Abstract
Background
Previous studies have suggested a link between Sleep Disordered Breathing (SDB) and dementia risk. In the present study, we analyzed the relationship between SDB severity, cerebrospinal fluid (CSF) Alzheimer's disease (AD) biomarkers, and the ApoE alleles.
Methods
95 cognitively normal elderly participants were analyzed for SDB severity, CSF measures of phosphorylated-tau (P-Tau), total-tau (T-Tau), and amyloid beta 42 (Aβ42), as well as ApoE allele status.
Findings
In ApoE3+ subjects, significant differences were found between sleep groups for P-Tau (F[df2]=4.3, p=0.017), and T-Tau (F[df2]=3.3, p=0.043). Additionally, among ApoE3+ subjects, the apnea/hypopnea with 4% O2-desaturation index (AHI4%) was positively correlated with P-Tau (r=0.30, p=0.023), T-Tau (r=0.31, p=0.021), and Aβ42 (r=0.31, p=0.021). In ApoE2+ subjects, AHI4% was correlated with lower levels of CSF Aβ42 (r=−0.71, p=0.004), similarly to ApoE4+ subjects where there was also a trend towards lower CSF Aβ42 levels
Interpretation
Our observations suggest that there is an association between SDB and CSF AD- biomarkers in cognitively normal elderly. Existing therapies for SDB such as CPAP could delay the onset to mild cognitive impairment or dementia in normal elderly.
Keywords: Alzheimer's disease, Sleep-Disordered Breathing, Elderly, Biomarkers, CSF, Amyloid beta 42, P-Tau, Cerebral Amyloid Angiopathy, ApoE
1. Introduction
Sleep disordered breathing (SDB) is a continuum of upper airway dysfunction that ranges from significant snoring to frequent episodes of complete airway obstruction. Severity is conventionally graded by the Apnea/Hypopnea Index (AHI). SDB is common, and has an estimated overall prevalence of 10–20% of adults in the US and a particularly high prevalence in the elderly (30–80%) (Roepke and Ancoli-Israel, 2010). Overall, SDB is associated with decreased quality of life and cardio/cerebrovascular morbidity (Selim et al., 2010), but the added burden of SDB in late life is unclear. Several studies have shown an indirect association between excessive daytime sleepiness and the development of cognitive decline, (Jaussent et al., 2012; Foley et al., 2001) but only recently did a polysomnography study of cognitively normal community-dwelling women find direct evidence that women with SDB were more likely to develop mild cognitive impairment (MCI) or dementia at follow-up (Yaffe et al., 2011).
Late onset Alzheimer's disease (LOAD) is the most common form of dementia, and is defined histologically postmortem by the presence of amyloid beta (Aβ) plaques, neurofibrillary tangles (NFT), neuronal loss, and inflammation (Braak and Braak, 1995). The strongest genetic risk factor to LOAD is the Apolipoprotein E4 (ApoE4) allele (Strittmatter et al., 1993), while the ApoE2 allele is protective (Corder et al., 1994) although it may predispose individuals to cerebral amyloid angiopathy and be a risk factor for brain hemorrhage and/or white matter disease (McCarron and Nicoll, 2000; Lemmens R et al., 2007). Prior work has shown that: a) SDB and LOAD share a number of risk factors, including vascular disease (De La Torre, 2010); b) there is an increased prevalence of SDB in LOAD patients (Gehrman et al., 2003); and, c) SDB and the ApoE4 allele may have additive effects (O'Hara et al., 2005; Spira et al., 2008). Therapeutically, it has been shown that treatment of SDB improves sleep patterns in LOAD patients, and has a positive effect on cognition (Ancoli-Israel et al., 2008). Additionally, the treatment of LOAD with donepezil improves SDB, possibly due to the fact that donepezil improves oxygen saturation (Moraes et al., 2008). However, research on the relationship between SDB and LOAD has been hampered by the fact that a definitive diagnosis of LOAD is only possible after autopsy. There is also disagreement on the type and level of respiratory abnormality that must be used as a “cut-off” for significant SDB in the elderly.
Decreases in cerebrospinal fluid (CSF) levels of Aβ42, as well as increases in phosphorylated-tau (P-Tau) and total-tau (T-Tau), have all proven to be useful in the diagnosis of LOAD. Moreover, recent studies show that alterations of these biomarkers may reflect LOAD pathology years prior to cognitive symptoms in older adults, and thus are useful in predicting future decline (Fagan et al., 2007). This point in the disease process has been termed preclinical AD (Sperling et al., 2011) and provides a critical stage for potential interventions as tissue damage is presumably mild.
In the present study, we hypothesized that: 1) cognitively normal elderly subjects with SDB would show greater CSF biomarker evidence for preclinical AD vs. non-SDB controls; and 2) these findings would be exacerbated in ApoE4 carriers with SDB.
2. Methods
2.1 Subject recruitment
Ninety five cognitively normal elderly participants were recruited at NYU Center for Brain Health (CBH) from active NIH supported longitudinal studies of normal aging and CSF AD-biomarkers that have been ongoing between 2009 and 2013. The subjects agreed to undergo additional home-monitoring for SDB for the present study. Subjects had been recruited from multiple community sources including random sampling using voter registration records. Individuals with medical conditions or history of significant conditions that may affect brain structure or function, such as stroke, uncontrolled diabetes, traumatic brain injury, any neurodegenerative diseases, depression, and MRI evidence of intracranial mass or infarcts, were excluded. All subjects signed a separate IRB approved consent form and participated in a sleep study that included a detailed sleep interview, the Epworth Sleepiness Scale (ESS), and home-monitoring of SDB (read below). Sleep complaints were not part of the inclusion or exclusion criteria of any of the NIH studies that the subjects were recruited from, nor were subjects referred to the AD studies from the NYU Sleep Disorders Clinic.
2.2 Clinical and diagnostic evaluation
Subjects received a standardized diagnostic assessment that included medical, psychiatric, and neurological evaluations. The selected subjects had no history of medical conditions known to affect brain structure or function and were not on active treatment for SDB. Enrolled subjects were 68.0±7.6 years of age (range: 53.0–87.5), had a Clinical Dementia Rating (CDR) of 0, and a Geriatric Depression Scale score ≤5. Eligibility requirements for the present study included having had CSF collected from lumbar puncture and a diagnostic structural MRI scan completed within three years of the sleep examination. Groups were categorized according to widely used cut-off values for SDB: normal (NL) (AHI4%<5), mild SDB (AHI4%≥5 and <15), and moderate-severe SDB (AHI4% ≥15) irrespective of reported associated consequences of SDB such as cardiovascular disease, hypertension or complaints of sleepiness. ApoE genotype was determined using standard polymerase chain reaction procedures.
2.3 Cognitive evaluation
All subjects were administered a standard neuropsychological test battery which has published norm values (De Santi et al., 2008). The measures include subtests of the Guild Memory Scale: verbal paired associates (initial: PRDI, and delayed: PRDD), and immediate (PARI), and delayed paragraph recall subtest (PARD) to measure declarative memory. Subtests of the Wechsler Intelligence Scale Revised (WAIS-R) were used to assess working memory (digits forward: WAISDIG-F, and backward: WAISDIG-B), and attention (digit symbol substitution test: DSST). The Mini Mental State Examination (MMSE) was also included.
2.4 Cerebrospinal fluid
Lumbar punctures were performed between 11:00 a.m. and 01:00 p.m. using a 25-gauge needle guided by fluoroscopy. All CSF samples were kept on ice until centrifuged for 10 minutes at 1500g at 4°C. Samples were aliquoted to 0.25 mL polypropylene tubes and stored at −80°C until assayed. CSF P-Tau (pg/mL), T-Tau (pg/mL), and Aβ42 (pg/mL) were blindly analyzed in batch mode using enzyme-linked immunosorbent assay (ELISA) (Blennow and Zetterberg, 2013).
2.5 Home-monitoring of SDB
Home-monitoring of SDB was completed using an ARES™ Unicorder during a two night period. The ARES™ Unicorder measures oxygen saturation (SpO2) and pulse rate from reflectance forehead oximetry, air flow from a nasal cannula and pressure transducer, head movement from actigraphy, and head position from accelerometers. The variables collected included: 1) AHI4%: defined as the sum of all apneas (>90% reduction in airflow for >10 seconds) and all hypopneas (>30% reduction in airflow) associated with >4% O2 desaturation, divided by the total time where both flow and oximetry signals were valid; 2) AHIall: defined as the sum of all apneas and all hypopneas identified divided by the total time where there was a valid flow signal; and 3) mean SpO2 saturation during the night. Both these AHI indices have been compared to the recommended definitions of AHI based on full in laboratory polysomnography that included EEG measures of sleep (Ayappa et al., 2008) and show good comparability.
2.6 Statistical analysis
Demographic measures between SDB groups were examined using ANOVA with post hoc Tukey tests for continuous variables and Pearson χ2 analysis for categorical variables. Regression-based z-scores corrected for age, sex, and education, derived from our normative sample (De Santi et al., 2008) were used for comparisons of cognitive measures. To test whether normal elderly subjects with SDB would show positive CSF biomarker evidence for preclinical AD, analyses of covariance (ANCOVA) controlling for BMI were used. Additionally, linear regression and correlation analyses were used to assess for the relations between continuous dependent (CSF AD-biomarkers) and explanatory variables (AHI4% and AHIall), as well as to obtain correlation coefficients. Age, BMI, CSF internal batch (for Aβ42 analyses), time interval between sleep study and lumbar puncture, and ApoE4 status were included in the models using backward elimination principle to exclude non-significant (p>0.05) factors. For right skewed data we applied log transformations to make the data distributed symmetrically. To test if ApoE4 or ApoE2 carriers had exacerbated associations between SDB and CSF biomarkers, the same statistical approaches were used for 3 subgroups of subjects: ApoE2+ (ApoE 2/2, 2/3 and 2/4 carriers), ApoE3+ (ApoE 3/3 carriers), and ApoE4+ (ApoE 2/4, 3/4 and 4/4 carriers). Four ApoE 2/4 subjects were shared between the ApoE2+ and ApoE4+ groups.. Moreover, an interaction term between AHIs and ApoE group (ApoE3+ vs. ApoE4+) was included to test if the associations between CSF biomarkers and AHIs were different between ApoE defined groups. Statistical significance was set at p<0.05. Analyses were done with SPSS 20.0 (Chicago, IL) and SAS v9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1 Participant characteristics
Demographic characteristics of the subjects are shown in Table 1. Among the 95 participants, 25 were considered free of SDB (AHI4%<5) and were included as normal controls (NL), 51 had mild SDB (AHI4% 5–14.99), and 19 had moderate to severe SDB (AHI4%>15). Both SDB positive groups had significantly higher mean BMI than controls (F[df2]=4.8; p<0.011) (see Table 1), but overall, it was not an overweight group (mean BMI of 25.8±5.4 and only 12 subjects with a BMI>30). There were no differences across NL and SDB groups for age, gender, years of education, hypertension, diabetes, cardiovascular disease or thyroid disease (see Table 1). Raw values with sleep characteristics of the three groups are shown in Table 1. Of note, daytime somnolence (ESS) was remarkably low in most subjects including the SDB groups (mean value: 5.6±4.0), with only 12 subjects with an ESS>10.
Table 1.
Patient sociodemographic, clinical, and sleep characteristics.
| SDB Clinical Groups | Normal (n=25) | SDB Mild (n=51) | SDB Moderate/Severe (n=19) |
|---|---|---|---|
| Age (mean±SD) | 65.3±8.2 | 67.8±7.0 | 70.1±8.8 |
| Female (%) | 68.0% | 58.8% | 57.9% |
| BMI (mean±SD) | 24.2±3.9 | 25.5±3.6† | 28.9±9.0** |
| Years of education (mean±SD) | 16.2±2.0 | 17.2±1.7 | 16.3±2.4 |
| Hypertension (%) | 24.0 | 29.4 | 31.6 |
| Cardiovascular disease (%) | 8.0 | 9.8 | 15.8 |
| Diabetes (%) | 8.0 | 7.8 | 5.3 |
| Thyroid disease (%) | 12.0 | 17.6 | 21.1 |
| ApoE4+ (%) | 28.0 | 31.4 | 31.6 |
| ApoE3+ (%) | 52.0 | 62.7 | 57.9 |
| Epworth Sleepiness Scale (mean±SD) | 5.5±4.2 | 5.4±3.6 | 6.4±4.7 |
| AHI4% (mean±SD) | 2.3±1.2 | 8.3±2.7*** | 30.7±14.5*** |
| AHIall (mean±SD) | 11.0±3.8 | 22.6±6.5*** | 43.5±17.9*** |
| Mean nocturnal SpO2 (mean±SD) | 95.8±0.7 | 95.1±1.1* | 93.9±1.5*** |
SDB: Sleep Disordered Breathing; BMI: Body Mass Index; AHI4%: Apnea/Hypopnea Index with hypopneas restricted to respiratory events associated with 4% desaturation; AHIall: Apnea/Hypopnea Index irrespective of desaturation; SpO2: mean oxygen saturation measured by a forehead reflectance oximeter.
p<0.05 comparing with SDB moderate/severe group,
p<0.05,
p<0.025,
p<0.01 comparing with normal group.
3.2 Cognitive evaluation
Cognitive characteristics (mean±SD) of the subjects are shown in Table 2. There were no statistically significant differences across NL and SDB groups on any of the tests administered (see Table 2).
Table 2.
Patient neuropsychological characteristics.
| SDB Clinical Groups | Normal (n=25) | SDB Mild (n=51) | SDB Moderate/Severe (n=19) |
|---|---|---|---|
| MMSE (raw) | 28.8±1.4 | 29.2±1.2 | 29.2±1.2 |
| PARI (z-scores) | −0.06±1.0 | 0.06±1.0 | 0.03±0.8 |
| PARD (z-scores) | −0.0±1.15 | −0.04±1.01 | 0.2±1.0 |
| PRDI (z-scores) | 0.5±1.4 | 0.6±1.3 | 0.8±1.2 |
| PRDD (z-scores) | 0.6±1.3 | 0.6±1.3 | 0.9±1.5 |
| WAISDIG-F (z-scores) | −0.07±1.1 | −0.04±1.4 | −0.3±0.9 |
| WAISDIG-B (z-scores) | −0.2±1.2 | −0.3±1.2 | −0.3±0.9 |
| DSST (z-scores) | 0.03±1.1 | −0.02±1.0 | 0.1±1.1 |
MMSE: Mini Mental State Examination; PARI: initial immediate recall of orally presented paragraphs; PARD: delayed immediate recall of orally presented paragraphs; PRDI: verbal paired associates initial; PRDD: verbal paired associates delayed; WAISDIG-F: WAIS test digits forward; WAISDIG-B: WAIS test digits backward; DSST: digit symbol substitution test.
3.3 Cerebrospinal fluid (CSF)
The mean time interval between the lumbar puncture and the home monitoring of SDB was 0.8±1.0 years. Summary statistics of CSF levels of Aβ42, P-Tau, and T-Tau are shown in Table 3. There were not statistical differences among the three sleep groups, even after controlling for BMI (data not shown). Moreover, no significant linear associations or correlations were found between AHI4% or AHIall, and AD-biomarkers (see Figures 1–3). Age, BMI, time interval and ApoE status did not improve the linear regression models.
Table 3.
Mean raw and normalized cerebrospinal fluid (CSF) levels of Aβ42, P-Tau, and T-Tau in all subjects.
| SDB Clinical Groups | Normal (n=25) | SDB Mild (n=51) | SDB Moderate/Severe (n=19) | |
|---|---|---|---|---|
| CSF analytes raw values | Aβ42 | 607.1±209.4 | 629.6±252.8 | 619.4±252.3 |
| P-Tau | 39.3±14.6 | 44.5±17.3 | 46.4±18.6 | |
| T-Tau | 250.6±115.7 | 290.0±145.3 | 289.1±130.0 | |
| CSF analytes normalized values † | Ln(P-Tau) | 3.6±0.4 | 3.7±0.4 | 3.7±0.4 |
| Ln(T-Tau) | 5.4±0.4 | 5.5±0.5 | 5.5±0.5 | |
SDB: Sleep-Disordered Breathing.
Comparisons of CSF analytes were only performed for normally distributed and normalized (logarithmic transformed) values.
Figure 1.
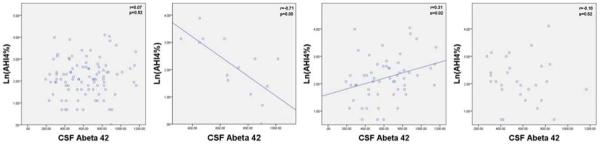
Graphs of AHI4% and CSF Aβ42 for all subjects (n=95), ApoE 2+ subjects (n=14), ApoE3+ subjects (n=56), and ApoE4+ subjects (n=29).
Y Axis: Natural Log of AHI4%; X Axis: CSF levels of Aβ42.
Within ApoE allele groups, ApoE2+ subjects showed a significant difference between sleep groups for Aβ42 (F[2,11]=13.3, p=0.001) and no significant differences between sleep groups for P-Tau and T-Tau (see Table 4). Ln(AHI4%) was significantly negatively correlated with Aβ42 (r=−0.71, p=0.004) (see Figure 1), adjusting for BMI further improved the correlation (r=−0.74, p=0.004). Ln(AHIall) was negatively correlated with Aβ42 (r=−0.66, p=0.010). Among the ApoE2+ subjects no statistically significant correlation was found between ln(AHI4%) or ln(AHIall) and ln(P-Tau) or ln(T-Tau). These findings in the ApoE2+ group were not driven by the inclusion of the four ApoE 2/4 subjects. Excluding ApoE 2/4 subjects, a significant difference was still found in ApoE2+ between sleep groups for Aβ42 (F[2,7]=5.0, p=0.045), Ln(AHI4%) was significantly correlated with Aβ42 (r=−0.64, p=0.047), and adjusting for BMI further improved the correlation (r=−0.71, p=0.034).
Table 4.
Mean raw and normalized cerebrospinal fluid (CSF) levels of Aβ42, P-Tau, and T-Tau in ApoE2+ subjects.
| SDB Clinical Groups ApoE2+ | Normal (n=5) | SDB Mild (n=4) | SDB Moderate/Severe (n=5) | |
|---|---|---|---|---|
| CSF analytes raw values | Aβ42 | 842.4±113.5 | 811.75±158.4† | 465.2±111.8*** |
| P-Tau | 48.4±11.6 | 47.75±21.6 | 46.4±13.0 | |
| T-Tau | 340.7±146.5 | 310.0±169.6 | 328.3±111.2 | |
| CSF analytes normalized values † | Ln(P-Tau) | 3.9±0.2 | 3.8±0.5 | 3.8±0.3 |
| Ln(T-Tau) | 5.8±0.5 | 5.6±0.6 | 5.7±0.3 | |
SDB: Sleep-Disordered Breathing.
Comparisons of CSF analytes were only performed for normally distributed and normalized (logarithmic transformed) values.
p<0.01 comparing with SDB moderate/severe group,
p<0.01 comparing with normal group.
ApoE3+ subjects showed significant differences between sleep groups for ln(P-Tau) (F[2,53]=4.3, p=0.017), ln(T-Tau) (F[2,53]=3.6, p=0.033), and a non-significant trend in Aβ42 (F[2,53]=2.0, p=0.14) (see Table 5). Ln(AHI4%) was positively correlated with ln(P-Tau) (r=0.30,p=0.023), ln(T-Tau) (r=0.31, p=0.021), and Aβ42 (r=0.31, p=0.021) (see Figures 1–3). Adjusting for BMI further improved the correlation between ln(AHI4%) and CSF Aβ42 levels (r=0.39, p=0.003). No statistically significant correlations were found between AHIall and the AD-biomarkers among ApoE3+ subjects.
Table 5.
Mean raw and normalized cerebrospinal fluid (CSF) levels of Aβ42, P-Tau, and T-Tau in ApoE3+ subjects.
| SDB Clinical Groups ApoE3+ | Normal (n=13) | SDB Mild (n=32) | SDB Moderate/Severe (n=11) | |
|---|---|---|---|---|
| CSF analytes raw values | Aβ42 | 505.6±182.8 | 637.1±257.6 | 696.4±273.4 |
| P-Tau | 32.0±11.2 | 45.3±18.7 | 51.0±20.6 | |
| T-Tau | 189.7±67.5 | 296.1±142.0 | 303.7±141.4 | |
| CSF analytes normalized values † | Ln(P-Tau) | 3.4±0.4 | 3.7±0.4* | 3.8±0.5* |
| Ln(T-Tau) | 5.2±0.4 | 5.6±0.5* | 5.6±0.6 | |
SDB: Sleep-Disordered Breathing.
Comparisons of CSF analytes were only performed for normally distributed and normalized (logarithmic transformed) values, with
p<0.05 for ANCOVA analysis adjusting for BMI comparing with normal reference group.
In ApoE4+ subjects there were no differences between sleep groups for P-Tau, T-Tau, and Aβ42 (see Table 6). No significant correlation was found between ln(AHI4%) and any CSF analyte. Interestingly, the presence of the ApoE4 allele seemed to decrease CSF levels of Aβ42 depending on the severity of AHI4% similarly to ApoE2 carriers, instead of increasing Aβ42 levels as shown in ApoE3+ subjects. AHIall was not a significant predictor of any AD-biomarkers among ApoE4+ subjects. Regression analysis of ln(AHI4%) and AD biomarkers in both ApoE3+ and ApoE4+, including a 2-way interaction between each AD biomarker and ApoE group showed that the associations between ln(AHI4%) and AD-biomarkers significantly differed between ApoE3 vs. ApoE4 groups for ln(P-Tau) (F[1,81]=4.1, p=0.045), T-Tau (F[1,81]=4.4, p=0.039), and there was a trend for Aβ42 (F[1,81]=2.8, p=0.099).
Table 6.
Mean raw and normalized cerebrospinal fluid (CSF) levels of Aβ42, P-Tau, and T-Tau in ApoE4+ subjects.
| SDB Clinical Groups ApoE4+ | Normal (n=7) | SDB Mild (n=16) | SDB Moderate/Severe (n=6) | |
|---|---|---|---|---|
| CSF analytes raw values | Aβ42 | 627.7±176.2 | 570.3±241.0 | 504.0±212.1 |
| P-Tau | 46.5±16.4 | 40.7±14.0 | 41.5±16.6 | |
| T-Tau | 299.4±111.9 | 264.6±152.5 | 282.2±137.1 | |
| CSF analytes normalized values † | Ln(P-Tau) | 3.8±0.3 | 3.6±0.4 | 3.6±0.4 |
| Ln(T-Tau) | 5.6±0.3 | 5.4±0.5 | 5.5±0.5 | |
SDB: Sleep-Disordered Breathing. Comparisons of CSF analytes were only performed for normally distributed and normalized (logarithmic transformed) values.
4. Discussion
Sleep disordered breathing was common and affected 74% of our cognitively normal, elderly, community cohort. Severity was generally mild (group mean AHI4% 12.3±14.1, range 0–60) and subjects had few complaints of sleepiness. When groups were stratified by ApoE allele phenotypes, we observed unexpected positive and negative interactions between SDB severity and CSF AD-biomarkers.
In ApoE3+ subjects, AHI4% but not AHIall was associated with increases in P-Tau, T-Tau, and Aβ42. AHIall reports on respiratory events during sleep as well, but unlike the AHI4%, it additionally records respiratory-effort related arousals that disrupt sleep without significant oxygen desaturations, indicating that intermittent hypoxia and not sleep fragmentation is more likely related to these biomarker increases in the ApoE3+ group. There is evidence of increased levels of Aβ42 in cell cultures and transgenic AD mice after exposure to intermittent hypoxia (Shiota et al., 2013), activation of tau-phosphorylation after chronic hypoxia in transgenic mice (Gao et al., 2013), CSF and serum T-Tau increases after cardiac arrest in humans (Randall et al., 2013), as well as P-Tau increases in hypertensive patients with reductions in blood pressure possibly reflecting hypoperfusion (Glodzik et al., 2014), but to our knowledge, this would be the first report of an association between intermittent hypoxia and increases in CSF T-Tau, P-Tau and Aβ42 in ApoE3+ cognitively normal elderly. It is noteworthy that in the ApoE3+ subjects, we did not find evidence for an increased amyloid burden (decreases in CSF Aβ42) associated with SDB despite finding elevations in CSF Aβ42. Total CSF Aβ42 concentrations stay stable or increase slightly in midlife in healthy ApoE3+ adults, whereas in ApoE4+ subjects there is a decline in CSF Aβ42 concentration beginning in young adulthood, followed by marked acceleration of this decline in midlife (Peskind et al., 2006). In agreement with these findings, some authors have described a model in which brain CSF Aβ42 concentrations rise with age in presymptomatic disease, to a “tipping point” at the onset of fibrillar Aβ deposition, followed by CSF Aβ42 decreases (Bayer-Carter et al., 2011). In this model, recently verified in familial AD mutation carriers (Reiman et al., 2012), any condition that increases levels of Aβ42 in the early presymptomatic stages of the disease would increase the risk for developing LOAD, and our findings could be interpreted as early Aβ42 increases secondary to intermittent hypoxia before the consequential decreases in Aβ42 that follow amyloid plaque formation.
In ApoE4+ subjects, severity of SDB trended towards lower CSF Aβ42 levels although there were no significant SDB group differences for Aβ42, T-Tau or P-Tau, but this could be due to different stages of preclinical disease in this group. Normal breathing (AHI4%<5) ApoE4+ subjects had significantly higher P-Tau levels than their ApoE3+ normal breathing counterparts (F[df1]=5.8, p=0.027). These results suggest that some cognitively normal ApoE4+ subjects in our dataset might be more advanced in the preclinical staging of LOAD (Sperling et al., 2011) than the ApoE3+ subjects, and that the interactions between intermittent hypoxia and AD-biomarkers are detectable only in early stages of brain damage. This would also be in agreement with existing literature showing that ApoE4+ subjects have systematically been found to present with lower concentrations of Aβ42 and higher T-Tau and P-Tau than ApoE3+ at any cognitively defined disease stage. In other words, the presence of ApoE4 carriers disproportionately changes the test performances of CSF AD-biomarkers and the ability to distinguish between the different cognitive conditions such as normal or MCI (Leoni, 2011) (or, in our case, between normal and SDB groups). The question of whether the presence of intermittent hypoxia is related to CSF Aβ42 changes or a faster progression of the disease in ApoE4+ subjects will need to be tested in longitudinal studies with larger sample sizes or in younger cohorts. In ApoE2 subjects, the finding of a strong negative correlation between SDB and CSF Aβ42 levels was unexpected, but in agreement with neuropathological reports of similar levels of cerebral amyloid angiopathy (CAA) in ApoE2 and ApoE4 carriers with accumulation of Aβ in parenchymal and meningeal blood vessels in ApoE2+. (Nelson et al., 2013) and presence of decreased CSF Aβ42 levels in subjects with CAA (Verbeek et al., 2009; Renard et al., 2012).
Alternatively, a recent study in mice reported a 60% increase in interstitial space fluid (ISF) that occurs during slow wave sleep, modulated by adrenergic signaling. The authors interpreted this as allowing CSF to expand into the brain tissue and clear Aβ42, P-Tau and other waste byproducts of cellular activity during sleep through passive transport (Xie et al., 2013). Using this model, increased sympathetic tone stemming from intermittent hypoxia would disrupt sleep and affect the clearance of brain metabolic byproducts increasing ISF levels of Aβ42. A decreased clearance of Aβ from the ISF could lead to damage and loss of synapses and to progressive accumulation of extracellular Aβ42. In the presence of cortical amyloid plaques (ApoE4+) or CAA (ApoE2+), trapping of Aβ42 would lead to selective reduction in CSF levels of this protein, while it would translate into increases in those subjects without amyloid deposition (ApoE3+). Other studies on Aβ42 dynamics have described the presence of an Aβ circadian pattern in CSF (Huang et al., 2012) and plasma (Huang et al., 2012b). However, it is unclear in the human if this rhythm is regulated by the sleep-wake cycle and mediated by active transport, and how this could be affected by the presence of SDB or the different ApoE allele phenotypes.
Our observations are partially consistent with our hypothesis that there is an association between SDB and preclinical-AD in cognitively normal elderly, although the causal relationships and directionality of these associations cannot be inferred from our data. It may be that SDB increases the risk for preclinical-AD but it is also possible that existing preclinical-AD brain lesions increase the risk for SDB. The first option is the most attractive, and would imply that existing therapies for SDB such as CPAP could delay the onset to MCI or dementia in elderly with SDB. The high prevalence of mild SDB in cognitively normal elderly (74% in our sample) adds to the importance of this possibility.
Strengths of our study include that our community residing subjects were not recruited for the study based on sleep complaints, and thus should have been free of selection biases potentially affecting sleep-clinic based cohorts. We also utilized a state-of-the-art method for home-monitoring of SDB, and standardized CSF biomarkers. One potential weakness of the study is the number of variables that we analyzed, given only a modest number of subjects and the small number of ApoE2 and ApoE4 carriers. For this reason, these findings need to be confirmed in larger datasets.
5. Conclusion
In summary, this study is the first study to document that SDB in ApoE3+ and ApoE2+ normal elderly is associated with changes in specific biomarkers of LOAD. The implication of these findings is that we have identified a highly prevalent condition (74% in our sample) that likely either contributes to tau pathology (and possibly hippocampal damage) and amyloid beta deposition or is affected by AD-type damage on the path to cognitive decline. Our data support testing whether clinical interventions aimed at SDB, such as treatment with CPAP, could be implemented during the phase in which tissue damage precedes clinical symptoms and neuronal dysfunction, to mitigate the progression of cognitive impairment.
Figure 2.
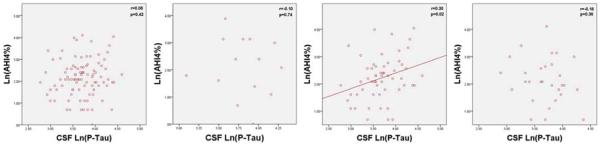
Graphs of AHI4% and Natural Log of CSF P-Tau for all subjects (n=95), ApoE 2+ subjects (n=14), ApoE3+ subjects (n=56), and ApoE4+ subjects (n=29).
Y Axis: Natural Log of AHI4%; X Axis: Natural Log of CSF levels of P-Tau
Figure 3.
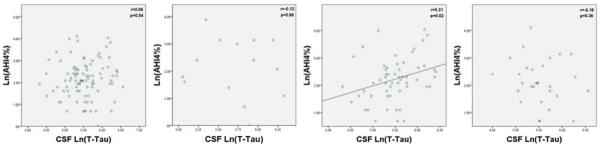
Graphs of AHI4% and Natural Log of CSF T-Tau for all subjects (n=95), ApoE 2+ subjects (n=14), ApoE3+ subjects (n=56), and ApoE4+ subjects (n=29).
Y Axis: Natural Log of AHI4%; X Axis: Natural Log of CSF levels of T-Tau
Acknowledgements
We would like to thank Dr. Pauline McHugh M.D and Schantel Williams for patients' evaluations, John Murray, Catherine Randall, and Ana Rejon for study coordination, Dr. Yi Li and Wai Tsui for MRI review, and Professor. Blas Frangione for his valuable comments on the writing of the research summarized here. Supported by grants from: Foundation for Research in Sleep Disorders, 2012 NYU COE Seed Grant, NIRG 11-205479, NYU ADC Pilot Study Grants, NIH: R01 AG13616, R01 AG022374, R01 AG12101, R01 HL118624-01 and CTSI UL1TR000038.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement RO, JM, TG, AV, AM, OB, ZT, ED, NS, MB, EP, HL, HZ, KB, SL, LM, and LG do not have any conflicts of interest to disclose.
IA has received support for research from the industry in the past 24 months: grants and clinical trials from Fisher & Paykel Healthcare, Ventus Medical. IA holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Fisher & Paykel Healthcare and Advanced Brain Monitoring.
KB has served at Advisory Boards for Innogenetics, Pfizer, and Roche.
DR has received support for research from the industry in the past 24 months: grants and clinical trials from Fisher & Paykel Healthcare, Ventus Medical, and speaking and consulting engagements for Fisher & Paykel Healthcare. DR holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher & Paykel Healthcare, Advanced Brain Monitoring, and Tyco (Health C'Aire).
ML serves on the external advisory board of Roche Pharmaceuticals and holds patents issued through NYU related to the image analysis of PET and MRI scans.
Reference List
- Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–81. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayappa I, Norman RG, Seelall V, Rapoport DM. Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med. 2008;4:26–37. [PMC free article] [PubMed] [Google Scholar]
- Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Walter BK, Tsai E, Plymate SR, Postupna N, Wilkinson CW, Zhang J, Lampe J, Kahn SE, Craft S. Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Arch Neurol. 2011;68:743–52. doi: 10.1001/archneurol.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Zetterberg H. The application of cerebrospinal fluid biomarkers in early diagnosis of Alzheimer disease. Med Clin North Am. 2013;97:369–76. doi: 10.1016/j.mcna.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of alzheimer's disease-related neurofibrillary changes. Neurobiology of Aging. 1995;16:271–8. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–14. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- De La Torre JC. The vascular hypothesis of Alzheimer's disease: bench to bedside and beyond. NeurodegenerDis. 2010;7:116–21. doi: 10.1159/000285520. [DOI] [PubMed] [Google Scholar]
- De Santi S, Pirraglia E, Barr WB, Babb J, Williams S, Rogers K, Glodzik L, Brys M, Mosconi L, Reisberg B, Ferris S, de Leon MJ. Robust and conventional neuropsychological norms: Diagnosis and prediction of age-related cognitive decline. Neuropsychology. 2008;22:469–84. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun M, Morris JC, Holtzman DM. Cerebrospinal Fluid tau/beta-Amyloid42 Ratio as a Prediction of Cognitive Decline in Nondemented Older Adults. Arch Neurol. 2007;64:343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- Gao L, Tian S, Gao H, Xu Y. Hypoxia Increases Abeta-Induced Tau Phosphorylation by Calpain and Promotes Behavioral Consequences in AD Transgenic Mice. J Mol Neurosci. 2013;51:138–47. doi: 10.1007/s12031-013-9966-y. [DOI] [PubMed] [Google Scholar]
- Gehrman PR, Martin JL, Shochat T, Nolan S, Corey-Bloom J, Ancoli-Israel S. Sleep-disordered breathing and agitation in institutionalized adults with Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:426–33. [PubMed] [Google Scholar]
- Glodzik L, Rusinek H, Pirraglia E, McHugh P, Tsui W, Williams S, Cummings M, Li Y, Rich K, Randall C, Mosconi L, Osorio R, Murray J, Zetterberg H, Blennow K, de Leon M. Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiol Aging. 2014;35:64–71. doi: 10.1016/j.neurobiolaging.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, Kasten T, Morris JC, Mintun M, Duntley S, Bateman RJ. Effects of age and amyloid deposition on Aβ dynamics in the human central nervous system. Arch Neurol. 2012;69:51–8. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Potter R, Sigurdson W, Kasten T, Connors R, Morris JC, Benzinger T, Mintun M, Ashwood T, Ferm M, Budd SL, Bateman RJ. β-Amyloid Dynamics in Human Plasma. Arch Neurol. 2012b;69:1591–7. doi: 10.1001/archneurol.2012.18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaussent I, Bouyer J, Ancelin ML, Berr C, Foubert-Samier A, Ritchie K, Ohayon MM, Besset A, Dauvilliers Y. Excessive Sleepiness is Predictive of Cognitive Decline in the Elderly. Sleep. 2012;35:1201–7. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens R, Görner A, Schrooten M, Thijs V. Association of apolipoprotein E epsilon2 with white matter disease but not with microbleeds. Stroke. 2007;38:1185–8. doi: 10.1161/01.STR.0000259816.31370.44. [DOI] [PubMed] [Google Scholar]
- Leoni V. The effect of apolipoprotein E (ApoE) genotype on biomarkers of amyloidogenesis, tau pathology and neurodegeneration in Alzheimer's disease. Clin Chem Lab Med. 2011;49:375–83. doi: 10.1515/CCLM.2011.088. [DOI] [PubMed] [Google Scholar]
- McCarron MO, Nicoll JA. Apolipoprotein E genotype and cerebral amyloid angiopathy-related hemorrhage. Ann N Y Acad Sci. 2000;903:176–9. doi: 10.1111/j.1749-6632.2000.tb06366.x. [DOI] [PubMed] [Google Scholar]
- Moraes W, Poyares D, Sukys-Claudino L, Guilleminault C, Tufik S. Donepezil improves obstructive sleep apnea in Alzheimer disease: a double-blind, placebo-controlled study. Chest. 2008;133:677–83. doi: 10.1378/chest.07-1446. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Pious NM, Jicha GA, Wilcock DM, Fardo DW, Estus S, Rebeck GW. APOE-ε2 and APOE-ε4 correlate with increased amyloid accumulation in cerebral vasculature. J Neuropathol Exp Neurol. 2013;72:708–15. doi: 10.1097/NEN.0b013e31829a25b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara R, Schroder CM, Kraemer HC, Kryla N, Cao C, Miller E, Schatzberg AF, Yesavage JA, Murphy GM., Jr Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurol. 2005;65:642–4. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, Farlow MR, DeCarli C, Raskind MA, Schellenberg GD, Lee VM, Galasko DR. Age and Apolipoprotein E4 Allele Effects on Cerebrospinal Fluid beta-Amyloid 42 in Adults With Normal Cognition. Arch Neurol. 2006;63:936–9. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- Randall J, Mortberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S, Blennow K, Zetterberg H, Wilson DH. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84:351–6. doi: 10.1016/j.resuscitation.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ, Alexander GE, Langbaum JB, Kosik KS, Tariot PN, Lopera F. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–56. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard D, Castelnovo G, Wacongne A, Le Floch A, Thouvenot E, Mas J, Gabelle A, Labauge P, Lehmann S. Interest of CSF biomarker analysis in possible cerebral amyloid angiopathy cases defined by the modified Boston criteria. J Neurol. 2012;259:2429–33. doi: 10.1007/s00415-012-6520-8. [DOI] [PubMed] [Google Scholar]
- Roepke SK, Ancoli-Israel S. Sleep disorders in the elderly. Indian J Med Res. 2010;131:302–10. [PubMed] [Google Scholar]
- Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31:203–20. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Shiota S, Takekawa H, Matsumoto SE, Takeda K, Nurwidya F, Yoshioka Y, Takahashi F, Hattori N, Tabira T, Mochizuki H, Takahashi K. Chronic intermittent hypoxia/reoxygenation facilitate amyloid-β generation in mice. J Alzheimers Dis. 2013;37:325–33. doi: 10.3233/JAD-130419. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, Yaffe K. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–81. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, Kremer BP, Rikkert MO, Van Domburg PH, Skehan ME, Greenberg SM. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009;66:245–9. doi: 10.1002/ana.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]


