Abstract
Background
Cancer stem cells (CSCs) play a key role in the posthepatectomy recurrence of hepatocellular carcinoma (HCC). CD133+ HCC cells exhibit liver CSC–like properties, and CSC differentiation–inducing therapy may lead these cells to lose their self-renewal ability and may induce terminal differentiation, which may in turn allow their malignant potential to be controlled. Because arsenic trioxide (As2O3) increases remission rates and prolongs survival among patients with acute promyelocytic leukemia by inducing differentiation and apoptosis of leukemic cells, we hypothesized that As2O3 might also inhibit HCC recurrence and prolong survival time after hepatectomy by inducing differentiation of HCC CSCs.
Methods
We evaluated the As2O3 induced differentiation of human HCC CSCs and its mechanism in vitro, and we investigated the effects of treatment with As2O3 on recurrence rates and median survival in a mouse xenograft model.
Results
We found that As2O3 induced HCC CSC differentiation by down-regulating the expression of CD133 and some stemness genes, thus inhibiting the cells’ self-renewal ability and tumorigenic capacity without inhibiting their proliferation in vitro. In vivo experiments indicated that As2O3 decreased recurrence rates after radical resection and prolonged survival in a mouse model. As2O3, which shows no apparent toxicity, may induce HCC CSC differentiation by down-regulating the expression of GLI1.
Conclusions
We found that As2O3 induced HCC CSC differentiation, inhibited recurrence, and prolonged survival after hepatectomy by targeting GLI1expression. Our results suggest that the clinical safety and utility of As2O3 should be further evaluated.
Keywords: Arsenic trioxide, Hepatocellular carcinoma, Cancer stem cell, Differentiation, GLI1
Background
Worldwide, hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer death in men, and the seventh most common cancer and the sixth leading cause of cancer death in women [1]. At present, surgical resection is the first choice for treatment of HCC, but the long-term prognosis remains unsatisfactory; the recurrence rate is high, owing to the lack of an effective adjuvant therapy [2]. Fan et al. reported that the presence of circulating liver cancer stem cells (CSCs) is closely related to HCC recurrence and metastasis after resection [3]. Recent studies have suggested that antiangiogenic therapy can stimulate tumor invasion and metastasis [4,5], and the mechanism of this stimulation may also be related to CSCs [6].
CSCs have the ability to self-renew, to differentiate into defined progeny, and, most importantly, to initiate and sustain tumor growth, and they play a key role in tumor progression, metastasis, and recurrence [7]. CSCs also show resistance to chemotherapeutics and radiation [8]. In HCC, CD133+ cells exhibit liver CSC–like properties, such as high clonogenicity, tumorigenicity, and resistance to radiation [9-13]. In addition to CD133, EpCAM [14], CD44 [13] and CD90 [15] have also been used as markers for the identification of HCC CSCs. Tang et al. reported that CD133 expression overlaps extensively with expression of EpCAM and CD44, which suggests that stem cells marked by CD133/EpCAM and possibly CD133/CD44 may have similar characteristics and regulatory mechanisms [11]. Tang et al. also showed that CD90 is expressed at very low levels in HCC cell lines and clinical specimens. Other studies have shown that the presence of CD133+ CSCs in HCC patients after surgery is correlated with early recurrence and poor prognosis [16,17]. Two studies have reported that hepatocyte nuclear factor 4 alpha and bone morphogenetic protein 4 can promote the differentiation of CD133+ HCC stem cells, inhibition of self-renewal, and resistance to chemotherapy [18,19]; and these results suggest that inducing CSC differentiation is a promising approach to the treatment of HCC. However, the use of differentiation-inducing drugs for HCC has not been well explored.
A number of studies have shown that the Hedgehog (HH) signaling pathway plays an important role in CSC self-renewal and inhibition of tumor differentiation in various cancers [20-23]. Targeting HH signaling has been shown to inhibit self-renewal of melanoma CSCs in vitro and to decrease their tumor initiation ability in vivo[24]. In a clinical study, advanced basal cell carcinoma patients were found to benefit from inhibition of HH signaling [25]. In HCC, GLI1 expression is significantly correlated with tumor size and TNM stage; GLI1 expression is high in patients with early recurrence and poorer overall survival [26]. Therefore, GLI1 may be a useful target for the prevention of HCC recurrence after surgery.
Arsenic trioxide (As2O3) induces differentiation and apoptosis of acute promyelocytic leukemia (APL) cells by binding to the PML-RARα oncoprotein [27], and treatment with As2O3 has been shown to increase remission rates and prolong survival in patients [28]. Some studies have shown that As2O3 inhibits the growth and metastasis of HCC by inducing apoptosis [29,30], but a clinical trial showed that treatment with As2O3 is not effective for patients with advanced HCC [31]. There have been no reports of As2O3 preventing HCC recurrence or inducing differentiation of HCC CSCs.
We hypothesized that As2O3 could induce differentiation of CD133+ HCC cells through the HH-GLI pathway, and also investigated whether As2O3 could inhibit HCC recurrence and prolong survival time after hepatectomy in a mouse model.
Results
As2O3 reduced CD133 expression in HCC CSCs but did dot inhibit proliferation at lower dosages
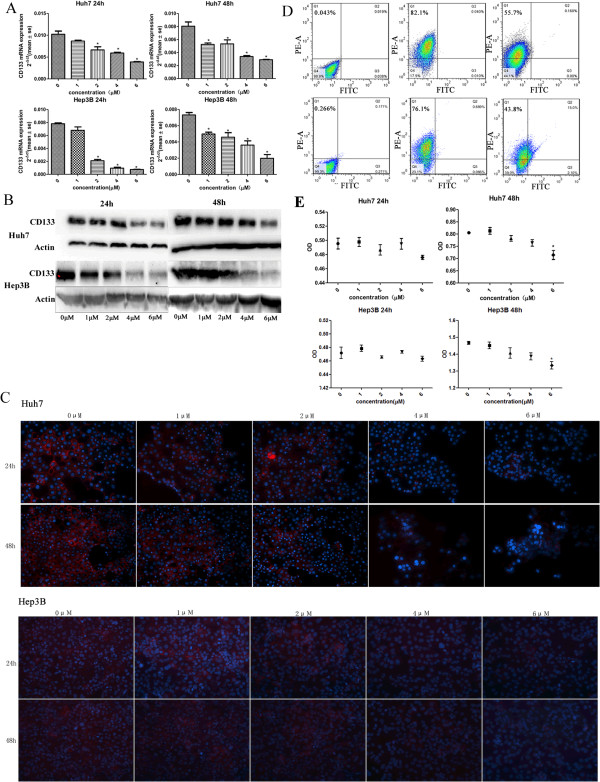
We used unmodified human hepatoma cell lines (Huh7-wt and Hep3B-wt), which have a relatively high percentage of CD133+ cells, to analyze the biologic effects of As2O3 on HCC CSCs. Various dosages of As2O3 (0–6 μM) were used to explore its effects on CSC differentiation. We found that in CD133+ cells, As2O3 significantly reduced expression of CD133 mRNA and protein and that mRNA expression decreased with increasing As2O3 concentration (Figure 1A–C). Because 4 μM As2O3 significantly down-regulated CD133 expression, we used this concentration for subsequent tests. Flow cytometry results indicated that treatment with As2O3 decreased the percentage of CD133+ cells by more than 20% (Figure 1D).
Figure 1.
As2O3 down-regulated CD133 expression in vitro and did not inhibit cell proliferation at lower dosages. (A–C) Treatment of CD133+ HCC cells, with 2 μM As2O3 reduced CD133 mRNA expression after 24 hours and treatment with 1 μM As2O3 did so after 48 hours (A, *P < 0.05) ; treatment with As2O3 also reduced CD133 protein expression (B,C; red, CD133; blue, 4′,6-diamidino-2-phenylindole; magnification, ×200). (D) After treatment with 4 μM As2O3 for 5 days, the percentage of CD133+ Huh7-wt cells decreased from 82.1% to 55.7% (upper panels) and the percentage of CD133+ Hep3B-wt cells decreased from 76.1% to 43.8% (lower panels). (E) In a 48-hour assay, 6 μM As2O3 inhibited proliferation of HCC CSCs, whereas As2O3 at 1–4 μM had little effect on proliferation over the course of 24 or 48 hours (*P < 0.05).
To determine whether As2O3 inhibited proliferation of CD133+ HCC cells, we performed a CCK8 cell proliferation assay, the results of which showed that As2O3 had little effect on CD133+ cell proliferation at 1–4 μM (Figure 1E).
As2O3 down-regulated expression of stemness-associated genes of HCC CSCs
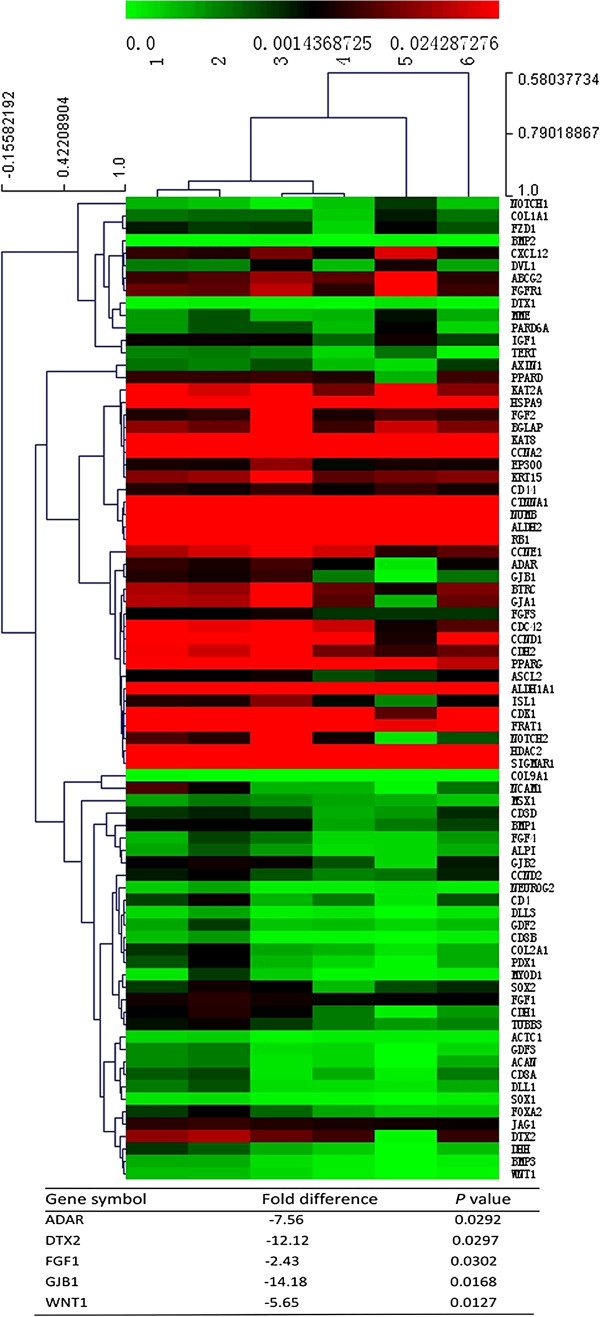
To evaluate the alteration of stemness gene expression during the process by which As2O3 induced differentiation of HCC CSCs, we analyzed stemness gene expression by means of a Human Stem Cell RT2 Profiler PCR Array after the CD133+ Huh7-wt cells had been treated with 4 μM As2O3 for 48 hours. The data showed that reduced expression of CD133 after As2O3 treatment was accompanied by down-regulation of 5 stemness genes that are involved in the maintenance of pluripotency or are richly expressed in human CSCs [13,32-35]: adenosine deaminase, RNA-specific (ADAR); gap junction protein, beta 1, 32 kDa (GJB1); fibroblast growth factor 1 (acidic) (FGF1); deltex homolog 2 (Drosophila) (DTX2); and wingless-type MMTV integration site family, member 1 (WNT1) (Figure 2). These 5 stemness genes were also down-regulated in CD133+ Hep3B-wt cells that had been treated with 4 μM As2O3 for 48 hours, as indicated by real-time polymerase chain reaction (Additional file 1: Table S1).
Figure 2.
As 2 O 3 down-regulated expression of 5 stemness genes in HCC CSCs.
As2O3 inhibited self-renewal and tumorigenic capacity of HCC CSCs
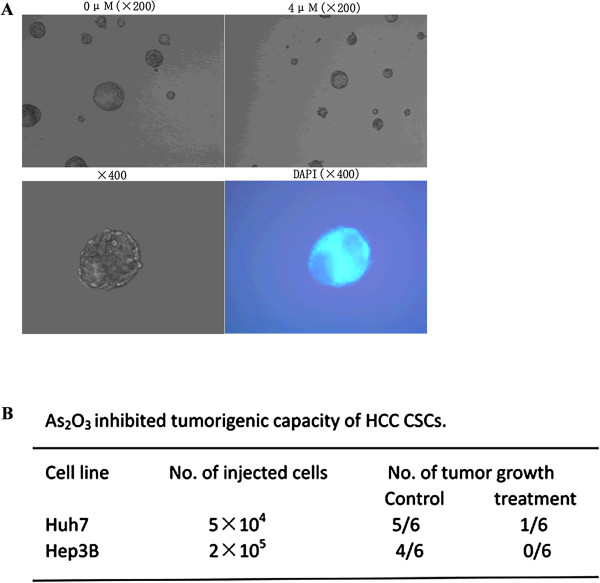
The fundamental properties of CSCs are their ability to self-renew and their ability to differentiate into defined progeny. To evaluate the self-renewal potential of CD133+ cells after treatment with As2O3in vitro, we used a sphere-forming assay. We tested the sphere-formation ability of CD133+ cells incubated with or without As2O3 for 5 days and found that tumor spheres generated from CD133+ cells treated with As2O3 were fewer in number and smaller in size than the spheres generated from untreated cells (Figure 3A).
Figure 3.
As2O3 inhibited self-renewal and decreased the tumorigenic capacity of CD133+ CSCs. (A) Pretreatment of CD133+ Huh7-wt and CD133+ Hep3B-wt cells with 4 μM As2O3 for 5 days inhibited CSC sphere formation. (B) As2O3 also decreased the tumorigenicity of CD133+ cells.
To compare the tumorigenic potential of As2O3-treated and untreated CD133+ HCC cells, we inoculated NOD/SCID mice subcutaneously with 5 × 104 CD133+ Huh7-wt cells or 2 × 105 CD133+ Hep3B-wt cells, and we found that tumor growth was inhibited by As2O3 treatment (Figure 3B).
As2O3 inhibited recurrence after curative resection and improved survival in a mouse model
The incidences of intrahepatic tumor recurrence in the control and As2O3-treated groups were 75% (9/12) and 33.33% (4/12) for the Huh7-GFP cell line and 91.67% (11/12) and 41.67% (5/12) for the Hep3B-GFP cell line, and the differences were statistically significant. No lung metastasis was observed in either group. No animal experienced a weight loss of more than 10%, fever, or anemia during the treatment regimen. We also found there were no significant pathological changes in the heart, spleen, or kidney of the mice in the As2O3 group.
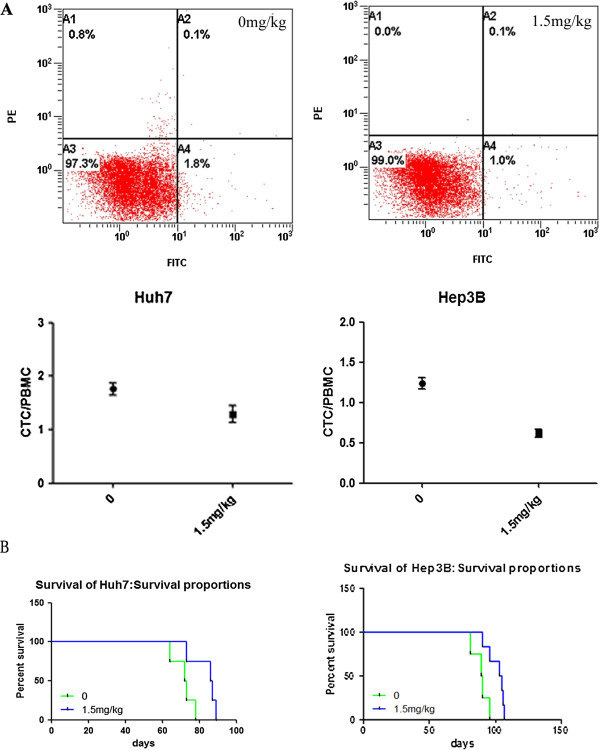
Recurrence after resection is closely related to the presence of circulating tumor cells (CTCs) in patients with HCC [36]. Therefore, we compared the numbers of CTCs in the As2O3 and control groups (Figure 4A) and found that As2O3 treatment significantly decreased the number of CTCs.
Figure 4.
As2O3 decreased the proportion of CTCs relative to peripheral blood mononuclear cells (PBMCs) and prolonged survival. (A) Flow cytometry analysis indicated that As2O3 significantly decreased the proportion of CTCs relative to PBMCs, In the Huh7-GFP cell line, the proportions of CTCs relative to PBMCs were 1.292% ± 0.164% in the As2O3 group and 1.758% ± 0.118% in the control group (P = 0.0305), and in the Hep3B-GFP cell line, the corresponding proportions were 0.619% ± 0.050% and 1.235% ± 0.072% (P < 0.0001). (B) In the Huh7-wt cell line, median survival increased from 72.5 to 86.5 days (P = 0.0344), and in the Hep3B-wt cell line, median survival increased from 89.5 to 104 days (P = 0.015).
In both the Huh7-wt model and the Hep3B-wt model, treatment with As2O3 for 4 weeks significantly prolonged survival (Figure 4B).
As2O3 down-regulated GLI1 expression via the HH signaling pathway
To explore how As2O3 induced differentiation of CD133+ HCC CSCs, we analyzed stem cell signaling–related gene expression by using a Human Stem Cell Signaling RT2 Profiler PCR Array after the CD133+ Huh7-wt cells had been treated with 4 μM As2O3 for 48 hours. We found that As2O3 treatment down-regulated expression of GLI1 and PTCH1 (−10.13 fold, P = 0.0279); (Figure 5). Combined with the results of the Human Stem Cell RT2 Profiler PCR Array, these results indicate that As2O3 down-regulated the expression of GLI1 and its downstream target genes PTCH1 and WNT1, so we hypothesized that As2O3 inhibited the expression of CD133 by down-regulating GLI1. This hypothesis was confirmed by Western blotting analysis in the CD133+ Huh7-wt and CD133+ Hep3B-wt models (Figure 6A). Furthermore, we found that knockdown of GLI1 expression or forced over-expression of GLI1 significantly decreased or increased, respectively, CD133 expression (Figure 6B). Huh7-LV-shGLI1 and Hep3B-LV-shGLI1 cells were treated with 4 μM As2O3 for 48 hours, the expression of CD133 did not decrease significantly (Figure 6C), and this results support the notion that As2O3 may decrease CD133 expression by targeting GLI1.
Figure 5.
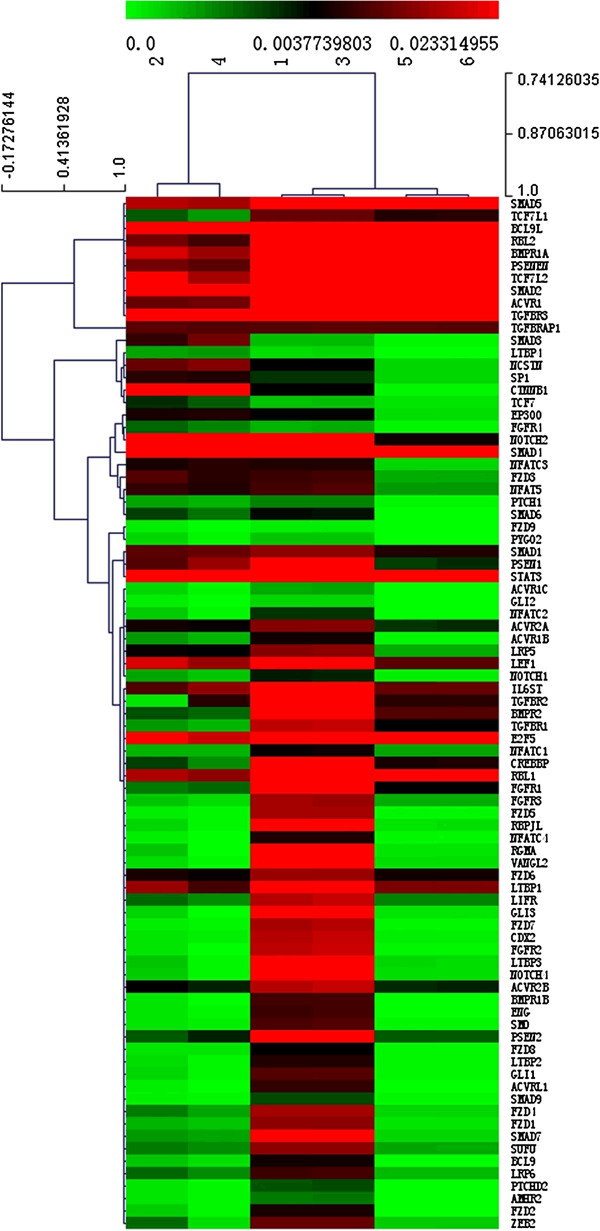
The results of Human Stem Cell Signaling RT 2 Profiler PCR Array analysis indicated that As 2 O 3 down-regulated the expression of GLI1 and PTCH1 in CD133 + Huh7-wt cells.
Figure 6.
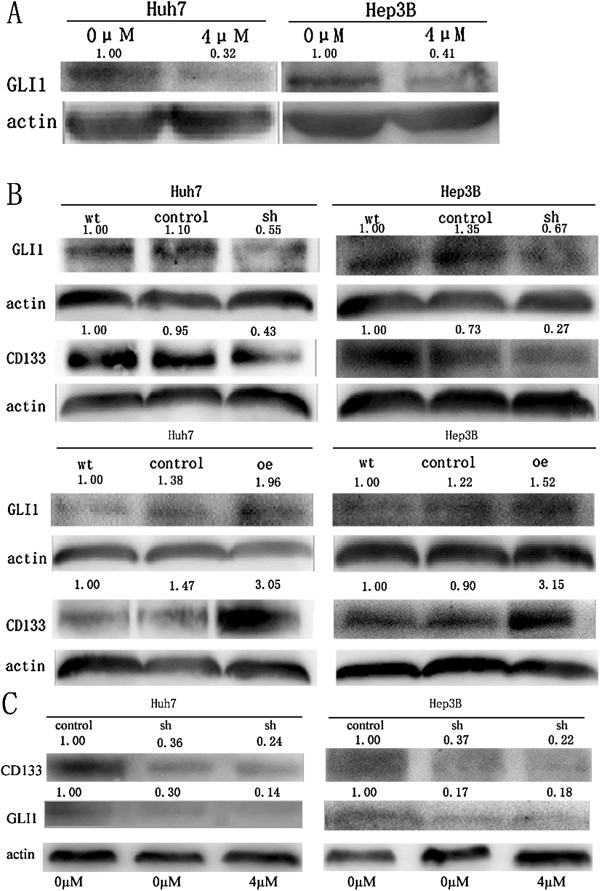
As2O3 down-regulated GLI1 expression via the Hedgehog signaling pathway. (A), As2O3 decreased GLI1 protein expression in CD133+ Huh7-wt and CD133+ Hep3B-wt cells, as indicated by Western blotting analysis. (B), Short hairpin RNA knockdown of GLI1 expression decreased CD133 expression in CD133+ Huh7-wt and CD133+ Hep3B-wt cells; similarly, up-regulation of GLI1 expression increased CD133 expression (wt = CD133+ Huh7-wt and CD133+ Hep3B-wt; control = Huh7-LV-shNon, Hep3B-LV-shNon, and Huh7-LV-oeNon, Hep3B-LV-oeNon; sh = Huh7-LV-shGLI1 and Hep3B-LV-shGLI1; oe = Huh7-LV-oeGLI1 and Hep3B-LV-oeGLI1). (C), Huh7-LV-shGLI1 and Hep3B-LV-shGLI1 cells were treated with 4 μM As2O3 for 48 hours, the expression of CD133 did not decrease significantly.
Discussion
The CSC theory is based on the fact that CSCs exhibit properties similar to those of normal stem cells, such as self-renewal, the ability to produce heterogeneous progeny, and the ability to divide in an unlimited way, giving rise to high tumorigenicity, chemotherapy and radiation resistance, metastasis, and cancer recurrence after therapy [8]. Therefore, CSCs have the potential to be effective targets for preventing relapse after resection, including in HCC. Therapy involving induced differentiation of CSCs could lead to the cells losing their self-renewal ability and could induce terminal differentiation. To date, the best example of the clinical use of differentiation-inducing therapy is treatment of APL with all-trans retinoic acid, which enhances the effects of chemotherapy and significantly improves patient outcome [37]. Targeting induction of CSC differentiation may be a powerful therapy for HCC. CD133+ HCC cells are considered to be CSCs because of their higher tumorigenicity [10] and lower expression of mRNAs for mature hepatocyte markers [9]. As2O3, which is an FDA-approved agent for the treatment of APL, can induce APL cells differentiation. Our results revealed that CD133 expression and the percentage of CD133+ cells were dramatically decreased in both CD133+ Huh7-wt and cd133+ Hep3B-wt cells treated with As2O3. But real-time PCR analysis revealed that after the CSCs were treated with As2O3 for 48 hours, a cluster of hepatocyte marker genes—including phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, cytochrome P450, glutamine synthetase, biliverdin reductase, aldolase B, apolipoprotein c III, apolipoprotein A I, 4-hydroxyphenylpyruvate dioxygenase, and glycogen synthetase 2—were not re-expressed (data not shown). Furthermore, we found that As2O3 treatment decreased the expression of some stemness genes, which are important for the maintenance of CSC self-renewal ability and tumorigenicity [13,38]. We also found that high-dose As2O3 reduced CSC proliferation, whereas low-dose As2O3 had no such effect. CSCs are considered to be more resistant to chemotherapeutic agents than non-CSCs, and consistent with this, low-dose As2O3 has been found to inhibit Huh7-wt cell proliferation [39]. In short, low-dose As2O3 may induce HCC CSCs differentiation into non-CSCs,which is very important for the clinical application of As2O3 because of its toxicity.
It should be possible to treat cancer by differentiation-inducing therapy targeted at CSCs. If CSCs could be induced to differentiate, then their malignant potential could be controlled [34]. We have shown that As2O3 induced HCC CSC differentiation in vitro, and thus we postulate that As2O3 may be useful for inhibiting recurrence of HCC after resection and may prolong survival in vivo. The HCC recurrence rate was decreased by systemic administration of As2O3, and survival time was significantly prolonged. However, in a phase II clinical trial, patients with advanced HCC did not benefit from As2O3 treatment [31], and we believe that the effective treatment of advanced HCC must involve a combination of differentiation-inducing therapy and conventional chemotherapies to eradicate the tumor mass, and this belief is partly supported by the results of Wang et al. [13]. Due to the toxic effects of As2O3 limits its clinical application, we found that low-dosage of As2O3 can induce differentiation of HCC CSCs and inhibit recurrence, so the application of As2O3 to prevent recurrence of HCC patients after surgery may be an effective way. We also found CTCs were decreased by As2O3 treatment. Because the presence of CTCs is associated with metastasis to distant organs and is strongly associated with poor overall survival [40], we suggest that As2O3 could decrease metastasis and prolong survival. This suggestion could be tested with other models.
The HH-GLI pathway is an important mechanism for determining embryonic pattern and regulating cell fate [11]. This pathway has been implicated in several types of tumors [41] and plays an important role in the differentiation of CSCs [20-23]. Differentiation of CSCs by inhibition of the HH-GLI pathway may be a promising therapeutic strategy for human tumors. Currently, all the therapeutics in clinical development that function by inhibiting the HH-GLI pathway are targeted at SMO[41]. However, in some types of tumors, specifically those in which increased GLI expression or activation is induced in a SMO-independent manner, SMO inhibitors are ineffective, and inhibitors that modulate GLI may be useful [42-44]. In HCC, expression of GLI1 mRNA has been reported to adversely affect recurrence and survival of patients with HCC after resection [26], which is consistent with our results, As2O3 may induce CSC differentiation by down-regulating the expression of GLI1, thus inhibiting recurrence and prolonging survival.
Conclusion
In conclusion, As2O3 may induce HCC CSC differentiation, inhibit cancer recurrence after radical resection, and prolong survival as a result of down-regulation of GLI1 expression. In in vivo tests, As2O3 shows no apparent toxicity, but its clinical safety and utility must be evaluated.
Methods
Cell culture and reagents
Unmodified Huh7 and Hep3B cells (Huh7-wt and Hep3B-wt) were obtained from American Type Culture Collection. Cells from both cell lines were transfected with GFP (Huh7-GFP and Hep3B-GFP, respectively) as described previously [45]. Huh7-LV-shGLI1 and Hep3B-LV-shGLI1 cells were obtained by infecting CD133+ Huh7-wt and CD133+ Hep3B-wt with a lentiviral vector encoding short hairpin RNA for GLI1 (sc-37911-V, Santa Cruz Biotechnology, Santa Cruz, CA, USA) to silence its expression. As controls, Huh7-LV-shNon and Hep3B-LV-shNon cells were obtained by infection of CD133+ Huh7-wt and CD133+ Hep3B-wt with a different lentiviral vector (sc-108080, Santa Cruz Biotechnology). Huh7-LV-oeGLI1 and Hep3B-LV-oeGLI cells were obtained by infecting CD133+ Huh7-wt and CD133+ Hep3B-wt with lentiviral vectors that overexpress GLI1 (Genechem, Shanghai, P.R. China). Huh7-LV-oeNon and Hep3B-LV-oeNon cells were also used as controls (Genechem). All cell lines were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum.
As2O3 (SL Pharm, Beijing, P.R. China) was dissolved at a concentration of 0.05 mg/ml in phosphate buffered saline (PBS) for the in vitro study. For the in vivo study, As2O3 was dissolved in normal saline.
Cell isolation by fluorescence-activated cell sorting
Huh7-wt and Hep3B-wt cells were labeled directly with phycoerythrin-conjugated anti-human CD133/1 antibody (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer’s instruction and were sorted by fluorescence-activated cell sorting to obtain CD133+ cell subpopulations; a sorted cell purity exceeding 90% was deemed acceptable for in vitro experiments.
Cell proliferation assay
Cell proliferation was counted by a CCK8 assay (Dojindo, Tokyo, Japan). Three thousand CD133+ cells were seeded in 96-well culture plates. After adhesion for 24 hours, the cells were treated with As2O3 at a final concentration ranging from 1 to 6 μM, and the treated cells were cultured for another 24 or 48 hours. Cells that were not exposed to As2O3 were used as controls. The cell proliferation assay was carried out as previously described [5]. The optical density values were read by an enzyme-linked immunosorbent assay reader at 450 nM.
Real-time polymerase chain reaction analysis
Real-time PCR analysis was carried out as described as elsewhere [5]. The following primers were used for amplification of human genes: CD133 [18], forward 5′- ACATGAAAAGACCTGGGGG-3′, reverse 5′- GATCTGGTGT CCCAGCATG-3′; β-actin, forward 5′-GCTCTGCAGACTTCAGACCA-3′, reverse 5′-GGCCGGACTCATCGTACTCCTGC-3′.
Western blotting assay
The procedure used for Western blotting assay is described elsewhere [5]. Primary antibodies included anti-CD133/1 (Miltenyi Biotec), anti-GLI1 (Santa Cruz Biotechnology), and anti-β-actin (Kangcheng Technology, Shanghai, P.R. China).
Immunofluorescence
To assess the distribution and changes of CD133 in HCC CSCs after treatment with As2O3, we stained CD133+ Huh7-wt and CD133+ Hep3B-wt cells for visualization by means of an immunofluorescence assay. Briefly, the cells were seeded on slides, allowed to adhere for 24 hours, treated with As2O3 at a final concentration ranging from 1 to 6 μM, and then cultured for another 24 or 48 hours. Cells that were not exposed to As2O3 were used as controls. The slides were washed with PBS and fixed with 4% paraformaldehyde. The cells were incubated with primary antibody to CD133/1 (Miltenyi Biotec) overnight at 4°C after being blocked with 5% bovine serum albumin, and then goat anti-mouse tetramethyl rhodamine isothiocyanate-conjugated secondary antibody was added before staining with 4′,6-diamidino-2-phenylindole. Fluorescence images were visualized with an inverted fluorescence microscope (Olympus, Melville, NY, USA). For a negative control, primary antibodies were replaced with PBS.
Flow cytometry analysis
The percentages of CD133+ cells were determined by flow cytometry to evaluate the expression of CD133. Isolated CD133+ HCC cells were incubated with or without As2O3 for 5 days. The cells were trypsinized, washed, and resuspended in PBS. Then the cells were incubated with phycoerythrin-conjugated anti-CD133/1 antibody (Miltenyi Biotec) on ice for 30 min and analyzed by means of flow cytometry (BD Biosciences, USA).
Culture of HCC CSC spheres
The self-renewal capability of HCC cells was evaluated by testing their sphere-formation ability. Specifically isolated CD133+ HCC cells treated with 4 μM As2O3 treatment for 5 days and untreated cells were cultured in a methyl cellulose–based medium in low-adherent 96-well culture plates (Corning, Corning, NY, USA) under serum-free conditions and supplemented with 20 μg/ml insulin, 20 μg/ml epidermal growth factor, and 10 μg/ml basic fibroblast growth factor (RD Systems, Minneapolis, MN, USA) according to a previously published procedure [46]. Single-cell suspensions of 100 CD133+ HCC cells were seeded, and epidermal growth factor, basic fibroblast growth factor, and insulin were added every second day for 2 weeks.
Tumorigenic capacity of HCC CSCs
The tumorigenic capacity of 4 μM As2O3-treated and untreated CD133+ HCC cells for 5 days were analyzed in an NOD/SCID mouse xenograft model. Male NOD/SCID mice (4–6 weeks old) were injected subcutaneously in the lateral flanks with 5 × 104 CD133+ Huh7-wt cells and 2 × 105 CD133+ Hep3B-wt cells. Tumorigenic capacity was evaluated after the cells had been allowed to undergo implantation for 7 weeks.
Animal model
Male BALB/c nu/nu nude mice (Shanghai Institute of Materia Medica, Chinese Academy of Science, Shanghai, P.R. China) were housed in laminar-flow cabinets under specific pathogen-free conditions and used when they weighed approximately 20 g and were 4–6 weeks old. The experimental protocol was approved by the Shanghai Medical Experimental Animal Care Committee. The animal model was established as previously described [47]. Briefly, Huh7-wt, Hep3B-wt, Huh7-GFP, and Hep3B-GFP cells (1 × 107) were subcutaneously inoculated into the right flanks of the nude mice. After 3–4 weeks, a piece of non-necrotic tumor tissue about 2 mm in size was orthotopically implanted into the liver. On the fourteenth day after implantation, the tumor was excised, with the length between incisional margin and tumor edge being ≥ 2 mm.
As2O3 treatment and grouping
The in vivo experiment involved 2 groups: a recurrence rate group, which used the Huh7-GFP and Hep3B-GFP cell lines, and a survival observation group, which used the Huh7-wt and Hep3B-wt cell lines. In the recurrence rate group, treatment was started on the fifth day after resection. The mice were randomly assigned to 2 groups (n = 12 for each group): one group received a daily intraperitoneal dose of 1.5 mg/kg As2O3, and the other group received an equal volume of normal saline (control group). The treatment was continued for 2 weeks, and after another 3 weeks, the mice were anesthetized and orbital blood was obtained. The mice were then sacrificed by cervical dislocation to determine the recurrence rate.
In the survival group, treatment was started on the fifth day after resection. The mice were randomly assigned to 2 groups (n = 8 for each group): one group received a daily intraperitoneal dose of 1.5 mg/kg As2O3, and the other group received an equal volume of normal saline (control group). The treatment was administered for 2 weeks, then stopped for 1 week to allow the mice to recover, and then administered for an additional 2 weeks; then the median survival was determined.
Detection of CTCs
CTCs in the peripheral blood of mice in the recurrence group were counted by flow cytometry, with GFP as a marker. The protocol was carried out as previously reported [15]. The GFP-positive cells were gated with processed blood from a mouse that had not been subjected to the xenograft procedure.
PCR microarray analysis of stemness gene expression and pathway exploration
Total RNA was extracted from isolated As2O3-treated and untreated CD133+ HCC cells and was analyzed by means of a Human Stem Cell RT2 Profiler PCR Array ( http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-405Z.html) and a Human Stem Cell Signaling RT2 Profiler PCR Array ( http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-047Z.html), according to the manufacturer’s instructions.
Statistical analysis
Values for continuous variables are expressed as means ± SDs and were compared by means of the unpaired 2-tailed Student t test, unless otherwise specified, Multiple comparisons of Kaplan-Meier curves were made using the log-rank test. All statistical tests were performed using SPSS for Windows (ver. 120.0, SPSS, Inc). P < 0.05 (2-tailed) was considered to indicate statistical significance.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZKZ designed the study, established the animal model, carried out the Western blotting assays, performed the statistical analysis, and drafted the manuscript. ZQB and ZQB participated in the design of the study, data analysis, and drafting the manuscript. SHC participated in the design of the study and helped to draft the manuscript. AJY, CZT, ZXD, LL, ZYY, BY, and KLQ helped to acquire experimental data. TZY conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Real-time PCR primers for the 5 stemness genes that were down-regulated in CD133+ Hep3B-wt cells after treatment with As2O3 for 48 hours.
Contributor Information
Ke-Zhi Zhang, Email: zkz97@163.com.
Qiang-Bo Zhang, Email: zqb-sd@163.com.
Quan-Bao Zhang, Email: zhangquanbao1979@hotmail.com.
Hui-Chuan Sun, Email: sun.huichuan@zs-hospital.sh.cn.
Jian-Yang Ao, Email: 13777817222@126.com.
Zong-Tao Chai, Email: runout1983@gmail.com.
Xiao-Dong Zhu, Email: zhuxiaodong@gmail.com.
Lu Lu, Email: luluyixin1980@gmail.com.
Yuan-Yuan Zhang, Email: bby570@gmail.com.
Yang Bu, Email: boyang1976@163.com.
Ling-Qun Kong, Email: konglingqun2008@gmail.com.
Zhao-You Tang, Email: zytang88@163.com.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (grant nos. 81101564, 81020108025), the Shanghai Natural Science Fund for Youth Scholars (12ZR1442300), and the National Key Project for Infectious Diseases (2012ZX10002012-004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg. 2011;254:569–576. doi: 10.1097/SLA.0b013e3182300a1d. [DOI] [PubMed] [Google Scholar]
- Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sun HC, Wang WQ, Zhang QB, Zhuang PY, Xiong YQ, Zhu XD, Xu HX, Kong LQ, Wu WZ, Wang L, Song TQ, Li Q, Tang ZY. Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology. 2012;143:1641–1649. doi: 10.1053/j.gastro.2012.08.032. e1645. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Arii S. Molecular targeted therapy for hepatocellular carcinoma in the current and potential next strategies. J Gastroenterol. 2011;46:289–296. doi: 10.1007/s00535-011-0387-9. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Tong CM, Ma S, Guan XY. Biology of hepatic cancer stem cells. J Gastroenterol Hepatol. 2011;26:1229–1237. doi: 10.1111/j.1440-1746.2011.06762.x. [DOI] [PubMed] [Google Scholar]
- Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer J int du cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, Ng IO, Man K, To KF, Lai PB, Lo CM, Guan XY, Chan KW. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55:807–820. doi: 10.1002/hep.24739. [DOI] [PubMed] [Google Scholar]
- Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, Sung PS, Song MJ, Lee BC, Hwang D, Yoon SK. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129–137. doi: 10.1016/j.canlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Warrell RP Jr, Frankel SR, Miller WH Jr, Scheinberg DA, Itri LM, Hittelman WN, Vyas R, Andreeff M, Tafuri A, Jakubowski A, Gabrilove J, Gordon MS, Dmitrovsky E. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid) N Engl J Med. 1991;324:1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Li H, Tao K, Li R, Song Z, Zhao Q, Zhang F, Dou K. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62:1212–1218. doi: 10.1111/j.1742-1241.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- Giampieri R, Scartozzi M, Loretelli C, Piva F, Mandolesi A, Lezoche G, Del Prete M, Bittoni A, Faloppi L, Bianconi M, Cecchini L, Guerrieri M, Bearzi I, Cascinu S. Cancer stem cell gene profile as predictor of relapse in high risk stage II and stage III, radically resected colon cancer patients. PloS one. 2013;8:e72843. doi: 10.1371/journal.pone.0072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Lin Y, Zhang X, Chen YX, Zeng X, Yue HY, Hou JL, Deng X, Zhang JP, Han ZG, Xie WF. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4alpha gene. Hepatology. 2008;48:1528–1539. doi: 10.1002/hep.22510. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sun H, Zhao F, Lu P, Ge C, Li H, Hou H, Yan M, Chen T, Jiang G, Xie H, Cui Y, Huang X, Fan J, Yao M, Li J. BMP4 administration induces differentiation of CD133+ hepatic cancer stem cells, blocking their contributions to hepatocellular carcinoma. Cancer Res. 2012;72:4276–4285. doi: 10.1158/0008-5472.CAN-12-1013. [DOI] [PubMed] [Google Scholar]
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz I, Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Yue W, Wei B, Wang N, Li T, Guan L, Shi S, Zeng Q, Pei X, Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PloS one. 2011;6:e17687. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang Y, Mao JH, Hsieh D, Kim IJ, Hu LM, Xu Z, Long H, Jablons DM, You L. Inhibition of CK2alpha down-regulates Hedgehog/Gli signaling leading to a reduction of a stem-like side population in human lung cancer cells. PloS one. 2012;7:e38996. doi: 10.1371/journal.pone.0038996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Rodova M, Roy SK, Sharma J, Singh KP, Srivastava RK, Shankar S. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013;330:22–32. doi: 10.1016/j.canlet.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini R, Vinci MC, Pandolfi S, Penachioni JY, Montagnani V, Olivito B, Gattai R, Pimpinelli N, Gerlini G, Borgognoni L, Stecca B. Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells. 1808–1818;2012:30. doi: 10.1002/stem.1160. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM, Lum BL, Darbonne WC, Marsters JC Jr, de Sauvage FJ, Low JA. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- Jeng KS, Sheen IS, Jeng WJ, Lin CC, Lin CK, Su JC, Yu MC, Fang HY. High expression of patched homolog-1 messenger RNA and glioma-associated oncogene-1 messenger RNA of sonic hedgehog signaling pathway indicates a risk of postresection recurrence of hepatocellular carcinoma. Ann Surg Oncol. 2012. [DOI] [PubMed]
- Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, De The H, Chen SJ, Chen Z. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010;328:240–243. doi: 10.1126/science.1183424. [DOI] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oketani M, Kohara K, Tuvdendorj D, Ishitsuka K, Komorizono Y, Ishibashi K, Arima T. Inhibition by arsenic trioxide of human hepatoma cell growth. Cancer lett. 2002;183:147–153. doi: 10.1016/S0304-3835(01)00800-X. [DOI] [PubMed] [Google Scholar]
- Kito M, Matsumoto K, Wada N, Sera K, Futatsugawa S, Naoe T, Nozawa Y, Akao Y. Antitumor effect of arsenic trioxide in murine xenograft model. Cancer Sci. 2003;94:1010–1014. doi: 10.1111/j.1349-7006.2003.tb01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL, Yang CH. Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Invest New Drugs. 2007;25:77–84. doi: 10.1007/s10637-006-9004-9. [DOI] [PubMed] [Google Scholar]
- Nieto-Miguel T, Calonge M, de la Mata A, Lopez-Paniagua M, Galindo S, de la Paz MF, Corrales RM. A comparison of stem cell-related gene expression in the progenitor-rich limbal epithelium and the differentiating central corneal epithelium. Mol Vis. 2011;17:2102–2117. [PMC free article] [PubMed] [Google Scholar]
- Barbash O, Zamfirova P, Lin DI, Chen X, Yang K, Nakagawa H, Lu F, Rustgi AK, Diehl JA. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer cell. 2008;14:68–78. doi: 10.1016/j.ccr.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chang L, Patel N, Yang J, Lowe L, Burns DK, Zhu Y. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Luo Y, Dallaglio K, Chen Y, Robinson WA, Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris DA, Roop DR, Vasiliou V, Fujita M. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JW, Hsu YC, Kao CY, Su HL, Chiu IM. Leukemia inhibitory factor-induced Stat3 signaling suppresses fibroblast growth factor 1-induced Erk1/2 activation to inhibit the downstream differentiation in mouse embryonic stem cells. Stem Cells Dev. 2013;22:1190–1197. doi: 10.1089/scd.2012.0229. [DOI] [PubMed] [Google Scholar]
- Krause DS, Crispino JD. Molecular pathways: induction of polyploidy as a novel differentiation therapy for leukemia. Clin Cancer Res. 2013;19:6084–6088. doi: 10.1158/1078-0432.CCR-12-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2012;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- Kito M, Akao Y, Ohishi N, Yagi K, Nozawa Y. Arsenic trioxide-induced apoptosis and its enhancement by buthionine sulfoximine in hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2002;291:861–867. doi: 10.1006/bbrc.2002.6525. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1 + CD11b + myeloid cells that promote metastasis. Cancer cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JM, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwerner JP, Joo J, Warner KL, Christensen L, Hu-Lieskovan S, Triche TJ, May WA. The EWS/FLI1 oncogenic transcription factor deregulates GLI1. Oncogene. 2008;27:3282–3291. doi: 10.1038/sj.onc.1210991. [DOI] [PubMed] [Google Scholar]
- Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, Endo Y, Rubin JS, Toretsky J, Uren A. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–9082. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, Hanahan D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Tan J, Li J, Kivimae S, Yang X, Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, Cheyette BN, Yu Q. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer cell. 2008;13:529–541. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PloS one. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tang ZY, Qin LX, Wu XF, Sun HC, Xue Q, Ye SL. High-dose and long-term therapy with interferon-alfa inhibits tumor growth and recurrence in nude mice bearing human hepatocellular carcinoma xenografts with high metastatic potential. Hepatology. 2000;32:43–48. doi: 10.1053/jhep.2000.8525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real-time PCR primers for the 5 stemness genes that were down-regulated in CD133+ Hep3B-wt cells after treatment with As2O3 for 48 hours.