Abstract
The objectives of this study are to compare the results of newer performance-based functional assessments in the study of HIV-associated neurocognitive disorders (HAND) and to correlate these functional assessments with specific levels of severity of HAND. One hundred fourteen HIV+ subjects in an existing cohort were evaluated with a medical history, neurological exam, neuropsychological test battery as well as subjective and novel objective measures of functional abilities. Self-reported measures of functional performance included the Karnofsky Performance Scale, a questionnaire for instrumental activities of daily living, and a questionnaire for physical quality of life measures. The newer objective functional performance assessments in cluded the Columbia Medication Management and the San Diego Finances tests. These newer performance-based measures of function were assessed for their ability to predict level of HAND. The two objective measures of functional performance, The Columbia Medication Management Scale and the San Diego Finances Test, were both associated with levels of severity of HAND. The Karnofsky Performance Scale and the questionnaires for role and physical quality of life were subjective measures that were also associated with specific levels of HAND. Newer measures of functional performance can be used to objectively evaluate functional impairment in HAND and validate different levels of HAND.
Keywords: HIV, HIV-associated neurocognitive disorders, Functional outcome measures
Introduction
After the advent and utilization of highly active antiretroviral therapy, HIV-associated dementia (HAD) and HIV-associated neurocognitive disorders (HAND) have become less frequent, but HIV-positive patients are nonetheless still at risk for these neurological complications. Approximately 2% to 5% of HIV positive individuals may develop HAD and a higher percentage (around 37%) have less severe forms of HAND (Sacktor 2002; Sacktor et al. 2002). The American Academy of Neurology (AAN) originally published criteria for defining different levels of HAND in 1991 with subsequent revisions in 2007 to reflect the disorder more accurately in the post HAART era (Antinori et al. 2007). These guidelines provided a framework for clinicians to diagnose HAND and for researchers to categorize patients with HAND in clinical research trials and studies. Criteria from 1991 described two levels of HAND which included minor cognitive motor disorder (MCMD) and HIV-associated dementia (HAD). MCMD and HAD were similar in that they both required impairment in two neuropsychological domains but differed in degree of functional impairment such that impairments in work or in everyday activities of daily living were required for the diagnosis of HAD. In the revised criteria, the category of MCMD was further subdivided to include asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND). According to these criteria, patients with ANI, MND, and HAD all must have impairment in at least two neuropsychological domains. Again, level of functional impairment define these levels of HAND such that patients with ANI have no functional limitations, MND have mild functional impairment, and those with HAD have marked functional impairment.
The classification of HIV-related neurocognitive status is thus critically dependent on accurate assessment of an individual's functional performance status. Common measures of functional assessment in HIV cohort studies include the Karnofsky performance scale, instrumental activities of daily living scales of Lawton and Body (e.g., dressing, eating, preparing food, and bathing), Katz ADL/Lawton self-maintenance scale, role functioning items of the medical outcomes study, and medical outcomes study physical function subscale (Karnofsky et al. 1948; Schifitto et al. 2001). The principal limitation of these rating scales is that the score depends on the subjective reporting of the patient or the provider. Self-report of functional performance is used for most of the scales except the Karnofsky performance scale which is also subjective by virtue of the provider/rater. In a large study of 270 HIV+ patients, Schifitto and colleagues found that traditional subjective measures of functional status showed poor correlation with neuropsychiatric outcomes in patients with HAND (Schifitto et al. 2001). One possible explanation of the poor correlation was that the traditional functional measures were too subjective and relied on self-report from patients.
Efforts to provide more objective measures of functioning were introduced in a study by Heaton and colleagues (2004). In this study, 267 HIV-positive patients were assessed using subjective functional scales, but in addition, they underwent rigorous objective laboratory assessments of functioning. These included tests of functional abilities regarding shopping, cooking, vocational skills, management of finances, and management of medications. Overall, neuropsychologicaly impaired HIV-positive patients were found to be more functionally impaired on both subjective and objective measures of functional performance. Even though these objective laboratory measures did provide improved assessment over subjective measures, the feasi bility of large-scale laboratory functional measures would be impractical for both clinical studies and trials.
The Clinical Outcomes Core cohort was established to evaluate functional performance measures in HIV-positive patients with HAND in order to have more practical and objective predictors of functional performance, hence functional impairment. The objective of the current study is to evaluate the association between performance on objective performance measures and neurocognitive impairment as assessed by the revised AAN rating scale.
Methods
Participants
The study included 114 HIV-positive individuals at the General Clinical Research Clinic at Johns Hopkins Hospital in Baltimore, Maryland who were evaluated from 2007 to 2010. The study was approved by the Johns Hopkins IRB. HIV-positive patients were recruited from two cohorts: the Northeast AIDS Dementia (NEAD) cohort (McArthur et al. 2004) and the Oxidative Stress Cohort (Gandhi et al. 2010; Mohamed et al. 2010) and were chosen using the following inclusion criteria: adults older than 18 years, HIV-1-seropositive status, ability to provide written informed consent, and ability to ambulate at first clinic visit. Exclusion criteria were the following: history of or current opportunistic central nervous system infection, history or current schizophrenia, current severe affective disorder believed to account for the subject's cognitive impairment, and history of chronic neurological disorders such as epilepsy or multiple sclerosis. Substance abusers and heavy alcohol users, remote or active, were not excluded but were not examined if judged by the examiner to be intoxicated during the visit.
Patient consents
Written informed consent was obtained from all patients participating in the study.
Procedures
Participants underwent the same procedures every 6 to 12 months in accordance with their scheduled NEAD and Oxidative Stress cohort visits when possible. Standardized questionnaires were used to capture medical information and demographics, along with medical, psychiatric, and neurological history.
Clinical exam
A macro-neurologic examination created for the AIDS Clinical Trials Group and the motor subscale (part III) of the Unified Parkinson's Dementia Rating Scale (Fahn et al. 1987) to assess extrapyramidal signs associated with HAD were used.
Depression assessment
Mood was assessed with the self-administered Beck Depression Inventory II scale. A score greater than or equal to 16 was defined as the presence of depression symptomatology.
Laboratory assessment
Baseline CD4 lymphocyte count and levels of HIV RNA in CSF and plasma were recorded when available. HIV RNA levels were determined using the Roche PCR assay (Amplicor HIV-1 Monitor assay, version 1.5; Roche Diagnostics). Hepatitis C viral status was obtained via history and evaluation of laboratory findings for hepatitis C virus antibodies.
Diagnosis of HIV-associated neurocognitive disorder
Dementia assessment was performed according to the 2007 Revised AAN classification using previously published methodology for neurocognitive testing and definition of functional impairment based on instrumental activities of daily living (IADL) assessment (Lawton and Brody 1969).
Functional performance assessments
Subjective measures of functional performance included Karnofsky Performance Scale, questionnaire for IADLs, and questionnaire for role and physical quality of life (QOL) measures (Sacktor 2002; Schifitto et al. 2001). The Karnofsky scale was rated from 0–100 with 100 being the highest score or normal. All 114 participants received baseline Karnofsky ratings. The IADL scale was rated from 0–30 with 0 being no abnormality.
Participants were asked to report their current and previous best abilities to perform fourteen common daily tasks. The participant chose the description that best fit his or her functional level out of several options listed under each task. The choices for each task were scaled from 0 (no deficit) to 2 or 3 (marked functional impairment). The role QOL scale consisted of four yes/no questions regarding problems with work as a result of physical health. Each “yes” response indicated a functional deficit and was assigned a value of 1 for a maximum role QOL score of 4. The physical QOL scale consisted of nine yes/no questions regarding limitation of activities due to health and were scored the same way as the role QOL resulting in a maximum score of 9. A total of 58 participants completed both QOL scales.
The newer functional performance assessments included the Columbia Medication Management test and the San Diego Finances test (Heaton et al. 2004; Saxton et al. 2005). The Columbia Medication Management test evaluates patients on their ability to answer questions about five different imitation medications placed in pill containers with modified labels. The participant must count pills, answer questions about dosing, and demonstrate the use of a pill box. The range of scores for this test was from 0–16 with 16 being the highest or no mistakes on testing. The San Diego Finances Test requires patients to identify money, count money, write a check, and balance a checkbook ledger. Each of the tasks is scored, and total score range from 0–22 with 22 being the highest or no mistakes on the task.
Data analysis
Mean scores on all subjective and objective functional performance measures were obtained and categorized by level of neurocognitive impairment using the revised AAN rating scale for HAND. Group differences on demographic features were tested using analysis of variance (continuous measures) and Chi-squared test of association (categorical measures).
The ability of functional performance measures to differentiate between strata on the rating scales was tested using an ordinal logistic regression model. The rating scale served as the multinomial dependent variable. Functional performance measures served as the continuous independent variable. In all analyses, the presence of depression (BDI≥16) was included in the model to statistically adjust for baseline differences. Odds ratio for progressing to more severe levels of HAND were also calculated.
Results were considered statistically significant if the p value was ≤0.05. Statistical calculations were performed using Stata 10.0 software (StataCorp LP, College Station, TX).
Results
Study demographics
Table 1 contains a summary of the demographics of the 114 HIV-positive patients evaluated in this study. The mean age was 46.8 years (standard deviation (SD) 6.4 years). A majority of the patients were men (67%) and were African-American (92%). Mean (SD) CD4 T-cell count was 360.4 (206.1) cells/μL. Self-reported symptoms of depression defined as a BDI score greater than 16 were present in 21 (18%) of participants. Coinfection with hepatitis C was reported in 67 (59%) of participants. Twenty-two (19%) participants revealed active substance abuse, defined as use of marijuana, cocaine, heroin, or methamphetamine within 30 days prior to the visit based on self-report, as well as medical records and urine toxicology when available. Forty participants (35%) reported being employed, the majority of whom were working part-time, and 91 (80%) received financial support for disability.
Table 1.
Baseline demographics for the clinical outcomes cohort
| Patient demographics (n = 114) | |
|---|---|
| Age, years, mean (SD) | 46.8 (6.4) |
| Education, years, mean (SD) | 12.7 (2.2) |
| Male, n (%) | 76 (67%) |
| Race, n (%) | |
| African American | 105 (92%) |
| Caucasian | 9 (8%) |
| CD4 T-cell count, cells/μL, mean (SD) (n =113) | 360.4 (206.1) |
| HIV RNA, log10 copies/mL | |
| plasma, mean (SD) (n=47) | 3.4 (1.1) |
| CSF, mean (SD) (n=27) | 2.7 (0.9) |
| Depression (BDI≥16), n (%) | 21 (18%) |
| Hepatitis C virus positive, n (%) | 67 (59%) |
| Active substance abuse, n (%) | |
| Karnofsky, mean (SD) (n = 114) | 81.0 (9.4) |
| CMMT, mean (SD) (n=93) | 11.7 (3.0) |
| SDFT, mean (SD) (n=113) | 19.3 (2.2) |
| Role QOL, mean (SD) (n=58) | 1.7 (1.7) |
| Function QOL, mean (SD) (n=58) | 5.9 (4.7) |
| IADL, mean (SD) | |
| Best performance (n=66) | 0.45 (0.90) |
| Current performance (n = 110) | 1.59 (2.93) |
| Best-current performance (n=66) | 1.23 (2.69) |
| NART-R, mean (SD) (n=105) | 96.0 (9.7) |
SD standard deviation, n number
All 114 participants received baseline Karnofsky ratings. Due to protocol differences between the NEAD and Oxidative Stress cohorts, baseline response rates were limited to 110 for the measurement of current performance on the IADL scale and 66 for the measurement of previous best performance. A total of 58 participants completed both the Role QOL and Physical QOL scales at baseline.
Table 2 summarizes the demographics for the cohort stratified by the revised AAN ratings for HAND. Individuals classified as having HIV dementia were more likely to have symptoms of depression (p=0.015). There were no differences among the HAND groups in CD4 cell count, plasma HIV RNA, CSF HIV RNA, hepatitis C positivity, or active substance abuse.
Table 2.
Demographics for different levels of HAND using the revised AAN criteria
| Revised AAN rating for HAND (n=114) | Normal (n =16) | ANI (n=37) | MND (n=22) | HAD (n=39) |
|---|---|---|---|---|
| Age, years, mean (SD) | 45.4 (6.0) | 45.9 (6.5) | 46.8 (6.4) | 48.3 (6.3) |
| Education, years, mean (SD) | 12.3 (1.8) | 13.0 (2.8) | 12.7 (1.3) | 12.6 (2.1) |
| Male, n (%) | 12 (75%) | 27 (73%) | 10 (45%) | 27 (69%) |
| Race, n (%) | ||||
| African American | 13 (81%) | 34 (92%) | 21 (95%) | 37 (95%) |
| Caucasian | 3 (19%) | 3 (8%) | 1 (5%) | 2 (5%) |
| CD4 T-cell count, cells/μL, mean (SD) (n = 113) | 328.3 (126.7) | 353.0 (212.6) | 417.9 (264.1) | 347.9 (190.2) |
| HIV RNA, | ||||
| Plasma, tested, n (N=113) | 16 | 36 | 22 | 39 |
| Plasma, detectable VL, n (%) | 8 (50%) | 17 (47%) | 8 (36%) | 13 (33%) |
| Plasma, log10 copies/mL, mean (SD) | 3.5 (3.6) | 4.3 (4.5) | 4.4 (4.6) | 4.9 (5.3) |
| CSF, tested, n (N=81) | 11 | 29 | 17 | 24 |
| CSF, detectable VL, n (%) | 3 (27%) | 11 (38%) | 4 (24%) | 9 (38%) |
| CSF, log10 copies/mL, mean (SD) | 2.5 (2.3) | 3.6 (3.9) | 3.3 (3.5) | 4.0 (4.3) |
| Depression, % BDI>16, n (%) | 1 (6%) | 4 (11%) | 2 (9%) | 14 (36%) |
| Hepatitis C virus positive, n (%) | 8 (50%) | 21 (57%) | 13 (59%) | 25 (64%) |
| Active substance abuse, n (%) | 1 (6.3%) | 9 (24%) | 3 (14%) | 9 (23%) |
Categories that are bold and italicized represent differences that are statistically significant by ordered logistic regression model to an alpha level <0.05 SD standard deviation, n number
Utility of subjective and performance-based functional measures in HAND
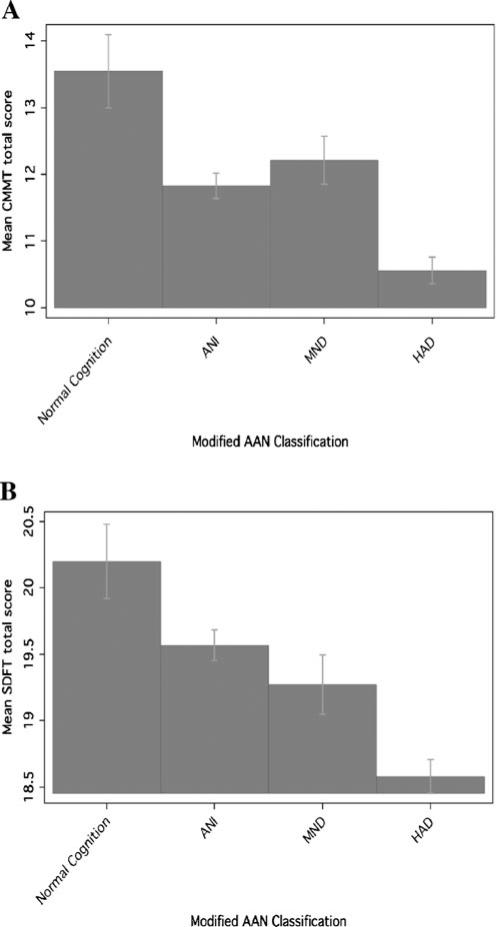
Mean performance for each functional performance measure is shown in Table 3, stratified by the revised AAN rating for HAND. We noted significant differences in Columbia Medication Management Test (CMMT; p=.007; see Fig. 1a), San Diego Finances Test (SDFT; p=.009; see Fig. 1b), Karnofsky (p<.001), and IADL current performance (p<.001). Higher scores on the CMMT (OR 0.84 95% CI 0.73, 0.95), SDFT (OR 0.81 95% CI 0.69, 0.95), and Karnofsky (OR 0.91 95% CI 0.88, 0.95) were associated with a decreased odds of cognitive impairment, whereas increased scores (more functional impairment) on IADL current performance (OR 1.36 95% CI 1.13, 1.64) were associated with an increased odds of cognitive impairment after adjusting for the Beck Depression Inventory total score (Table 3).
Table 3.
Mean scores for functional performance scales for different levels of HAND as rated by the revised AAN criteria
| Revised AAN rating for HAND (n=82) | Normal | ANI | MND | HAD | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|---|---|---|---|
| CMMT, mean (SD) (n=93) | 13.6 (2.5) | 11.8 (2.6) | 12.2 (3.1) | 10.6 (3.2) | 0.84 | 0.73, 0.95 | .007 |
| SDFT, mean (SD) (n =112) | 20.2 (1.8) | 19.6 (2.1) | 19.3 (2.3) | 18.6 (2.3) | 0.81 | 0.69, 0.95 | .009 |
| Karnofsky, mean (SD) (n =114) | 85.6 (8.9) | 84.1 (7.6) | 81.4 (8.3) | 75.9 (9.7) | 0.91 | 0.88, 0.95 | <.001 |
| Role QOL, mean (SD) (n=58) | 0.6 (1.1) | 1.4 (1.6) | 2.1 (1.8) | 2.1 (1.8) | 1.33 | 1.01, 1.76 | .044 |
| Physical QOL, mean (SD) (n=58) | 2.7 (3.2) | 4.2 (3.8) | 6.6 (5.0) | 8.0 (4.9) | 1.18 | 1.06, 1.31 | .003 |
| IADLs, mean (SD) | |||||||
| Best performance (n=66) | 0.71 (0.95) | 0.14 (0.36) | 0.44 (0.81) | 0.68 (1.21) | 1.32 | 0.73, 2.37 | .211 |
| Current performance (n = 110) | 0.79 (1.25) | 0.47 (2.02) | 1.09 (1.95) | 3.24 (3.79) | 1.36 | 1.23, 1.64 | <.001 |
| Best-current performance (n=66) | 0.43 (0.53) | 0.67 (2.61) | 0.88 (1.59) | 3.24 (3.79) | 1.25 | 0.99, 1.58 | .162 |
Linear regression models were adjusted for Beck Depression Inventory total score SD standard deviation, n number
Fig. 1.
Differences in mean score performance for the Columbia Medication Management test (a) and for the San Diego Finances Test (b), a CMMT Columbia Medication Management test, ANI asymptomatic neurocognitive impairment, MND mild neurocognitive disorder, HAD HIV-associated dementia, AAN American Academy of Neurology; b SDFT San Diego Finances test, ANI asymptomatic neurocognitive impairment, MND mild neurocognitive disorder, HAD HIV-associated dementia, AAN American Academy of Neurology
Discussion
Functional impairment in HAND can present in multiple ways. The ability to work, drive, manage medications, and manage finances can be impaired when neurocognitive performance declines. HIV-positive patients with HAND are more likely to be unemployed in comparison to HIV-positive patients without cognitive dysfunction (Benedict et al. 2000; van Gorp et al. 1999). Other aspects of functional performance including medication management and management of finances impact not only the day-to-day living of HIV-positive patients but also survival and viral control if HIV-positive patients are not able to obtain and adhere to highly active antiretroviral regimens (Fogarty et al. 2002; Gross 2007; Hinkin et al. 2002; Munakata et al. 2006).
Objective measures of functional performance have been developed to provide a better means to predict functioning by virtue that these measures do not depend on self-report. Heaton and colleagues examined a series of laboratory assessments of function which included working, managing finances, managing medications, cooking, and shopping (Heaton et al. 2004). Our study utilized two objective measures including the CMMT and the SDFT. Our results showed that the CMMT task and the SDFT were useful in predicting different stages of HAND such that worsening scores on these tests were predictive of increased severity of HAND. Furthermore, these performance-based objective measures may validate the subjective functional measures, particularly when informant-report is not available.
Scores obtained from the subjective measures of performance (Karnofsky, Role QOL, Physical QOL, and IADLs) showed that lower scores on these measures correlated with more severe levels of HAND. The primary caveat is that their predictive power is limited by the fact that these measures are used to categorize the patients into different levels of HAND. Essentially, these subjective measures go into the stratification process for HAND. Thus, it is likely that some of their predictive power could be overestimated. In contrast, the two objective functional measures (CMMT and SDFT) were not used in the classification of HAND for cohort participants.
While the subjective measures can provide some information about the functioning of the patient, they should be evaluated cautiously. Self report from depressed, impaired, or demented patients are often very different from the patient's actual level of functional independence, primarily because they lack appropriate insight into their functional deficits (Graham et al. 2005). In our model, we adjusted for differences in depression symptomatology and differences in each functional measure stratified by the revised AAN rating for HAND remained significant. Research in Alzheimer's disease and mild cognitive impairment has shown that self-report and informant-report can vary substantially in regards to the level of deficits in an individual (Ready et al. 2004a, b; Fuh and Wang 2006; Vogel et al. 2006). In fact, it has been suggested that the informant-report is a better assessment of a cognitively impaired patient's functioning rather than self-report and even assessment by a clinician (Snow et al. 2005). Integration of self-report and informant-report is now widely used in research on Alzheimer's disease and mild cognitive impairment and is considered superior to unaggregated measures (Ready and Ott 2007). To date, there has been no research regarding informant-report in patients with HAND. Perhaps one of the main reasons that informant-report is lacking in research of patients with HAND is the continued but unwarranted social stigma of HIV infection. Furthermore, the support system for patients with HIV and HAND is likely different from patients with Alzheimer's disease lending again to the lack of informant reporting.
In summary, our data suggest that the Columbia Medication Management test and San Diego Finances test are two useful performance-based measures of function that can be utilized as a brief battery to objectively evaluate functional performance in HIV+ individuals with HAND.
Acknowledgments
Supported by the Johns Hopkins NIMH Center for Novel Therapeutics of HIV Associated Cognitive Disorders (MH 075673), (MH71150), and (NS 049465)
Footnotes
Disclosure The authors report no conflicts of interest.
Contributor Information
Nishiena S. Gandhi, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Richard L. Skolasky, Department of Orthopedic Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Katherine B. Peters, Department of Neurology, Duke University, Durham, NC, USA
Richard T. Moxley, IV, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jason Creighton, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Heidi Vornbrock Roosa, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ola A. Selnes, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Justin McArthur, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ned Sacktor, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Neurology, Johns Hopkins Bayview Medical Center, 4940 Eastern Avenue, 301 Building, Suite 2100, Baltimore, MD 21224, USA.
References
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neuro-cognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RH, Mezhir JJ, Walsh K, Hewitt RG. Impact of human immunodeficiency virus type-1-associated cognitive dysfunction on activities of daily living and quality of life. Arch Clin Neuropsychol. 2000;15:535–544. [PubMed] [Google Scholar]
- Fahn S, Marsden C, Caine D. Recent developments in Parkinson's disease. Macmillan Healthcare Information; Florham Park: 1987. [Google Scholar]
- Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns. 2002;46:93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- Fuh JL, Wang SJ. Assessing quality of life in Taiwanese patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2006;21:103–107. doi: 10.1002/gps.1425. [DOI] [PubMed] [Google Scholar]
- Gandhi NS, Moxley CJ, Vornbrock Roosa H, Skolasky RL, Selnes OA, McArthur J, Sacktor N. Comparison of scales to evaluate the progression of HIV-associated neurocognitive disorder. HIV Therapy. 2010;4:373–379. doi: 10.2217/hiv.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DP, Kunik ME, Doody R, Snow AL. Self-reported awareness of performance in dementia. Brain Res Cogn Brain Res. 2005;25:144. doi: 10.1016/j.cogbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Gross R. Predicting and monitoring antiretroviral adherence. LDI Issue Brief. 2007;13:1–4. [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Thrasher D, Goetz MB, Stefaniak M. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky D, Abelman W, Craver L, Burchenal J. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- McArthur JC, McDermott M, McClernon D, St Hillaire C, Conant K, Marder K, Schifitto G, Selnes OA, Sacktor N, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein LG. Attenuated CNS infection in advanced HIV/AIDS with highly active antireteroviral therapy. Arch Neurol. 2004;61:1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- Mohamed MA, Barker PB, Skolasky RL, Moxley RT, Pomper MG, Sacktor NC. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study. Magn Reson Imaging. 2010;28(9):1251–1257. doi: 10.1016/j.mri.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata J, Benner JS, Becker S, Dezii CM, Hazard EH, Tierce JC. Clinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virus. Med Care. 2006;44:893–899. doi: 10.1097/01.mlr.0000233679.20898.e9. [DOI] [PubMed] [Google Scholar]
- Ready RE, Ott BR. Integrating patient and informant reports on the cornell-brown quality-of-life scale. Am J Alzheimers Dis Other Demen. 2007;22:528–534. doi: 10.1177/1533317507307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready RE, Ott BR, Grace J. Validity of informant reports about AD and MCI patients’ memory. Alzheimer Dis Assoc Disord. 2004a;18:11–16. doi: 10.1097/00002093-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Ready RE, Ott BR, Grace J. Patient versus informant perspectives of quality of life in mild cognitive impairment and alzheimer's disease. Int J Geriatr Psychiatry. 2004b;19:256–265. doi: 10.1002/gps.1075. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Saxton J, Morrow LA, Baumann S, Zuccolotto A, Schneider W, Offerman J, Dekosky ST. The computer-based assessment of mild cognitive impairment (CAMCI). Thirty-Third Annual International Neuropsychological Society Conference. 2005;33 [Google Scholar]
- Schifitto G, Kieburtz K, McDermott MP, McArthur J, Marder K, Sacktor N, Palumbo D, Selnes O, Stern Y, Epstein L, Albert S. Clinical trials in HIV-associated cognitive impairment: cognitive and functional outcomes. Neurology. 2001;56:415–418. doi: 10.1212/wnl.56.3.415. [DOI] [PubMed] [Google Scholar]
- Snow AL, Graham DP, Molinari VA, Orengo CA, Doody RS, Norris MP, Kunik ME. Factors affecting deficit awareness in persons with dementia. Dement Geriatr Cogn Disord. 2005;20:133–139. doi: 10.1159/000086945. [DOI] [PubMed] [Google Scholar]
- Van Gorp WG, Baerwald JP, Ferrando SJ, McElhiney MC, Rabkin JG. The relationship between employment and neuropsycho-logical impairment in HIV infection. J Int Neuropsychol Soc. 1999;5:534–539. doi: 10.1017/s1355617799566071. [DOI] [PubMed] [Google Scholar]
- Vogel A, Mortensen EL, Hasselbalch SG, Andersen BB, Waldemar G. Patient versus informant reported quality of life in the earliest phases of Alzheimer's disease. Int J Geriatr Psychiatry. 2006;21:1132–1138. doi: 10.1002/gps.1619. [DOI] [PubMed] [Google Scholar]