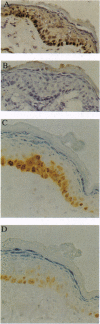
Abstract
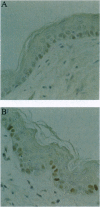
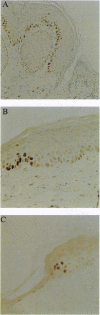
High levels of the p53 protein are immunohistochemically detectable in a majority of human nonmelanoma skin cancers and UVB-induced murine skin tumors. These increased protein levels are often associated with mutations in the conserved domains of the p53 gene. To investigate the timing of the p53 alterations in the process of UVB carcinogenesis, we used a well defined murine model (SKH:HR1 hairless mice) in which the time that tumors appear is predictable from the UVB exposures. The mice were subjected to a series of daily UVB exposures, either for 17 days or for 30 days, which would cause skin tumors to appear around 80 or 30 weeks, respectively. In the epidermis of these mice, we detected clusters of cells showing a strong immunostaining of the p53 protein, as measured with the CM-5 polyclonal antiserum. This cannot be explained by transient accumulation of the normal p53 protein as a physiological response to UVB-induced DNA damage. In single exposure experiments the observed transient CM-5 immunoreactivity lasted for only 3 days and was not clustered, whereas these clusters were still detectable as long as 56 days after 17 days of UVB exposure. In addition, approximately 70% of these patches reacted with the mutant-specific monoclonal antibody PAb240, whereas transiently induced p53-positive cells did not. In line with indicative human data, these experimental results in the hairless mouse model unambiguously demonstrate that constitutive p53 alterations are causally related to chronic UVB exposure and that they are a very early event in the induction of skin cancer by UVB radiation.
Full text
PDF

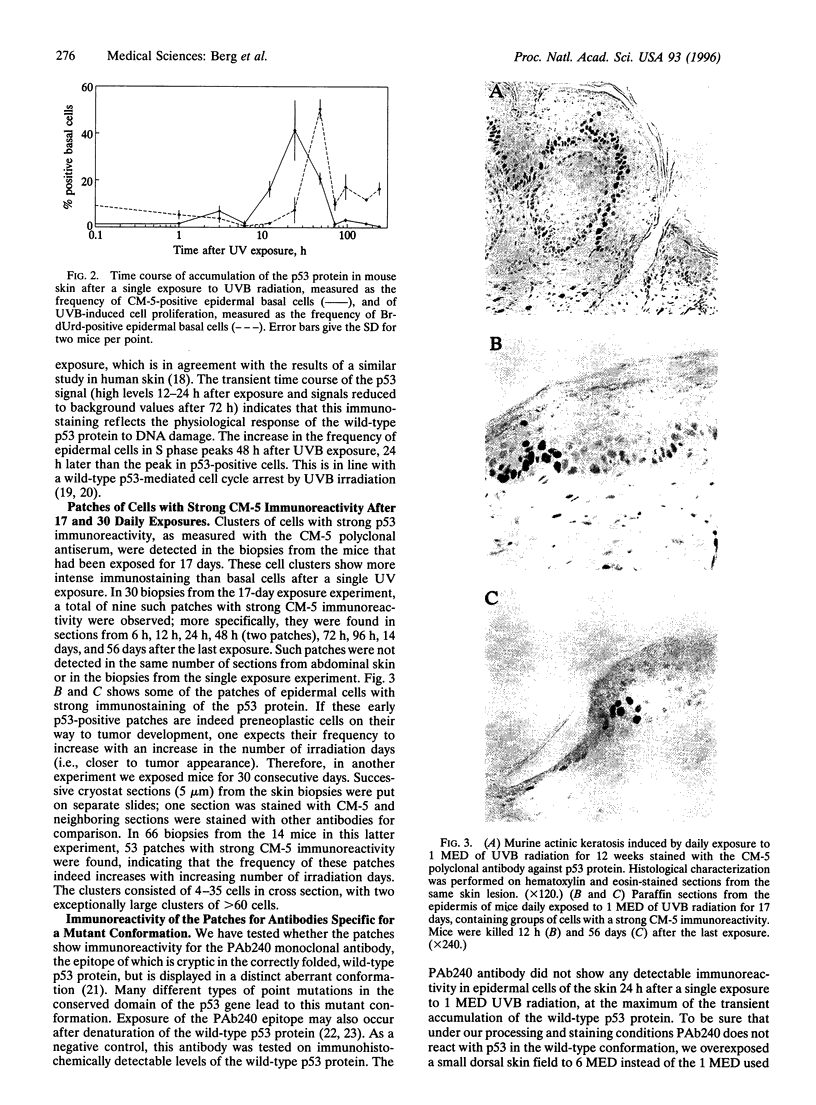

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gruijl F. R., Van Der Meer J. B., Van Der Leun J. C. Dose-time dependency of tumor formation by chronic UV exposure. Photochem Photobiol. 1983 Jan;37(1):53–62. doi: 10.1111/j.1751-1097.1983.tb04433.x. [DOI] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Hall P. A., Lane D. P. p53 in tumour pathology: can we trust immunohistochemistry?--Revisited! J Pathol. 1994 Jan;172(1):1–4. doi: 10.1002/path.1711720103. [DOI] [PubMed] [Google Scholar]
- Hall P. A., McKee P. H., Menage H. D., Dover R., Lane D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993 Jan;8(1):203–207. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kemp C. J., Donehower L. A., Bradley A., Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell. 1993 Sep 10;74(5):813–822. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lu X., Lane D. P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993 Nov 19;75(4):765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Nakazawa H., English D., Randell P. L., Nakazawa K., Martel N., Armstrong B. K., Yamasaki H. UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):360–364. doi: 10.1073/pnas.91.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte B., Reynders M. M., Bosman F. T., Blijham G. H. Effect of tissue fixation on anti-bromodeoxyuridine immunohistochemistry. J Histochem Cytochem. 1987 Nov;35(11):1343–1345. doi: 10.1177/35.11.3116075. [DOI] [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene. 1990 Jul;5(7):945–952. [PubMed] [Google Scholar]
- Stephen C. W., Lane D. P. Mutant conformation of p53. Precise epitope mapping using a filamentous phage epitope library. J Mol Biol. 1992 Jun 5;225(3):577–583. doi: 10.1016/0022-2836(92)90386-x. [DOI] [PubMed] [Google Scholar]
- Urano Y., Asano T., Yoshimoto K., Iwahana H., Kubo Y., Kato S., Sasaki S., Takeuchi N., Uchida N., Nakanishi H. Frequent p53 accumulation in the chronically sun-exposed epidermis and clonal expansion of p53 mutant cells in the epidermis adjacent to basal cell carcinoma. J Invest Dermatol. 1995 Jun;104(6):928–932. doi: 10.1111/1523-1747.ep12606204. [DOI] [PubMed] [Google Scholar]
- Vojtesek B., Dolezalova H., Lauerova L., Svitakova M., Havlis P., Kovarik J., Midgley C. A., Lane D. P. Conformational changes in p53 analysed using new antibodies to the core DNA binding domain of the protein. Oncogene. 1995 Jan 19;10(2):389–393. [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Jonason A. S., Leffell D. J., Simon J. A., Sharma H. W., Kimmelman J., Remington L., Jacks T., Brash D. E. Sunburn and p53 in the onset of skin cancer. Nature. 1994 Dec 22;372(6508):773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Leffell D. J., Kunala S., Sharma H. W., Gailani M., Simon J. A., Halperin A. J., Baden H. P., Shapiro P. E., Bale A. E. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl F. R., Forbes P. D. UV-induced skin cancer in a hairless mouse model. Bioessays. 1995 Jul;17(7):651–660. doi: 10.1002/bies.950170711. [DOI] [PubMed] [Google Scholar]
- de Gruijl F. R., Sterenborg H. J., Forbes P. D., Davies R. E., Cole C., Kelfkens G., van Weelden H., Slaper H., van der Leun J. C. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993 Jan 1;53(1):53–60. [PubMed] [Google Scholar]
- de Gruijl F. R., van der Leun J. C. Development of skin tumors in hairless mice after discontinuation of ultraviolet irradiation. Cancer Res. 1991 Feb 1;51(3):979–984. [PubMed] [Google Scholar]
- van Kranen H. J., de Gruijl F. R., de Vries A., Sontag Y., Wester P. W., Senden H. C., Rozemuller E., van Kreijl C. F. Frequent p53 alterations but low incidence of ras mutations in UV-B-induced skin tumors of hairless mice. Carcinogenesis. 1995 May;16(5):1141–1147. doi: 10.1093/carcin/16.5.1141. [DOI] [PubMed] [Google Scholar]