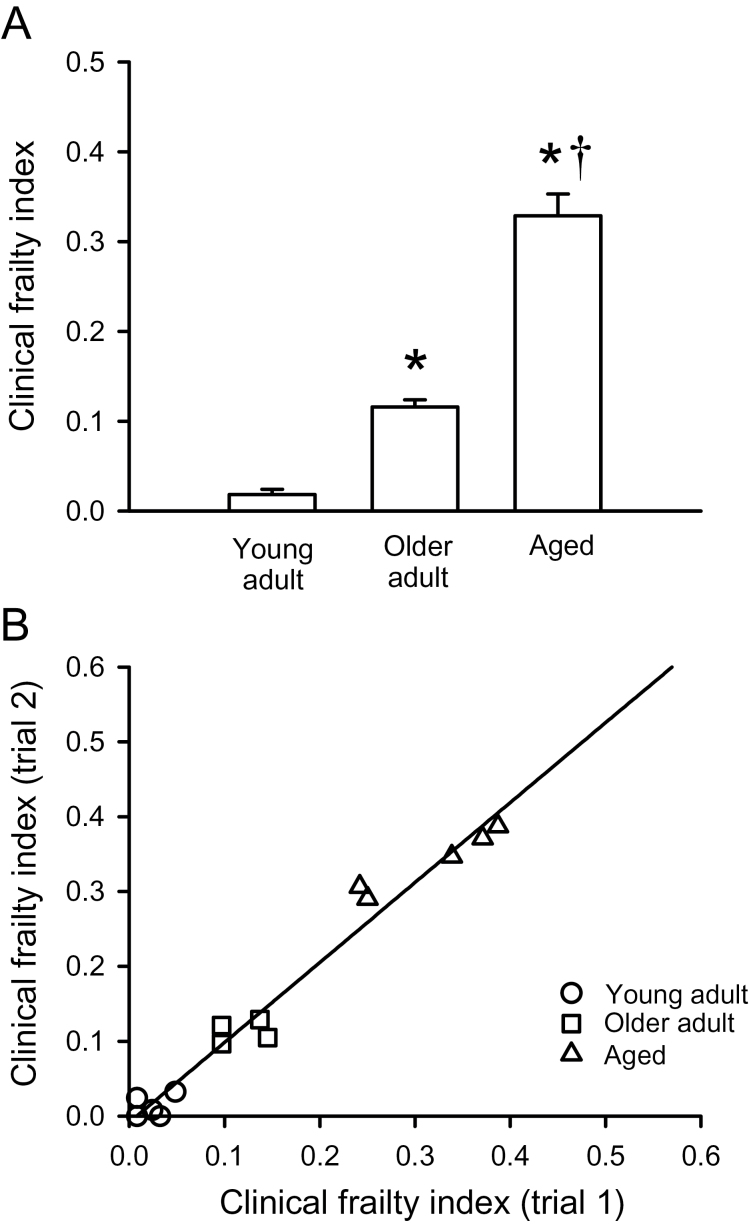
To examine the relationship between the clinically derived frailty index and age, we quantified frailty with a clinical frailty index as described in the Methods. Supplementary Table 4 shows a tally of the number of individual mice that received frailty scores of 0, 0.5, or 1 for each parameter in each of the three groups. It is readily apparent that the number of mice with frailty index scores of 0.5 or 1 for each parameter increased with age, in particular in the oldest group (Supplementary Table 4). The clinical frailty index scores for the first two trials were averaged for each animal and used to calculate a unique frailty index score for each mouse. Figure 3A shows that the average clinical frailty index scores increased progressively with age, and this increase was most pronounced in the oldest age group. The clinical frailty index scores were significantly higher in the older adult and aged groups compared with the young adult group (Figure 3A). Furthermore, unlike the performance-based eight-item frailty index, the frailty index scores were significantly higher in the aged group compared with the older adult group (Figure 3A). As with the eight-item frailty index, the standard error (Figure 3A) and standard deviation increased with age (values of mean ± SD were 0.018±0.013, 0.116±0.016, and 0.329±0.054 for young adult, older adult, and aged mice, respectively), whereas the coefficient of variation declined (from 0.72 in young adults to 0.14 and 0.16 in older adults and aged mice, respectively).
Figure 3.
Scores obtained with the clinical frailty index. (A) Mean (± SEM) clinical frailty index scores increased with age. (B) The clinical frailty index scores from trial #1 were plotted against those from trial #2. These data were fitted with a linear regression (r 2 = .97; p < .0001; n = 5 young adult mice, 4 older adult mice, and 5 aged mice). *Indicates significantly different from young adults; †Indicates significantly different from older adults (p < .05).
The data described in the preceding paragraph were obtained in female mice. We also investigated whether the clinical frailty index approach could be used to estimate frailty in a subset of male C57BL/6J mice. We found that the clinical frailty index scores were 0.013±0.003 (mean ± SEM; n = 3) in young adult male mice (~3 months). By contrast, the frailty index was 0.300±0.015 (n = 3) in aged male mice (~29 months). Interestingly, the frailty index scores in aged male mice were actually lower than the scores in 28-month-old female mice, suggesting that frailty may differ between the sexes.
The reproducibility of the clinical frailty index was evaluated by plotting the frailty index score for trial #1 against the score for trial #2, performed 11–15 days after the first. Figure 3B shows that the relationship between the two trials was clearly linear, as demonstrated by the regression line with an r 2 value of .97 (p < .0001). These data show that the clinical frailty index was highly reproducible from trial to trial.
Comparison of the Eight-Item Frailty Index and the Clinical Frailty Index
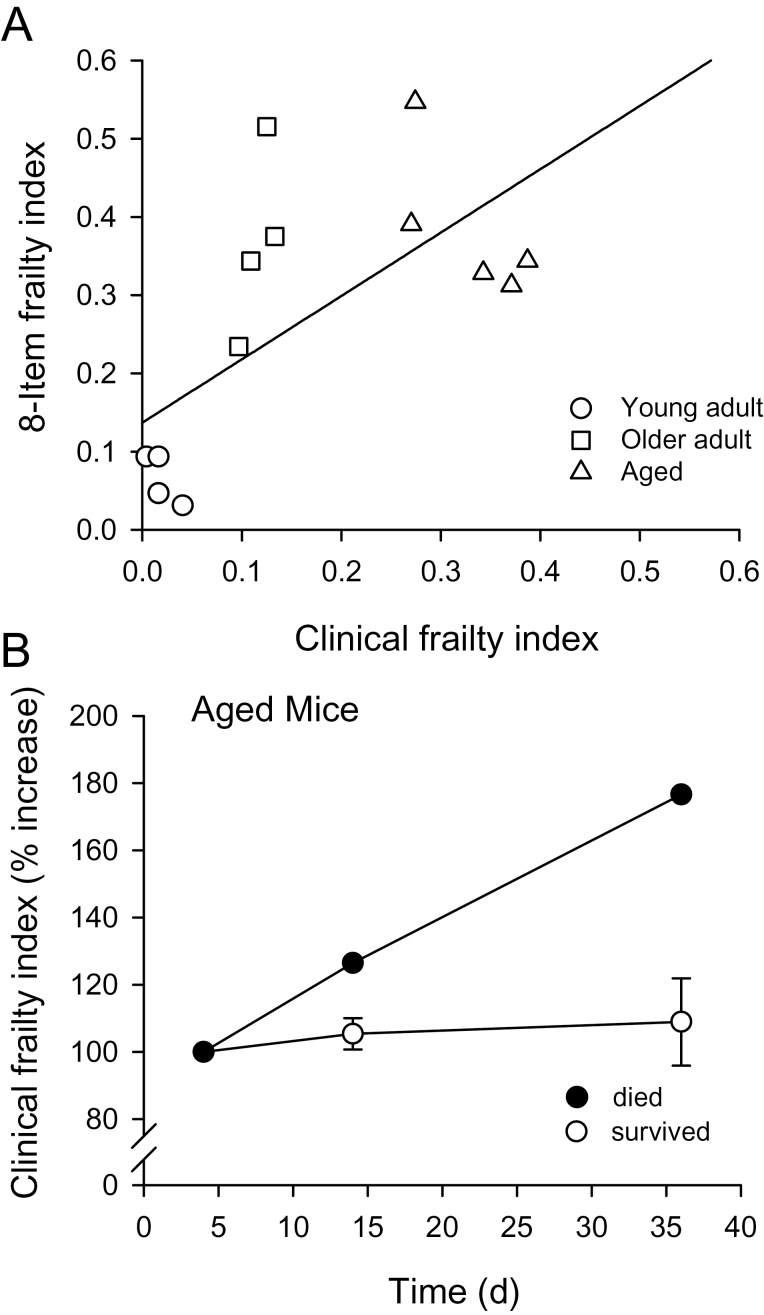
To compare the relationship between the performance-based eight-item frailty index and the clinical frailty index, we plotted the scores for the eight-item index against those for the clinical index as shown in Figure 4A. The relationship between these two indices was generally linear although the data were not a good fit to a straight line (r 2 = .43; p = .01; Figure 4A).
Figure 4.
Comparison of the eight-item frailty and clinical frailty indices. (A) The scores for the eight-item frailty index were plotted as a function of the clinical frailty index scores. A regression line fitted through these data has an r 2 value of .43 (p = .01). (B) The clinical frailty index was repeated on three separate trials in the oldest group. For 4/5 mice, the relationship showed little change with time (open symbols). However, one mouse that died 2 days after the final trial (filled symbols) showed a marked increase in the clinical frailty index.
Figure 4B shows the results obtained when clinical frailty index scores were evaluated a third time, 36 days after the first trial. The percent increase in the clinical frailty index score was plotted as a function of time for the aged mice used in this study. Interestingly, one of the aged mice died 2 days after the last frailty index measurement (Figure 4B, filled symbols). This mouse exhibited acceleration in the clinical frailty index immediately prior to death, unlike the aged mice that survived and showed little increase in frailty over this time (Figure 4B).
Relationship Between the Frailty Index and Age in Mice and Humans
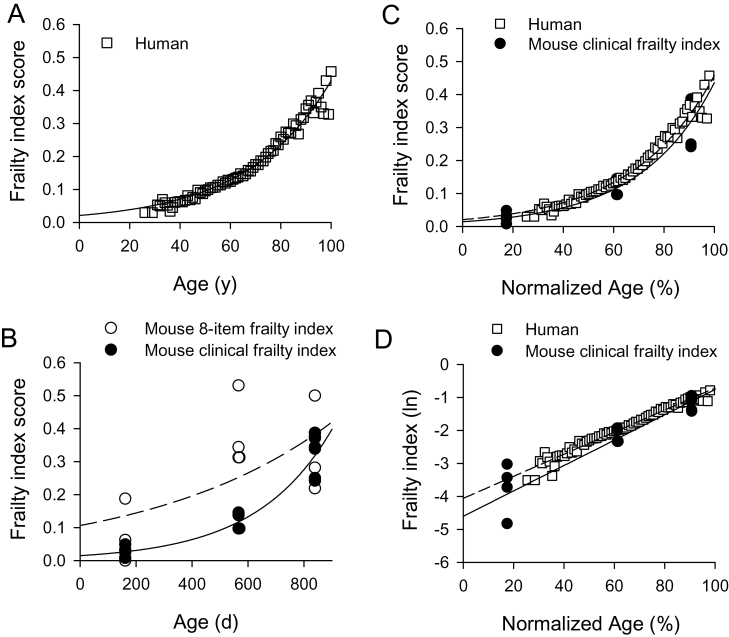
To compare the relationship between the frailty index and age in mice and humans, we used human frailty index scores from the SHARE survey and mouse frailty index scores described in this study. Figure 5A shows frailty index scores calculated from the SHARE database plotted as function of age; each point represents the average frailty at each age. These data demonstrate that the frailty index increased exponentially with age in humans (r 2 = .97; p < .0001; n = 30,025), as shown previously (38). Figure 5B shows values for the eight-item frailty index (open symbols) and the clinical frailty index (filled symbols) in mice plotted as a function of age. The clinical frailty index data were well described by an exponential function (r 2 = .91; p < .0001; n = 14). By contrast, the eight-item frailty index data were not a good fit to an exponential function (r 2 = .49) but were a better fit to a straight line (r 2 = .59; p < .001). Next, we directly compared the relationship between the clinical frailty index and age in mice and humans by normalizing age as a percentage of the 90% mortality values in each group. The 90% mortality value used for the human data was 102 years (39), whereas the 90% mortality value used for C57BL/6J mice was 925 days (40). As shown in Figure 5C, the relationship between the clinical frailty index and normalized age was similar in the two groups. Finally, the rate of deficit accumulation in mice and humans was compared by plotting the natural logarithm of the frailty index as a function of age and fitting the resulting relationship with a linear function (Figure 5D). This approach has previously been used to illustrate the rate of deficit accumulation in studies of older humans (38). The slopes of these lines, which represent the rate of deficit accumulation, were 0.034 and 0.038 in humans and mice, respectively (the y-intercepts were −4.06 in humans and −4.60 in mice). To test whether these lines differed between mice and humans, analysis of covariance was performed. Results showed that slope was slightly, but significantly, higher in the mouse compared with humans (p < .04; Figure 5D).
Figure 5.
Comparison of the relationship between the frailty index and age in mice and humans. (A) Frailty index scores from the Survey of Health, Ageing and Retirement in Europe survey were plotted as function of age fit with an exponential function. Frailty increased exponentially with age (r 2 = .97; n = 30,025 people). (B) Frailty index scores for the eight-item frailty index (open symbols) and the clinical frailty index (filled symbols) were plotted as a function of age and fit with an exponential function (n = 14 mice). The clinical frailty index data were well described by an exponential function (r 2 = .91), but the eight-item frailty index data were not (r 2 = .49). (C) When age was normalized to the 90% mortality level in each group, the relationship between the frailty index and age was similar in mice and humans. (D) The natural logarithm frailty index was plotted as a function of age. The slopes of the regression lines through these data, which represent the rate of deficit accumulation, were similar in mice and humans although analysis of covariance showed that the slope was significantly higher in mice than in humans.
Discussion
It is well established that frailty can be quantified in older humans based on deficit accumulation, where various deficits in health (eg, symptoms, signs, diseases, and disabilities) are simply counted and combined in a frailty index (41). Studies in humans have shown that a simple, practical frailty index score can be derived even from routinely used comprehensive geriatric assessment tools (42,43). Therefore, assessment of frailty in older adults in a clinical setting does not require specialized testing. Our group recently showed that the deficit accumulation approach also could be used to quantify frailty in aging mice (10). However, the parameters used to construct the full 31-item frailty index in our earlier study were measured with equipment that is not readily available in most research laboratories (10). Furthermore, as the full frailty index required the use of invasive techniques, it is not suitable for longitudinal studies of frailty in mice. A key advance in this study is the development of a simplified, noninvasive frailty index based on readily apparent signs of clinical deterioration that can be used to characterize frailty in aging mice. With the use of a simple 31-item check list, an individualized frailty index score was easily and quickly calculated for each mouse. Importantly, the values of frailty obtained with this new clinical frailty index were similar to those measured with the full 31-item index in our previous study. Scores between 0.3 and 0.5 were observed in 30-month-old female mice with the full frailty index in our original study (10), whereas scores between 0.3 and 0.4 were measured in 28-month-old female mice with our clinical frailty index. This simplified, noninvasive clinical frailty index may be useful not only in longitudinal studies of frailty in aging mice but also in studies of genetically manipulated mice and other murine models of disease.
Although we found that the eight-item and clinical frailty indices both increased with age, the clinical frailty index was less variable and exhibited little test-to-test variability compared with the performance-based eight-item index. The clinical frailty index also showed a progressive increase with age, whereas the eight-item index did not distinguish between different levels of frailty in the two oldest groups of mice. Thus, the clinical frailty index was able to detect graded levels of frailty in aging mice, whereas the eight-item index was not. One reason the clinical frailty index may perform better than the eight-item frailty index is because the 31-item index allows more pieces of information to contribute to the overall understanding of the level of frailty in an individual. Allowing the impact of small pieces of information to accumulate is well accepted in many mathematically based disciplines such as computer science (44) and in physical modeling of biologic systems (45,46). Interestingly, previous studies in humans have shown that graded levels of frailty cannot readily be distinguished when fewer than 30 variables are used to create a frailty index (10,11). Furthermore, the exact items that make up the frailty index appear to be less important than measuring at least 30 deficits (47,48). This suggests that small changes in many different items can accumulate and contribute to biologically important differences in the level of frailty in an individual.
A second reason that the clinical index may perform better than the eight-item index is that, where a few items are selected from many, the basis of their selection tends not to be stable, at least for the most part. In other words, applying the same statistical criteria in a second data set will tend to select different measures as being most informative; the results are internally valid but may not be generalizable (49–51). It is possible that some items in a frailty index integrate a great deal of information—mobility, for example, requires initiative, integration of pyramidal, extrapyramidal, cerebellar, spinal, motor unit, muscular, joint, bone, sensory, respiratory, cardiac, and metabolic inputs. Impairment in any of these items can result in motor slowing, which is likely to be selected by any data reduction technique. Even so, the information value will be less than knowing what is associated with the slowing. This too will vary by the population from which the sample is taken. For these reasons, we see a significant value to the frailty index in including a large number of items and then studying their behavior in the aggregate (52). In short, the frailty index integrates a great deal of information quantitatively, so as to get a relevant and nonarbitrary measure of the overall health status of individual animals. This is not achieved when very few parameters are measured. In this study, the clinical frailty index considers deficits from a large number of different systems in the body (eg, integument, musculoskeletal, vestibulocochlear, auditory, ocular, nasal, digestive, urogenital, respiratory). By contrast, the eight-item index is focussed almost exclusively on physical activity. A recent systematic review concluded that broadly based assessment tools like the frailty index are more likely to capture the multiple and dynamic factors that give rise to frailty in humans than instruments that rely on measures from one or two domains (7). Our data suggest that the more broadly based clinical frailty index in mice may provide a better estimate than the eight-item index that focuses on physical activity measures.
Frailty has been viewed as the variable vulnerability of adverse outcomes for organisms of the same age (2,4,53). In humans, there is controversy about how to operationalize this variable vulnerability. Although many different measurement instruments have been proposed, most commentary generally has focussed on two approaches, deficit accumulation and the frailty phenotype (reviewed by de Vries et al.) (7). The deficit accumulation approach we propose here for use in mice accords with the deficit accumulation approach used in many previous studies in humans (2,4,53). It includes integrative measures such as grooming, strength, mobility, and measures of discomfort, so it measures deficits constituted broadly. In this way, this clinical frailty index provides an estimate of frailty in ageing mice.
An important advance made in this study is our observation that the behavior of the clinical frailty index in mice was similar to the frailty index in humans. Previous studies in humans (54) have reported that the frailty index is less than 0.04 in younger adults (between the ages of 15 and 39 years), which compares to the young adult values of approximately 0.02 observed in young adult mice in our study. Rockwood et al. (54) also reported that the frailty indices increased to 0.125 in middle-aged adults (40–69 years) and rose to between 0.30 and 0.40 in the very old. These values are close to those reported here in mice, where we found an average frailty index near 0.112 in the older adults and a frailty index of 0.329 in the aged group. We also found that the frailty index increased exponentially with age in mice as it does in humans aged 15–102 years (54). Indeed, both murine and human frailty indices showed similar exponential increases with age when age was normalized to the 90% mortality levels. Furthermore, when the rate of deficit accumulation was compared by plotting the natural logarithm of the frailty index as a function of age, the slopes of the lines fitted through these data were comparable in humans and in mice. Previous studies have used this approach to show that community-dwelling older adults accumulate deficit at a rate of approximately 3% per year, which corresponds to a slope of approximately 0.03 (38). This study found the slope of the line was 0.034 for older adults in the SHARE database, which is similar to results reported previously (38). Interestingly, this is slightly lower than the slope of 0.038 observed in mice. It is possible that the rate of deficit accumulation with age is actually somewhat higher in mice than in humans although additional experiments in a larger sample and across a wider age range would be required to investigate this fully.
Our study provides additional evidence for similarities between the murine and human frailty index data. Previous studies in humans have shown that the absolute heterogeneity of the frailty index, indicated by measures such as the standard deviation or standard error, increases with age (55,56), as shown in our study in mice. By contrast, the relative heterogeneity, as indicated by the coefficient of variation, declines (56). Again, similar results were seen in this study in the mouse model. The age-related decline in the heterogeneity of the frailty index has been interpreted to indicate a loss of redundancy as the system ages (56). Our results suggest that this loss of physiological reserve or redundancy in frailty may also occur in the mouse. Our study also suggests that the frailty index may be higher in female mice than in males. Interestingly, this agrees with studies in humans that have reported that women have a higher frailty index than men (57,58) although evidence for a sex difference in the frailty index at the youngest (<75 years) and oldest (>95 years) ages is inconstant (59). However, clearly this finding is preliminary, and a larger study of sex differences in frailty in the mouse model is now warranted.
The clinical frailty index used in this study was developed by considering established signs of clinical deterioration in mice (15–18,20). Although most of these signs can occur in all aging mouse strains, our study specifically evaluated aging C57BL/6J mice. Other mouse strains may exhibit characteristic diseases and signs of clinical deterioration (60), and these could be used to tailor the frailty index to particular mouse strains. It also is possible that this general approach for creating a frailty index has more widespread applicability. For example, it may be useful to quantify frailty in other animal models of aging such as rats although the index would need to be adapted to each specific species of interest.
Previous studies have provided evidence that inflammation plays a key role in the development of frailty in humans (reviewed by Fulop et al. and Walston et al.) (53,61). Furthermore, the interleukin-10 knockout mouse (IL10tm/tm) has been proposed as a model for human frailty as these animals exhibit inflammation and develop an age-related decrease in skeletal muscle strength (62–65). As we designed our frailty assessment tool to be noninvasive, we did not directly evaluate the level of inflammation in the mice used in our study. Even so, we did find that the occurrence of dermatitis, which has been linked to inflammation (66), increased markedly with age. This provides indirect evidence that the level of inflammation may increase with age in our frail older mice. It would be interesting to experimentally evaluate the link between inflammation and frailty in mice with this new clinical frailty index.
There are several limitations to the experimental approach outlined in this study. Due to the relatively small sample size, we did not determine whether the frailty index was associated with underlying pathology or whether it was a predictor of mortality. We did observe acceleration of mortality in one animal that died shortly after the last frailty index measure was completed, but a large scale study is needed to explore this idea fully. In addition, the oldest mice we used were approximately 2.5 years old by the end of our study, an age where fewer than 40% of the mice would be expected to be alive based on our survival data. Still, it would have been interesting to obtain frailty index scores on even older animals to explore the limits of frailty in the murine model. Finally, it is possible that investigator bias may play a role in determination of the clinical frailty index, and this may affect the ability to compare results across studies. The question of interrater reliability should be addressed experimentally in future studies.
In summary, the results of this study demonstrate that a simple noninvasive clinical frailty index can be used to quantify frailty in aging mice. This approach was much simpler than our initial 31-item frailty index, which required specialized equipment and invasive measures that would limit its use in longitudinal studies. Unlike our earlier performance-based eight-item murine frailty index, this novel clinical frailty index was highly reproducible from trial to trial and revealed graded increases in frailty with age. We also found that there were important similarities between the clinical frailty index developed here for use in mice and the frailty index used to characterize frailty in people. This clinical frailty index may be useful to quantify frailty in experimental studies designed to investigate novel treatments for frailty in mouse models.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This study was supported by grants from the Canadian Institutes for Health Research (MOP 126018) and the Fountain Innovation Fund of the Queen Elizabeth II Health Sciences Foundation. K.R. receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research.
Acknowledgments
The authors express their appreciation for excellent technical assistance provided by Peter Nicholl.
References
- 1. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howlett SE, Rockwood K. New horizons in frailty: ageing and the deficit-scaling problem. Age Ageing. 2013;42:416–423 [DOI] [PubMed] [Google Scholar]
- 3. Rockwood K, Fox RA, Stolee P, Robertson D, Beattie BL. Frailty in elderly people: an evolving concept. CMAJ. 1994;150:489–495 [PMC free article] [PubMed] [Google Scholar]
- 4. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci. 2009;64:209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heuberger RA. The frailty syndrome: a comprehensive review. J Nutr Gerontol Geriatr. 2011;30:315–368 [DOI] [PubMed] [Google Scholar]
- 7. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114 [DOI] [PubMed] [Google Scholar]
- 8. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parks RJ, Fares E, Macdonald JK, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67:217–227 [DOI] [PubMed] [Google Scholar]
- 11. Ridda I, Lindley R, MacIntyre RC. The challenges of clinical trials in the exclusion zone: the case of the frail elderly. Australas J Ageing. 2008;27:61–66 [DOI] [PubMed] [Google Scholar]
- 12. Martinsson L, Eksborg S. Activity Index - a complementary ADL scale to the Barthel Index in the acute stage in patients with severe stroke. Cerebrovasc Dis. 2006;22:231–239 [DOI] [PubMed] [Google Scholar]
- 13. Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci. 2013;68:301–308 [DOI] [PubMed] [Google Scholar]
- 14. Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals. 2nd ed. Ottawa, ON: Canadian Council on Animal Care; 1993:1; 1984:2 [Google Scholar]
- 15. Fahlström A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging. 2011;32:1868–1880 [DOI] [PubMed] [Google Scholar]
- 16. Van Meer P, Raber J. Mouse behavioural analysis in systems biology. Biochem J. 2005;389(Pt 3):593–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foltz CJ, Ullman-Culleré MH. Guidelines for assessing the health and condition of mice. Lab Anim. 1999;28:28–32 [PubMed] [Google Scholar]
- 18. Brayton C, Justice M, Montgomery CA. Evaluating mutant mice: anatomic pathology. Vet Pathol. 2001;38:1–19 [DOI] [PubMed] [Google Scholar]
- 19. Strozik E, Festing MF. Whisker trimming in mice. Lab Anim. 1981;15:309–312 [DOI] [PubMed] [Google Scholar]
- 20. Ullman-Culleré MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci. 1999;49:319–323 [PubMed] [Google Scholar]
- 21. Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol. 1995;23:570–582 [DOI] [PubMed] [Google Scholar]
- 22. Simmler MC, Zwaenepoel I, Verpy E, et al. Twister mutant mice are defective for otogelin, a component specific to inner ear acellular membranes. Mamm Genome. 2000;11:960–966 [DOI] [PubMed] [Google Scholar]
- 23. Shiga A, Nakagawa T, Nakayama M, et al. Aging effects on vestibulo-ocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiol Neurootol. 2005;10:97–104 [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Yan H, Löfgren S, Tian X, Lou MF. Ultraviolet radiation-induced cataract in mice: the effect of age and the potential biochemical mechanism. Invest Ophthalmol Vis Sci. 2012;53:7276–7285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolf NS, Li Y, Pendergrass W, Schmeider C, Turturro A. Normal mouse and rat strains as models for age-related cataract and the effect of caloric restriction on its development. Exp Eye Res. 2000;70:683–692 [DOI] [PubMed] [Google Scholar]
- 26. Van Winkle TJ, Balk MW. Spontaneous corneal opacities in laboratory mice. Lab Anim Sci. 1986;36:248–255 [PubMed] [Google Scholar]
- 27. Lehmann K, Schmidt KF, Löwel S. Vision and visual plasticity in ageing mice. Restor Neurol Neurosci. 2012;30:161–178 [DOI] [PubMed] [Google Scholar]
- 28. Gelatt KN. Visual disturbance: where do I look? J Small Anim Pract. 1997;38:328–335 [DOI] [PubMed] [Google Scholar]
- 29. Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144(suppl 1):S146–S158 [DOI] [PubMed] [Google Scholar]
- 30. Percy DH, Barthold SW, eds. Pathology of Laboratory Rodents and Rabbits. 3rd ed. Ames, IA: Wiley-Blackwell; 2007 [Google Scholar]
- 31. Brayton C. Spontaneous diseases in commonly used inbred mouse strains. In: Fox JG, ed. The Mouse in Biomedical Research. 2nd ed. Amsterdam: Elsevier, Academic Press; 2007:2:623–718 [Google Scholar]
- 32. Torres-González E, Bueno M, Tanaka A, et al. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol. 2012;46:748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Defensor EB, Corley MJ, Blanchard RJ, Blanchard DC. Facial expressions of mice in aggressive and fearful contexts. Physiol Behav. 2012;107:680–685 [DOI] [PubMed] [Google Scholar]
- 34. Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449 [DOI] [PubMed] [Google Scholar]
- 35. Trammell RA, Cox L, Toth LA. Markers for heightened monitoring, imminent death, and euthanasia in aged inbred mice. Comp Med. 2012;62:172–178 [PMC free article] [PubMed] [Google Scholar]
- 36. Wong AA, Brown RE. Age-related changes in visual acuity, learning and memory in C57BL/6J and DBA/2J mice. Neurobiol Aging. 2007;28:1577–1593 [DOI] [PubMed] [Google Scholar]
- 37. Theou O, Brothers TD, Rockwood MR, Haardt D, Mitnitski A, Rockwood K. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older Europeans. Age Ageing. 2013;42:614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189 [DOI] [PubMed] [Google Scholar]
- 39. Pardo Silva MC, Janssens AC, Hofman A, Witteman JC, van Duijn CM. Apolipoprotein E gene is related to mortality only in normal weight individuals: the Rotterdam Study. Eur J Epidemiol. 2008;23:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501 [DOI] [PubMed] [Google Scholar]
- 41. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727 [DOI] [PubMed] [Google Scholar]
- 42. Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933 [DOI] [PubMed] [Google Scholar]
- 43. Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010;58:318–323 [DOI] [PubMed] [Google Scholar]
- 44. von Neuman J. In: Shannon CE, McCarthy J, eds. Probabilistic Logic and the Synthesis of Reliable Organisms from Unreliable Components, Automata Studies. Princeton, NJ: Princeton University Press; 1956:43–98 [Google Scholar]
- 45. Scheffer M. Complex systems: Foreseeing tipping points. Nature. 2010;467:411–412 [DOI] [PubMed] [Google Scholar]
- 46. Scheffer M, Carpenter SR, Lenton TM, et al. Anticipating critical transitions. Science. 2012;338:344–348 [DOI] [PubMed] [Google Scholar]
- 47. Lindley RI. Drug trials for older people. J Gerontol A Biol Sci Med Sci. 2012;67:152–157 [DOI] [PubMed] [Google Scholar]
- 48. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26 [DOI] [PubMed] [Google Scholar]
- 49. Song X, Mitnitski A, Cox J, Rockwood K. Comparison of machine learning techniques with classical statistical models in predicting health outcomes. Stud Health Technol Inform. 2004;107(Pt 1):736–740 [PubMed] [Google Scholar]
- 50. Kulminski AM, Culminskaya I, Arbeev KG, et al. The role of lipid-related genes, aging-related processes, and environment in healthspan. Aging Cell. 2013;12:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yashin AI, Arbeev KG, Wu D, et al. How lifespan associated genes modulate aging changes: lessons from analysis of longitudinal data. Front Genet. 2013;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bennett S, Song X, Mitnitski A, Rockwood K. A limit to frailty in very old, community-dwelling people: a secondary analysis of the Chinese longitudinal health and longevity study. Age Ageing. 2013;42:372–377 [DOI] [PubMed] [Google Scholar]
- 53. Fulop T, Larbi A, Witkowski JM, et al. Aging, frailty and age-related diseases. Biogerontology. 2010;11:547–563 [DOI] [PubMed] [Google Scholar]
- 54. Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183:E487–E494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. García-González JJ, García-Peña C, Franco-Marina F, Gutiérrez-Robledo LM. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr. 2009;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rockwood K, Mogilner A, Mitnitski A. Changes with age in the distribution of a frailty index. Mech Ageing Dev. 2004;125:517–519 [DOI] [PubMed] [Google Scholar]
- 57. Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123:1457–1460 [DOI] [PubMed] [Google Scholar]
- 58. Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B:246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kulminski A, Ukraintseva SV, Akushevich I, Arbeev KG, Land K, Yashin AI. Accelerated accumulation of health deficits as a characteristic of aging. Exp Gerontol. 2007;42:963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol. 2012;49:85–105 [DOI] [PubMed] [Google Scholar]
- 61. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001 [DOI] [PubMed] [Google Scholar]
- 62. Akki A, Yang H, Gupta A, et al. Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr). 2013. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ko F, Yu Q, Xue QL, et al. Inflammation and mortality in a frail mouse model. Age (Dordr). 2012;34:705–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ho YY, Matteini AM, Beamer B, et al. Exploring biologically relevant pathways in frailty. J Gerontol A Biol Sci Med Sci. 2011;66:975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walston J, Fedarko N, Yang H, et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63:391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neuhaus B, Niessen CM, Mesaros A, Withers DJ, Krieg T, Partridge L. Experimental analysis of risk factors for ulcerative dermatitis in mice. Exp Dermatol. 2012;21:712–713 [DOI] [PubMed] [Google Scholar]