Abstract
Introduction
Directly observed therapy of highly active antiretroviral therapy (DOT-HAART) is a feasible adherence intervention. Prospective DOT-HAART studies have shown mixed results, and optimal target groups have yet to be defined. We performed a meta-analysis and systematic review to assess the effect of DOT-HAART on adherence and virologic and immunologic response.
Methods
We performed a comprehensive search through August 2009 to identify peer-reviewed controlled studies that involved outpatient DOT-HAART among adults and reported at least 1 outcome assessed in this meta-analysis. Random-effects meta-analyses were performed; differences in effect on virologic suppression were examined using stratified meta-analyses and meta-regression on several study characteristics.
Results
Seventeen studies met inclusion criteria. Compared with control groups, DOT-HAART recipients were more likely to achieve an undetectable viral load (random effects risk ratio 1.24, 95% confidence interval (CI): 1.08 to 1.41), a greater increase in CD4 cell count (random effects weighted mean difference 43 cells/μL, 95% CI: 12 to 74 cells/μL), and HAART adherence of ≥95% (random effects risk ratio 1.17, 95% CI: 1.03 to 1.32). Results varied with respect to virologic response. DOT-HAART did not have a significant effect on virologic suppression when restricted to randomized controlled studies. Post-treatment effect was not observed in a limited number of studies.
Conclusions
DOT-HAART had a significant effect on virologic, immunologic, and adherence outcomes, although its efficacy was not supported when restricting analysis to randomized controlled trials. DOT-HAART shows greatest treatment effect when targeting individuals with greater risk of nonadherence and when delivering the intervention that maximizes participant convenience and provides enhanced adherence support. Further investigation is needed to assess the postintervention effect and cost-effectiveness of DOT-HAART.
Keywords: directly observed therapy, DOT-HAART, HAART, meta-analysis, review
INTRODUCTION
Highly active antiretroviral therapy (HAART) is standard of care for individuals infected with HIV.1–4 Strict adherence to treatment is required to achieve optimal clinical responses.5–10 Unfortunately, nonadherence is common among HIV-positive patients because of the life-long nature of HAART,11,12 adverse events,13–15 and numerous psychosocial and economic stressors.11,16–21 Interventions to improve HAART adherence vary widely and often include education and counseling,22–25 patient reminders,26,27 behavioral therapy,27,28 and social support.28–30 Other interventions promote combined strategies.31–33 Recent meta-analyses show that individuals receiving an adherence intervention are more likely to achieve 95% adherence than those receiving standard of care across a broad range of intervention designs.34,35
Directly observed therapy (DOT) has been the cornerstone of a strategy endorsed by the World Health Organization to improve tuberculosis treatment adherence and outcomes worldwide.36–38 As early as 1996, HIV providers considered the utility of DOT for HAART (DOT-HAART).39 Critics of DOT-HAART have voiced concerns about the feasibility of applying DOT to life-long treatment, the acceptability of DOT given confidentiality concerns and HIV-related stigma, and the potential threat of generating excess drug resistance.40 Conversely, proponents have endorsed DOT-HAART because of its ability to provide intensive support to otherwise hard-to-reach HIV-infected populations.41 Although this debate persists,32,42 recent data demonstrate that DOT-HAART is feasible, acceptable, and does not seem to increase the risk of drug resistance among participants.30,32,43–50 As a result, DOT-HAART has gained increasing recognition as an important antiretroviral adherence strategy.51 Unlike many other HAART adherence interventions, DOT-HAART has been successfully “test-driven” in real-world settings and has been delivered to more than 12,000 individuals to date.28,44,51–69 However, efficacy data from controlled trials are mixed, and interventions vary widely in terms of the nature of DOT-HAART (eg, site and frequency of DOT, additional support provided, DOT worker background); target populations (eg, substance users, HAART-naive resource-poor settings); and assessment (eg, duration of follow-up, outcomes).51 The question of translating evidence into implementation70 is not “can DOT-HAART be implemented,” but rather, “should it, how, and for whom”?
Recognizing the growing attention toward DOT-HAART and the need to synthesize findings across a diverse array of studies, Ford et al71 recently conducted a systematic review and meta-analysis of DOT-HAART randomized clinical trials (RCTs). Their analysis did not show an intervention effect on virologic suppression at study completion, although benefit was observed among individuals at high risk of nonadherence and among trials with DOT lasting less than 6 months. The moderate heterogeneity observed among studies and the identification of certain treatment characteristics which may confer greater treatment effect invite further exploration of the growing experience of DOT-HAART.
We sought to expand the current scientific knowledge of DOT-HAART by performing a meta-analysis and systematic review of controlled DOT-HAART trials that differs from that of Ford et al71 in several important respects. First, we included nonrandomized studies in recognition of the complexity and flexibility of many DOT-HAART interventions, which have adapted over time in response to community needs. Second, we differentiated between on-treatment and post-treatment effects, rather than pooling measures conducted at study completion. Finally, we investigated the modifying effect of several aspects of intervention design and target population that were not considered by Ford et al.71
METHODS
Search Strategy
We followed PRISMA72 and MOOSE73 guidelines in this systematic review. We extensively searched the following databases to identify controlled studies that described the provision of HAART as directly observed: MEDLINE via PubMed, the Computer Retrieval of Information on Scientific Projects database, www.clinicaltrials.gov, www.controlled-trials.com, and Google Scholar from 1995 to August of 2009 and 2006 to 2009 proceedings from the Conference on Retroviruses and Opportunistic Infections, the International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, and the National Institute of Mental Health and the International Association of Physicians in AIDS Care International Conference on HIV Treatment Adherence. We limited our searches to the post-HAART era, which began in 1995. We chose to search relevant conferences because of recent proceedings presenting preliminary and/or pre-publication data from DOT-HAART clinical trials that have influenced DOT-HAART discussions. We limited conference searches to recent years with the rationale that data published before these years should have resulted in a published article. We used the following queries: “HIV” OR “HAART” AND “directly observed” OR “DOT” OR “mDOT” OR “directly supervised” OR “directly observed therapy” OR “DOT-HAART,” OR “DAART”. We also used the “related articles” search tool in PubMed and examined the bibliographies of all reviewed sources, including several review papers.34,35,46,51,70,74 We did not restrict our searches to English. We compared sources to exclude duplicate references (ie, same outcomes reported on the same cohort). We contacted authors and experts for additional studies and data not available in publications and abstracts.
Study Selection
Studies were included if they (1) described a DOT-HAART intervention (ie, involved direct observation of at least some proportion of HAART), (2) were peer reviewed, (3) included a randomized or non-randomized comparison group, (4) took place in an outpatient setting, (5) exclusively enrolled adults, and (6) included at least 1 of the outcome measures of this analysis (viral load suppression, change in CD4 cell count, and HAART adherence).
Although RCTs are considered the gold-standard design for evaluating efficacy, they may be limited in assessing of the effectiveness of DOT-HAART in a diversity of settings and may not inform the adaptation of an intervention to suit local needs.75 RCT enrollment may be biased, and better outcomes are often observed across all RCT arms compared with standard of care,76 particularly among vulnerable populations and/or settings of poor service infrastructure.76–80 Furthermore, although DOT-HAART may seem straightforward on first blush, the intervention can be both complex and heterogeneous when implemented in the real-world setting. DOT-HAART interventions deployed within RCTs may be inherently simpler and less flexible because of the need to standardize the intervention and monitor treatment fidelity. For many nonrandomized studies of DOT-HAART interventions, evaluation using a randomized study design may not have been possible or ethical. We therefore included nonrandomized reports to maximize the diversity of experiences represented in our systematic review.
Data Abstraction
Using standardized coding forms, 2 reviewers independently abstracted information from the articles and posters. Each study was coded for study design, intervention and control characteristics, sample size, retention, and outcome data. We had an inter-rater agreement of 96% on key variables. Discrepant abstractions were resolved through discussion, including arbitration of an additional reviewer.
Study Outcomes
Studies varied in their definitions of virologic, immunologic, and adherence endpoints. For instance, some reported virologic success as achieving either an undetectable viral load or at least a log10 drop in viral load at the end of the study. For meta-analysis, we chose three endpoints: virologic suppression (proportion achieving an undetectable HIV load based on the assay used for the study); immunologic response (mean change in CD4 cell count from baseline); and adherence (proportion of individuals achieving ≥95% adherence to prescribed doses). Because adherence measures varied across studies; we used adherence outcomes as measured by study authors as long as data were available using the threshold of ≥95%. If data could not be gathered from published information, including endpoints that were not reported according to our meta-analysis endpoint definitions, we contacted the authors and invited them to provide additional information.
For studies that included multiple intervention or control arms, we analyzed the 1 intervention arm that represented the most frequent administration of DOT and the 1 control arm that was most comparable to the DOT group. For instance, the study by Idoko et al59 involved 3 intervention groups: 1 receiving daily, another receiving twice-weekly, and the third receiving once-weekly DOT. We chose the daily DOT arm to use as the intervention group for the purposes of our meta-analysis. Among the 3 control groups in the study by Lucas et al,60 we chose the control most comparable to the intervention group (intravenous drug users on methadone). Gross et al81 also conducted a 3-arm study; we compared the DOT arm with the control group that received the same once-daily regimen under self-administration. For Wohl et al,69 who tested 2 interventions (DOT and intensive case management), the DOT group was compared with the control arm receiving standard of care.
Methodologic Assessment
We summarized the methodologic features of the studies based on the following variables: study design, comparability of control and intervention arms, study retention (including differential retention by trial arms), methods for handling missing data, and methods of measuring adherence. We did not exclude studies based on quality assessment. Given the limited validity of quality scores,82,83 we did not create a quality score or weight studies differentially based on quality assessments. Rather, we performed stratification and meta-regression on key quality-associated study characteristics.
Analytic Approach
We employed risk ratios (RRs) to describe the associations between DOT-HAART and undetectable viral load and DOT-HAART and adherence. We assessed the effect of DOT-HAART on CD4 cell count by comparing the mean differences of CD4 cell count in each arm. Standard deviations of mean change in CD4 cell count and the number of observations for each arm were used to compute the standard error of the difference in CD4 change. For studies that did not provide the standard deviations or the mean change in CD4, we inferred them from other data available (see Supplemental Digital Content 1, http://links.lww.com/QAI/A43). Our analyses employed observations from 1 time point for each study; for studies with multiple time points of assessment, we used the last available on-intervention measurements to assess intervention effect among all pooled studies. Post-intervention measures occurring more than 1 month after the intervention were pooled separately to assess durability of intervention effect. Further, to examine the trajectory of postintervention virologic effect of DOT-HAART, we plotted the log-transformed RRs against the time since duration for studies that made multiple assessments of virologic success after intervention.81,84–87
We assessed the heterogeneity of effect estimates using the Cochran Q test,88 and we quantified the magnitude of between-study heterogeneity using the Higgins I2 estimate.89 We performed the Dersimonian-Laird random-effects (REs) meta-analysis90 to aggregate the effects of DOT-HAART on undetectable viral load and ≥95% adherence across studies because the studies showed significant heterogeneity by the Cochran Q Test (P < 0.05). The Dersimonian-Laird RE meta-analysis was also performed to compute the weighted mean difference (WMD) in change of CD4 cell count as the studies showed significant heterogeneity with respect to immunologic outcome.
Because studies varied with respect to outcome ascertainment, intervention, and study population, we looked for possible effect modifiers through stratified meta-analyses and meta-regression, which tests the difference in effect between 2 groups. For both virologic and immunologic outcomes, we employed RE meta-analyses to summarize the data within each stratum. Meta-regression was performed by regressing the natural logarithm of the RR for virologic success of the studies by the study-specific values for the effect modifiers of interest, weighting the studies by the inverse of the sum of study-specific variance and between-study variance. A priori, we identified variables that we hypothesized were most likely to contribute to heterogeneity in 3 areas: (1) intervention design; (2) target population; and (3) study quality.
Effect modifiers pertaining to intervention design were DOT site (hospital or HIV clinic vs. methadone clinic vs. residence-based, ie, patient homes, mobile community van, prison, or hospice); and DOT intensity (enhanced DOT-HAART vs. not enhanced DOT-HAART). Guided by a systematic review on DOT for tuberculosis,91 we defined “enhanced DOT-HAART” as any intervention that included additional formal adherence support not offered to the control group (ie, material or financial incentives/enablers) or a behavioral intervention or ancillary services aimed at improving adherence. Because certain services were often provided as necessary and ethical consequences of DOT, we did not consider the following activities to constitute formal additional support: asking about side effects and adherence at DOT visits and reporting any problems to providers; prepackaging and delivering HAART via DOT visits; and referring patients to other social services unless additional staff (eg, case manager, social worker) was integrated into the DOT team. Differences in target population included percent HAART naive (≥50% vs. <50%); study setting (resource-poor vs. resource-rich setting, based on groupings of low and middle vs. high human development, respectively, from the United Nation’s Human Development Index92); and substance use (≥50% substance users vs. <50% substance users). For studies that did not specify the proportion of substance users, we assumed <50%. Variables reflecting study characteristics were study design (RCT vs. nonrandomized study); control comparability (baseline virologic or immunologic differences between arms vs. no difference); and differential attrition of <8% vs. ≥8%.
We tested for publication bias by the Begg (rank correlation),93 the Egger (weighted regression) tests94 and also used a modified Macaskill test, which avoids the problem of correlation between the logarithm of the RR and its standard errors.95 A funnel plot of standard error estimates vs. effect size estimates based on intervention effects on virologic suppression was created to visually assess for asymmetry as an indication of publication bias. We performed all statistical analyses using the “meta” and “rmeta” packages in R version 2.8.1.96
RESULTS
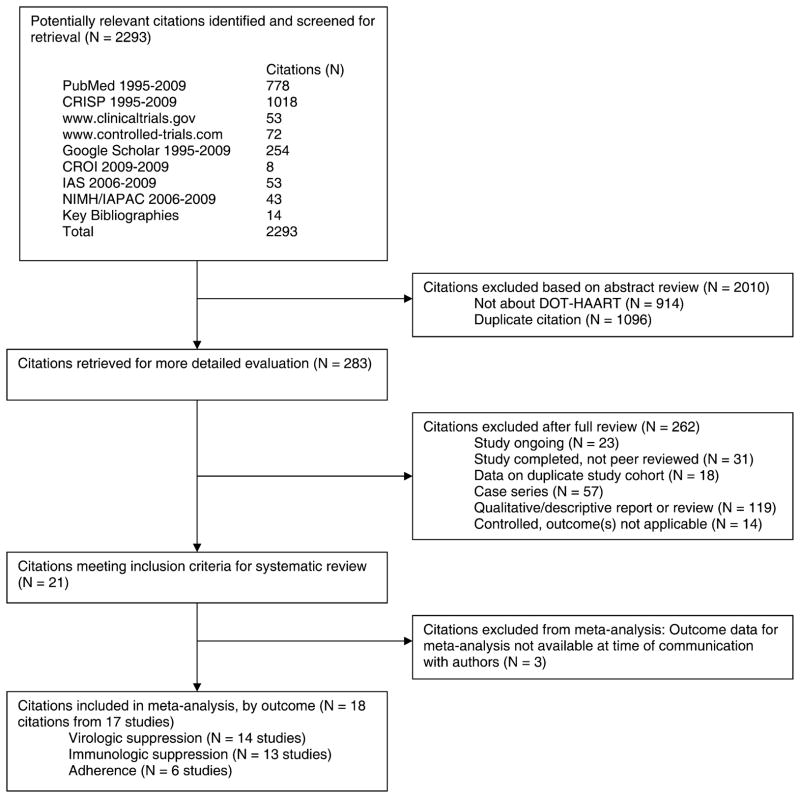
Figure 1 outlines the selection process used to identify studies that met our inclusion criteria. Of the 2293 citations returned from our queries, all but 283 were excluded after abstract review because they were not about DOT-HAART or were duplicate citations. Fifty-four of the 283 citations retrieved for further review were excluded because they described either ongoing studies or studies that were as yet complete but not peer reviewed. Other citations were excluded because they described case series, qualitative or descriptive reports, controlled studies that did not measure any of the meta-analysis outcomes, or were duplicate references. We identified 21 citations that met our criteria for inclusion; however, outcome data for 3 were not available yet per communication with authors.67,97,98 Two of the remaining 18 citations reported data from the same study.53,85 Therefore, we included 17 studies in our systematic review and meta-analyses.
FIGURE 1.
Flow diagram of selection process for systematic review and meta-analysis. CRISP, Computer Retrieval of Information on Scientific Projects database; CROI, Conference on Retroviruses and Opportunistic Infections; IAS, International AIDS Society; NIMH/IAPAC, National Institute of Mental Health and the International Association of Physicians in AIDS Care International Conference on HIV Treatment Adherence.
Study and Sample Characteristics
Characteristics of the 17 studies are summarized in Table 1. Cumulatively, these studies involved a total of 3169 patients (range 49–500, mean cohort size 186), of whom 38% were female. Twelve studies (71%) were published in peer-reviewed journals at the time of analysis and 5 were presented at conferences. Six studies (35%) were conducted in resource-poor countries. Eight studies (47%) targeted HAART-naive participants; 7 (41%) restricted inclusion to substance users.
TABLE 1.
Characteristics of Controlled DOT-HAART Studies Meeting Inclusion Criteria (n = 17)
| ID | Study | Setting | Study Size |
Salient Inclusion and Exclusion Criteria |
DOT Duration and Frequency |
DOT Site | Description of DOT, Other Support), in Addition to Standard of Care |
VL (Y/N), Sensitivity |
CD4 (Y/ N), Definition |
Adherence (Y/N), Method; Assessment Interval; Definition |
|---|---|---|---|---|---|---|---|---|---|---|
| A | Altice et al53 and Maru et al85 | Area HIV clinics in New Haven, CT | 141 | HIV positive; used heroin and/or cocaine in past 6 months; twice daily (or once daily HAART | 6 months, once daily on week days | Van travels with needle exchange to neighborhood sites | Enhanced community- based DOT of HAART and other medications by DOT specialist; beeper reminders & outreach; van with on- site clinician, drug treatment coordinator, case manager, outreach workers | (Y), <400 copies/mL | (Y), Mean change | (Y), Measured at baseline, 1, 3, & 6 months using 3-day ACTG self- reported adherence |
| B | Arnsten et al54 | Methadone clinics in New York City, NY | 65 | Stable methadone dose 5–6 day/week pick-up; receiving HIV care from methadone provider or affiliated clinic | 6 months, once daily 5–6 days/week | Methadone clinic | DOT-HAART at methadone clinic | (Y), <75 copies/mL | (N) | (Y), Pill count; defined as median number doses taken |
| C | Babudieri et al55 | Prisons in Italy | 84 | Incarcerated HIV-positive IDU | Variable (mean 8.7 months); all doses | Prison | DOT-HAART by prison nurse | (Y), <200 copies/mL | (Y), CD4 <200 cells/mL | (N) |
| D | Bangsberg et al84 | Community research site in San Francisco, CA | 81 | HIV viral load >400 copies/mL; nonadherence | 3 months, once daily 5 days/week | Community research site | Enhanced DOT-HAART and other medications by study staff; deliver missed doses to patients. DOT followed by 3 months of adherence case management | (Y) | (N) | (Y), Measured by 7-day self- report at baseline then monthly |
| E | Gross et al81 | HIV treatment centers at ACTG sites | 243 | HAART naïve; VL >2000 copies/mL; weight ≥40 kg | 6 months, once daily 5 days/week | ACTU or other clinic site | DOT-HAART by health professional (nurse or pharmacist, not a relative, partner, or close friend) | (Y), <200 copies/mL | (Y), Median change | (N) |
| F | Horta et al58 | Tertiary infectious diseases hospital in Porto, Portugal | 422 | HIV positive; active IDU | Variable duration; once daily all days | Hospital methadone clinic | Enhanced DOT of HAART & methadone; multidisciplinary support | (Y), <50 copies/mL | (Y), mean change | (Y), self-report and pharmacy refill |
| G | Idoko et al59 | Tertiary hospital in Jos, Nigeria | 175 | HAART naive. Excluded: medical condition that interferes with study; <1 year prognosis; substance users | 12 months, Three DOT groups: once daily all daysa; once daily twice weekly, once daily weekly | Patient home | Community-based DOT- HAART by patient- assigned treatment partner trained on record-keeping, side effects and follow-up | (Y), <400 copies/mL | (Y), median | (N) |
| H | Lanzafame et al101 | Tertiary hospital and prisons in Legnago, Italy | 49 | Naive to protease inhibitor therapy | 6 months, all doses | Prison | DOT-HAART by prison nurses | (Y), N/R | (Y), mean | (N) |
| I | Lucas et al60 | Methadone clinics in Baltimore, MD | 891 | Regular HIV treatment provider; methadone for >30 days without plans to discontinue; baseline VL >500 copies/mL; no triple- class resistance | Goal at least 12 months, once daily on methadone days | Methadone clinic | DOT-HAART by nurse or medical assistant; prepackaged doses for non-DOT days | (Y), <400 copies/mL | (Y), Median change | (N) |
| J | Macalino et al61 | HIV clinics in MA and RI | 87 | Active substance use; no genotypic resistance to a once-daily regimen | Up to 12 months; once daily for 3 months, then tapered | Location chosen by patient | Community-based DOT-HAART by trained outreach worker; packaged pills | (Y), <50 copies/mL | (Y), median change | (Y), self-report 30-day recall |
| K | Munoz et al100 | HIV clinic in tertiary hospitals Lima, Peru | 120 | HAART naïve; residence & receiving HIV care in study area. Priority given to tuberculosis coinfected patients and women | 12 months, all doses (all twice daily regimens) for 8 months then tapered | Patient home or alternative site per patient preference | Enhanced community-based DOT of HAART & other medications by lay health workers who also monitor side effects & provide social support; financial aid for tests, meds, transportation; nutritional support as needed | (Y), <400 copies/mL | (Y), Mean change | (Y), measured at baseline, then every 3 months using self-report 30-day recall |
| L | Nachega et al86 | Secondary hospital in Cape Town, South Africa | 274 | Stavudine/lamivudine/efavirenz or nevirapine regimen | 12 months, at least once daily | ART roll-out clinic at secondary hospital | DOT-HAART by trained treatment supporters | (Y), N/R | (Y), Mean change | (N) |
| M | Pearson et al65 | HIV clinic in tertiary hospital in Beira, Mozambique | 350 | Reside near clinic; no severe mental illness or dementia | 1.5 months, once daily on weekdays | HIV clinic in tertiary hospital | Enhanced DOT-HAART provided by HIV-positive peers trained based on Informational, Motivation and Behavioral Skills model; peers also provide social support and education, and encourage participation in support groups; transportation costs | (N) | (Y), Mean change | (Y), Self-report 7-day and 30-day recall |
| N | Sarna et al87 | HIV clinics in Mombasa, Kenya | 234 | reside in Mombasa; HAART naïve | 6 months, once daily twice weekly | Health center clinic | Enhanced DOT-HAART by clinic nurses who also provide adherence support; outreach if missed appointments; medication delivery to patient & travel costs provided if needed | (Y), < 400 copies/ml | (Y), median change | (Y), pill counts & self- reported 4-day recall measured every 8 weeks from weeks 1–48 then at week 72 |
| O | Taiwo et al99 | Tertiary hospital in Jos, Nigeria | 500 | HAART naive, Receiving care at PEPFAR Program at Jos University Hospital. Excluded: life expectancy <1 year despite HAART | 6 months, at least once daily all days | Patient home | Community-based DOT- HAART by patient-selected treatment partner who lives in same house or in close proximity. Partner receives adherence counseling and helps patient complete medication log, monitor side effects and medication supply | (Y), <400 copies/mL | (Y), Change | (Y), Assessed by prescription refill data, # reminder calls from pharmacist, medication log, visual analog scale of doses in past month |
| P | Tinoco et al66 | AIDS welfare homes & area reference hospitals in Cadiz, Spain | 98 | Stage C; IDU; enrolled in methadone program | 9 months, all doses | AIDS welfare homes | Enhanced DOT-HAART delivered by nurse volunteers; housing & psychological support, access to addiction- treatment & rehabilitation programs | (Y), <50 copies/mL | (Y), Mean change | (N) |
| Q | Wohl et al69 | HIV clinics in Los Angeles, CA | 250 | <1 prior failed regimen; ≥23 on Folstein Mini Mental Status Exam; live or work in study area; Excluded: advanced liver or kidney disease; DOT for tuberculosis | 6 months, once daily on weekdays | Home or other site decided by patient and community worker | Enhanced community-based DOT-HAART by bilingual community worker who also delivers non-DOT doses and addresses adherence problems; financial incentives up to $205 | (Y), <400 copies/mL | (Y), Median change | (Y), self-report with 24 hour, 3-day and 7-day recall |
IDU, intravenous drug users; N/R, not reported; VL, viral load; SAT, self-administered therapy.
Intervention and Control Characteristics
As shown in Table 1, interventions varied widely. Five studies (29%) provided DOT for all doses, another 4 reported daily DOT that did not explicitly cover all doses (24%), 6 (35%) provided once-daily DOT observed 5–6 times a week, 1 delivered DOT “on methadone days,” and 1 provided twice weekly DOT. The duration of DOT ranged from 6 weeks to 29 months, with a median duration of 6 months. The site of DOT also varied: 5 interventions required travel to a hospital or HIV clinic to receive DOT, 3 took place in methadone clinics, and 9 were residence based (ie, patient homes, mobile community van, hospice, or prison). DOTwas performed by nurses or clinic staff in 9 interventions, whereas 8 used lay workers (including family members) to deliver DOT. Seven studies (47%) provided enhanced DOT, with additional support ranging from case management to outreach for nonadherent patients to financial or material enablers. Standard of care varied by treatment site but did not involve direct observation of medications. Ten studies (59%) provided baseline adherence education and counseling to both study groups.
Methodologic Assessment
Indicators of study quality were also examined (see Table, Supplemental Digital Content 2, http://links.lww.com/QAI/A44). Eleven studies (65%) were RCTs. Six studies reported baseline virologic or immunologic differences between the DOT-HAART and comparison groups, and 7 studies experienced attrition of ≥8% during the study. Follow-up varied: 9 studies followed patients for 3–9 months, whereas the remaining 8 were 12 months or longer in duration. All studies reported on-treatment or immediate postintervention measures. Only 6 studies reported postintervention data, ranging from 6 to 12 months after completion of DOT. Of the 10 studies measuring HAART adherence, 6 relied on self-report, 1 used pill counts, and 3 combined multiple assessments including pill count, pharmacy refill, and self-report. Recall periods for adherence assessment ranged from 4 to 30 days.
Summary Effects
Viral Load
Although 16 studies assessed virologic response, for meta-analytic assessment, we included 14 studies for which data were available per our outcome definition: the proportion of patients with undetectable viral load at the time of DOT-HAART completion. We used viral load detection limits utilized by study authors (50 copies/mL,58,61,66 75 copies/mL,54 200 copies/mL,55,81 400 copies/mL,53,59,60,69,84,85,87,99,100 and 1 study did not specify86). Of data available, 67% (700 of 1049) of DOT-HAART participants and 53% (584 of 1110) of control participants achieved an undetectable viral load. Six studies55,58,60,84,99,100 showed significantly greater virologic suppression with DOT. As shown in Figure 2, DOT-HAART was associated with a 24% increase in virologic suppression (RE RR: 1.24, 95% confidence interval (CI): 1.08 to 1.41). However, the effect estimates were heterogeneous (P < 0.001, I2 = 80.3%, 95% CI: 67.8% to 87.9%), with between-study variability explaining 80% of the total variance. Meta-analysis of DOT-HAART durability on the studies that ascertained outcomes at least 1 month subsequent to the cessation of intervention81,84–87 revealed an RE RR of 0.95 (95% CI: 0.86 to 1.05), suggesting lack of postintervention effect. When RRs from studies with multiple outcome assessments were plotted against time since end of intervention (Fig. 3), the effect for virologic suppression decreased over time in the 1 study with a significant effect at the end of intervention.84
FIGURE 2.
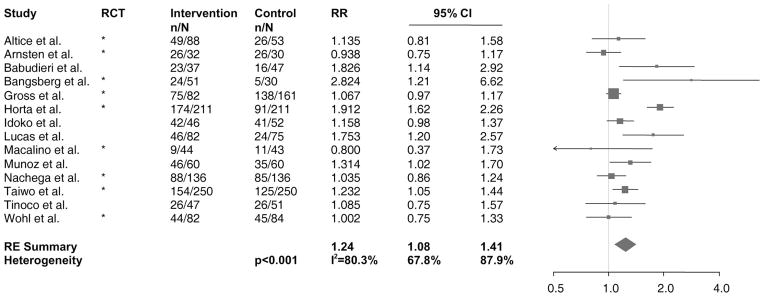
Forest plot of studies with results on the effect of DOT-HAART on virologic suppression. Values shown are among data available. The size of the squares is proportional to the inverse variance of log-transformed RRs. The arrow indicates that the 95% CI has been truncated to limits of the x axis scale. n, the number of patients achieving virologic suppression; N, the total number of patients in the study arm; RE, random effects.
FIGURE 3.
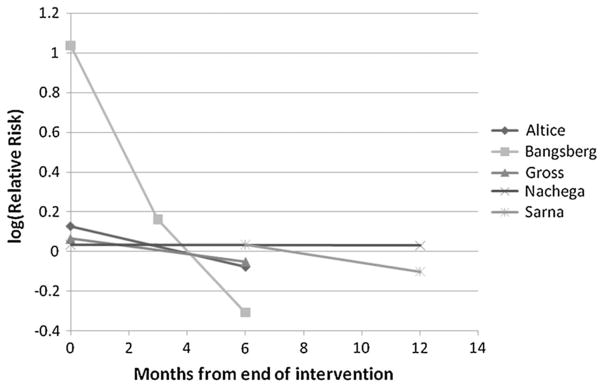
Durability of DOT-HAART. Postintervention change in effect of DOT-HAART on virologic suppression in studies that assessed durability of DOT-HAART.
CD4
Thirteen studies in the systematic review assessed CD4 cell count according to our definition of immunologic response: the mean change in CD4 cell count from baseline to the time of DOT-HAART completion.53,55,58–61,66,69,81,86,87,100,101 We obtained necessary data for computation of mean difference and its standard error from 9 studies and/or authors. For the remaining 4 studies,53,59,87,101 we inferred the mean change and standard deviations from other data available (see Supplemental Digital Content 1, http://links.lww.com/QAI/A43). Four studies showed significantly greater increase in the DOT-HAART group.53,58,60,66 As summarized in Figure 4, DOT-HAART was associated with greater increase in CD4 cell count (RE WMD 43 cells/μL, 95% CI: 12, 74 cells/μL) compared with standard of care. As with virologic suppression, the effects varied widely, as indicated by the significant Q test and a Higgin I2 value greater than 50% (P <0.001, I2 = 82.6%, 95% CI: 71.5% to 89.4%). Meta-analysis of DOT-HAART durability on change in CD4 included 5 studies for which postintervention data were available55,65,85–87 and did not show a significant effect (RE WMD: 40 cells/μL, 95% CI: −13 to 93 cells/μL).
FIGURE 4.
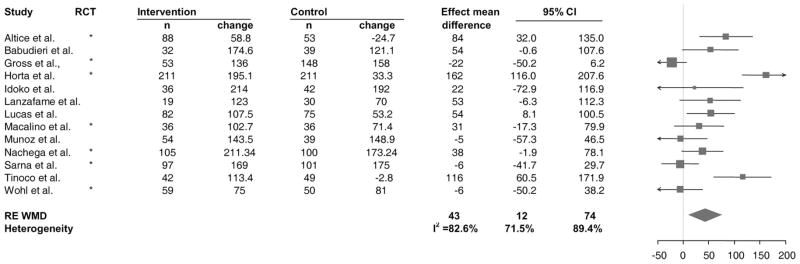
Forest plot of studies with results on the effect of DOT-HAART on mean change in CD4 cell count. Values shown are among data available. The size of the squares is proportional to the inverse variance of mean differences. The arrow indicates that the 95% CI has been truncated to limits of the x axis scale.
Adherence
Six of the studies included in the systematic review assessed adherence as taking at least 95% of prescribed doses at time of DOT-HAART completion.61,65,69,84,87,100 Of data available, we found that 88% (359 of 408) of those receiving the intervention compared with 75% (302 of 402) of patients in control groups achieved ≥95% adherence. As shown in Figure 5, the studies analyzed showed a positive intervention effect on adherence (RE RR: 1.17, 95% CI: 1.03 to 1.32). Again, the results varied from study to study (P = 0.01, I2 = 62.7%, 95% CI: 9.3% to 84.6%), with between-study variability explaining 63% of the total variance in the effect.
FIGURE 5.
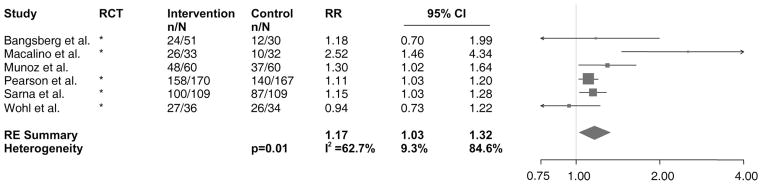
Forest plot of studies with results on the effect of DOT-HAART on ≥95% adherence to prescribed doses. Values shown are among data available. The size of the squares is proportional to the inverse variance of log-transformed RRs. The arrow indicates that the 95% CI has been truncated to limits of the x axis scale. n, the number of patients achieving ≥95% adherence; N, the total number of patients in the study arm.
Stratified Analysis and Meta-Regressions
To explore sources of heterogeneity in the effects of DOT-HAART on undetectable viral load, we stratified the studies and conducted meta-regressions by 8 variables, as shown in Table 2. Although meta-regression analyses were not statistically significant, several trends in treatment effects by stratified meta-analyses were notable. Treatment effect was greater among studies delivering DOT at patients’ residences compared with those delivering clinic-based DOT; in HAART-experienced individuals compared with HAART-naive individuals; in nonresource-poor settings compared with resource-poor settings; in substance-using populations compared with nonsubstance-using populations; and in those receiving enhanced DOT compared with those given nonenhanced DOT. Effect estimates were greater among nonrandomized observational studies compared with RCTs, although this meta-regression did not show evidence for a significant difference (P = 0.52). Associations with virologic suppression in RCTs did not meet statistical significance (RR = 1.18, 95%CI: 0.99, 1.42, P = 0.068). There were no differences in the effect of DOT-HAART by presence of baseline virologic or immunologic differences (P = 0.66) or by differential attrition (P = 0.96).
TABLE 2.
Stratification to Test for Effect Modification of Intervention Effect on Viral Load Suppression
| Categories | Subcategories | Number of Studies | Studies | Summary RR* | 95% CI of RR | P Heterogeneity for | I2,† | I2 95% CI | P for Meta-Regression |
|---|---|---|---|---|---|---|---|---|---|
| Intervention design | |||||||||
| DOT Site | Hospital/HIV Clinic | 3 | D,E,L | 1.10 | (0.91 to 1.34) | 0.05 | 67% | (0% to 90%) | REF |
| Residence | 8 | A,C,G,J,K,O,P,Q | 1.19 | (1.09 to 1.30) | 0.45 | 0% | (0% to 67%) | 0.36 | |
| Methadone Clinic | 3 | B,F,I | 1.46 | (0.88 to 2.41) | <0.0001 | 94% | (84% to 97%) | 0.99 | |
| DOT intensity | Enhanced | 6 | A,D,F,K,P,Q | 1.35 | (1.03 to 1.67) | 0.032 | 80% | (56% to 91%) | REF |
| Non-enhanced | 8 | B,C,E,G,I,J,L,O | 1.14 | (1.02 to 1.28) | 0.009 | 63% | (19% to 83%) | 0.31 | |
| Target population | |||||||||
| Proportion HAART naive | <50% | 9 | A,B,C,D,F,I,J,P,Q | 1.33 | (1.03 to 1.72) | <0.0001 | 82% | (67% to 90%) | REF |
| ≥50% | 5 | E,G,K,L,O | 1.12 | (1.04 to 1.21) | 0.24 | 28% | (0% to 72%) | 0.42 | |
| Study setting | Resource-poor setting | 4 | G,K,L,O | 1.17 | (1.07 to 1.28) | 0.0008 | 0% | (0% to 85%) | REF |
| Resource-rich setting | 10 | A,B,C,D,E,F,I,J,P,Q | 1.28 | (1.04 to 1.59) | 0.022 | 87% | (78% to 92%) | 0.58 | |
| Substance using proportion | <50% | 6 | E,G,K,L,O,Q | 1.11 | (1.04 to 1.19) | 0.33 | 13% | (0% to 78%) | REF |
| ≥50% | 8 | A,B,C,D,F,I,J,P | 1.39 | (1.04 to 1.84) | 0.024 | 82% | (66% to 91%) | 0.58 | |
| Study quality | |||||||||
| Study design | RCT | 9 | A,B,D,E,F,J,L,O,Q | 1.18 | (0.99 to 1.42) | <0.0001 | 86% | (75% to 92%) | REF |
| non-RCT | 5 | C,G,I,K,P | 1.32 | (1.11 to 1.58) | 0.08 | 52% | (0% to 82%) | 0.52 | |
| Baseline immunologic or virologic differences | No difference | 9 | B,C,D,I,J.K.L,O,P | 1.23 | (1.05 to 1.44) | 0.006 | 62% | (23% to 82%) | REF |
| Difference | 5 | A,E,F,G,Q | 1.23 | (0.96 to 1.57) | <0.0001 | 92% | (84% to 96%) | 0.66 | |
| Differential loss to follow-up‡ | ≥8% loss to follow-up | 5 | A,D,J,K,Q | 1.18 | (0.94 to 1.50) | 0.13 | 43% | (0% to 79%) | REF |
| <8% loss to follow-up | 7 | C,E,F,G,L,O,P | 1.27 | (1.06 to 1.52) | <0.0001 | 88% | (78% to 94%) | 0.96 | |
Random effects summary estimates within subcategories.
Proportion of total variance of summary estimate due to between studies variance.
Among data available.
REF, referent.
Publication Bias
There was no evidence of publication bias, as assessed by the Begg (P = 0.21) and Egger tests (P = 0.36). The modified Macaskill test confirmed this finding (P = 0.90). The funnel plot did not manifest any noticeable asymmetry (see Figure, Supplemental Digital Content 3, http://links.lww.com/QAI/A45).
DISCUSSION
In this systematic review of controlled DOT-HAART studies, we observed an overall beneficial effect of DOT-HAART on virologic, immunologic, and adherence outcomes. DOT-HAART was found to improve HAART adherence, supporting the presumed mechanism of DOT-HAART effectiveness on clinical outcomes through improved antiretroviral adherence.4,9,102,103 Qualitative data suggest that other mechanisms may also mediate DOT-HAARTeffectiveness, including positive effects on patients’ trust and communication with providers; increased patient motivation to engage in daily activities and become involved in the community; improved adherence to other aspects of medical care; and greater the utilization of other forms of social and adherence support.30,44,65,104–106
We encountered large variation in methodologic quality, intervention design, and population characteristics and explored their influence on the observed virologic effects through stratification and meta-regression. When stratified by study design, the positive effect of DOT-HAART on virologic and immunologic outcomes among RCTs was attenuated and not statistically significant, whereas the association remained significant in nonrandomized studies. The meta-analysis by Ford et al107 also found a lack of effect among RCTs (RR = 1.04 (95% CI: 0.91 to 1.20, P = 0.55), but this summary estimate was smaller than our findings. The potential reason for the difference may be that Ford et al107 included effect estimates from the postintervention period, during which the efficacy of DOT-HAART may have waned, as indicated by our findings. Experts often rely on RCTs for causal inference as randomization prevents the imbalance of confounding factors between intervention and control groups. Recognizing that those who were selected for DOT may have differed from those who received standard of care in characteristics that would affect outcomes, we investigated the impacts of baseline virologic or immunologic differences and of differential attrition on effect heterogeneity. Meta-regressions and stratifications did not detect any significant difference in effect based on these study characteristics. Thus, we cannot attribute the difference in effect between RCTs and nonrandomized studies to these factors. Instead, these findings may reflect true differences in effect by population characteristics or intervention design that varied between RCTs and non-randomized studies. Nonrandomized DOT-HAART experiences may have allowed greater flexibility in intervention design and modification and may have enrolled vulnerable populations in whom the intervention effect could be greatest.
Beyond methodological quality, there were considerable variations in DOT-HAART interventions and populations targeted. Meta-regression analyses failed to identify a clear source of heterogeneity. Nonetheless, some of the trends in intervention effect upon stratification merit further discussion. Greater effect on virologic outcome was observed among substance-using and HAART-experienced cohorts. These findings support the intuitive hypothesis that individuals at greatest risk of treatment nonadherence (including HAART-experienced individuals108,109 and substance users53,60,61,74,110) benefit most from this intervention. Residence and methadone-based DOT-HAART interventions demonstrated greater treatment effect compared with clinic-based interventions, although the effect among methadone-based interventions was not statistically significant. Choosing a convenient site—such as a methadone clinic or the patient’s residence—could enhance the effect of the intervention. Interventions delivered in patient homes, community-based vans, prisons, and methadone clinics may impose minimal additional burden on patients’ routines. On the other hand, the time and expenses of daily travel to a site (eg, HIV clinic, hospital) that is not part of a patient’s daily routine may pose important barriers to DOT-HAART adherence, in particular in resource-poor settings where the relative cost of traveling to health facilities may be even greater.
Not all DOT is the same. Enhanced DOT-HAART, defined as an intervention that provides additional material or behavioral adherence support not offered to the control group, seemed to enhance treatment effect. Consensus guidelines for treatment of tuberculosis endorsing DOT have pointed out that studies of DOTwith enablers have shown the highest treatment completion rates.91 Our findings suggest that the same observation may be true for DOT-HAART and that the use of additional motivations for adherence may improve outcomes,70,87,111 particularly among substance users.53,74,112–114 Ongoing RCTs such as that of Bangsberg et al84 and MOTIV8 are examples of enhanced DOT-HAART interventions intended to maximize the potential impact of DOT by administering other forms of adherence support, such as case management or adherence counseling based on motivational interviewing and cognitive behavioral techniques.51,84,104 Final data from such studies will provide important information on enhanced forms of DOT-HAART.
Although there were few studies that assessed post-intervention effect, we found that initial intervention effect may wane after completion of DOT support. Although these findings are consistent with a meta-analysis of a wide spectrum of HIV treatment adherence interventions,34 exploring this time-limited effect may be even more important for DOT-HAART, if the mechanism of action is through improved adherence via direct supervision. If DOT-HAART is to have a sustained effect on postintervention outcomes, interventions must be designed to engender psychosocial and behavioral changes in patients through DOT encounters, such as those described by several groups studying DOT-HAART.53,57,65,87,100,104,115 For this reason, although we did not identify a significant difference in intervention effect of enhanced vs. nonenhanced DOT on immediate outcomes, we speculate that enhanced DOT-HAART could lead to more lasting durability of intervention effect. Efforts to sustain the benefits of DOT postintervention may also require closer attention to the transition from DOT to self-administration and to individualizing DOT through varied frequency, intensity, and duration of support.116 If DOT-HAART effect is not durable, another option would be long-term or even life-long DOT-HAART for certain individuals or populations.41 Creating and implementing durable HAART adherence interventions remains an enormous challenge.34,85,87
Our review has several limitations. It was not feasible to blind abstractors to authors, institutions, or journals of the data reviewed; however, use of a standard extraction form, resolution of discordant abstractions, and involvement of third party minimized bias from lack of blinding. We could not overcome some of the heterogeneity across studies and differences in adherence measures, and we were unable to investigate the moderating effect of other potential variables, including unmeasured differences in DOT vs. control groups. As previously mentioned, the small number of studies limited the inferences that could be drawn from meta-regressions. To better understand findings across studies and to assemble data for the purposes of meta-analysis with greater ease in the future, we recommend that all forthcoming controlled studies on DOT-HAART report the 3 outcome measures as defined in this analysis.
Despite these limitations, our review of peer-reviewed controlled studies shows that DOT-HAART seems to be effective among selected patient populations, such as those with a history of prior HAART experience and/or substance use. Features of DOT-HAART which may increase treatment effect include nonclinic-based DOT and the provision of additional forms of adherence support. Because the impact of DOT-HAART on virologic response did not reach statistical significance when restricted to RCTs, the efficacy of DOT-HAART still remains in question. Areas for future research include assessment of long-term treatment effects and the refinement of DOT-HAART interventions to optimize the intensity, duration, and frequency according to patient need. Similar to the body of knowledge that has guided decisions on DOT for tuberculosis, efficacy trials should be complemented by outcomes data from large-scale DOT-HAART programs and cost-effectiveness analyses to inform public health decisions regarding whether and under what circumstances DOT-HAART should be employed.
Supplementary Material
Acknowledgments
P.C.D. received financial support for this study from a Harvard T32 training grant for HIV clinical research (PI: Ken Freedberg).
For their correspondence, input, and kind support, we would like to thank Sidney Atwood, Sergio Babudieri, David Bangsberg, Ronald Bosch, Kimberly Cullen, Allison DeLong, Molly Franke, Wendy Garland, José Girón González, Ana Horta, John Idoko, Massimiliano Lanzafame, Gregory Lucas, Grace Macalino, Donna Mildvan, Jennifer Mitty, Jean Nachega, Cynthia Pearson, Kathleen Ragland, Michael Rich, Avina Sarna, and Amy Wohl.
Footnotes
Preliminary findings were presented at the 4th International Conference on HIV Treatment Adherence, April 7, 2009, Miami, Florida by Dr. Sonya S. Shin.
We declare that we do not have any competing interests.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions this article on the journal’s Web site (www.jaids.com).
References
- 1.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. HIV/AIDS Department, World Health Organization; Geneva, Switzerland: 2006. [Accessed November 11, 2008]. revision. Available at: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [Google Scholar]
- 2.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 6.Gross R, Bilker WB, Friedman HM, et al. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15:2109–2017. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 7.Low-Beer S, Yip B, O’Shaughnessy MV, et al. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 8.Mannheimer SB, Matts J, Telzak E, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005;17:10–22. doi: 10.1080/09540120412331305098. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Berman SM, Swindells S, et al. Adherence of human immunodeficiency virus-infected patients to antiretroviral therapy. Clin Infect Dis. 1999;29:824–830. doi: 10.1086/520443. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett JA. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002;29(Suppl 1):S2–S10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson TF, Stewart KE, Funkhouser E, et al. Patient-perceived barriers to antiretroviral adherence: associations with race. AIDS Care. 2002;14:607–617. doi: 10.1080/0954012021000005434. [DOI] [PubMed] [Google Scholar]
- 13.Kumarasamy N, Venkatesh KK, Cecelia AJ, et al. Spectrum of adverse events after generic HAART in southern Indian HIV-infected patients. AIDS Patient Care STDS. 2008;22:337–344. doi: 10.1089/apc.2007.0093. [DOI] [PubMed] [Google Scholar]
- 14.Havlir DV, Currier JS. Complications of HIV disease and antiretroviral therapy. Top HIV Med. 2006;14:27–35. [PubMed] [Google Scholar]
- 15.Moreno-Cuerda VJ, Morales-Conejo M, Rubio R. Antiretroviral treatment associated life-threatening adverse events. Med Clin (Barc) 2006;126:744–749. doi: 10.1157/13088948. in Spanish. [DOI] [PubMed] [Google Scholar]
- 16.Wolf MS, Davis TC, Osborn CY, et al. Literacy, self-efficacy, and HIV medication adherence. Patient Educ Couns. 2007;65:253–260. doi: 10.1016/j.pec.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Shin S, Munoz M, Espiritu B, et al. Psychosocial impact of poverty on antiretroviral nonadherence among HIV-TB coinfected patients in Lima, Peru. J Int Assoc Physicians AIDS Care (Chic Ill) 2008;7:74–81. doi: 10.1177/1545109708315326. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee JS, Ivers L, Leandre F, et al. Antiretroviral therapy in resource-poor settings. Decreasing barriers to access and promoting adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S123–S126. doi: 10.1097/01.qai.0000248348.25630.74. [DOI] [PubMed] [Google Scholar]
- 19.Krain A, Fitzgerald DW. HIV antiretroviral therapy in resource-limited settings: experiences from Haiti. Curr HIV/AIDS Rep. 2005;2:98–104. doi: 10.1007/s11904-005-0025-3. [DOI] [PubMed] [Google Scholar]
- 20.Gordillo V, del Amo J, Soriano V, et al. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13:1763–1769. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 21.Bouhnik AD, Chesney M, Carrieri P, et al. Nonadherence among HIV-infected injecting drug users: the impact of social instability. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S149–S153. doi: 10.1097/00126334-200212153-00013. [DOI] [PubMed] [Google Scholar]
- 22.Rawlings MK, Thompson MA, Farthing CF, et al. Impact of an educational program on efficacy and adherence with a twice-daily lamivudine/zidovudine/abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34:174–183. doi: 10.1097/00126334-200310010-00007. [DOI] [PubMed] [Google Scholar]
- 23.Knobel H, Carmona A, Lopez JL, et al. Adherence to very active antiretroviral treatment: impact of individualized assessment. Enferm Infecc Microbiol Clin. 1999;17:78–81. in Spanish. [PubMed] [Google Scholar]
- 24.Jones DL, Ishii M, LaPerriere A, et al. Influencing medication adherence among women with AIDS. AIDS Care. 2003;15:463–474. doi: 10.1080/0954012031000134700. [DOI] [PubMed] [Google Scholar]
- 25.Goujard C, Bernard N, Sohier N, et al. Impact of a patient education program on adherence to HIV medication: a randomized clinical trial. J Acquir Immune Defic Syndr. 2003;34:191–194. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Safren SA, Hendriksen ES, Desousa N, et al. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care. 2003;15:787–793. doi: 10.1080/09540120310001618630. [DOI] [PubMed] [Google Scholar]
- 27.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15:841–847. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nachega JB, Knowlton AR, Deluca A, et al. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S127–S133. doi: 10.1097/01.qai.0000248349.25630.3d. [DOI] [PubMed] [Google Scholar]
- 29.Koenig LJ, Pals SL, Bush T, et al. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27:159–169. doi: 10.1037/0278-6133.27.2.159. [DOI] [PubMed] [Google Scholar]
- 30.Ciambrone D, Loewenthal HG, Bazerman LB, et al. Adherence among women with HIV infection in Puerto Rico: the potential use of modified directly observed therapy (MDOT) among pregnant and postpartum women. Women Health. 2006;44:61–77. doi: 10.1300/j013v44n04_04. [DOI] [PubMed] [Google Scholar]
- 31.Nicca D, Moody K, Elzi L, et al. Comprehensive clinical adherence interventions to enable antiretroviral therapy: a case report. J Assoc Nurses AIDS Care. 2007;18:44–53. doi: 10.1016/j.jana.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Koenig SP, Leandre F, Farmer PE. Scaling-up HIV treatment programmes in resource-limited settings: the rural Haiti experience. AIDS. 2004;18(Suppl 3):S21–S25. doi: 10.1097/00002030-200406003-00005. [DOI] [PubMed] [Google Scholar]
- 33.Fairley CK, Levy R, Rayner CR, et al. Randomized trial of an adherence programme for clients with HIV. Int J STD AIDS. 2003;14:805–809. doi: 10.1258/095646203322556129. [DOI] [PubMed] [Google Scholar]
- 34.Simoni JM, Pearson CR, Pantalone DW, et al. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41:285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 36.WHO. World Health Organization Global Tuberculosis Programme. Geneva: WHO; 1994. Framework for Effective Tuberculosis Control. [Google Scholar]
- 37.WHO. Treatment of Tuberculosis: Guidelines for National Programmes. 3. Geneva: World Health Organization; 2003. [Google Scholar]
- 38.Stop TB Partnership. Geneva: WHO; 2006. The Stop TB Strategy: Building on and enhancing DOTS to meet the TB-related Millennium Development Goals. [Google Scholar]
- 39.Woodward WC. Should directly observed therapy be considered for treatment of HIV? JAMA. 1996;276:1956. [PubMed] [Google Scholar]
- 40.Bangsberg DR, Mundy LM, Tulsky JP. Expanding directly observed therapy: tuberculosis to human immunodeficiency virus. Am J Med. 2001;110:664–666. doi: 10.1016/s0002-9343(01)00729-x. [DOI] [PubMed] [Google Scholar]
- 41.Farmer P, Leandre F, Mukherjee J, et al. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy) Bull World Health Organ. 2001;79:1145–1151. [PMC free article] [PubMed] [Google Scholar]
- 42.Liechty CA, Bangsberg DR. Doubts about DOT: antiretroviral therapy for resource-poor countries. AIDS. 2003;17:1383–1387. doi: 10.1097/00002030-200306130-00013. [DOI] [PubMed] [Google Scholar]
- 43.Lucas GM, Weidle PJ, Hader S, et al. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38(Suppl 5):S409–S413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- 44.Ma M, Brown BR, Coleman M, et al. The feasibility of modified directly observed therapy for HIV-seropositive African American substance users. AIDS Patient Care STDS. 2008;22:139–146. doi: 10.1089/apc.2007.0063. [DOI] [PubMed] [Google Scholar]
- 45.Macalino GE, Mitty JA, Bazerman LB, et al. Modified directly observed therapy for the treatment of HIV-seropositive substance users: lessons learned from a pilot study. Clin Infect Dis. 2004;38(Suppl 5):S393–S397. doi: 10.1086/421402. [DOI] [PubMed] [Google Scholar]
- 46.Machouf N, Lalonde RG. Directly observed therapy (DOT): from tuberculosis to HIV. Rev Epidemiol Sante Publique. 2006;54:73–89. doi: 10.1016/s0398-7620(06)76696-2. in French. [DOI] [PubMed] [Google Scholar]
- 47.Stenzel MS, McKenzie M, Mitty JA, et al. Enhancing adherence to HAART: a pilot program of modified directly observed therapy. AIDS Read. 2001;11:317–319. 324–328. [PubMed] [Google Scholar]
- 48.Wohl AR, Garland WH, Squires K, et al. The feasibility of a community-based directly administered antiretroviral therapy program. Clin Infect Dis. 2004;38(Suppl 5):S388–S392. doi: 10.1086/421401. [DOI] [PubMed] [Google Scholar]
- 49.Pearson CR, Micek M, Simoni JM, et al. Modified directly observed therapy to facilitate highly active antiretroviral therapy adherence in Beira, Mozambique. Development and implementation. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S134–S141. doi: 10.1097/01.qai.0000248339.82567.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maru DS, Kozal MJ, Bruce RD, et al. Directly administered antiretroviral therapy for HIV-infected drug users does not have an impact on antiretroviral resistance: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:555–563. doi: 10.1097/qai.0b013e318158c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goggin K, Liston RJ, Mitty JA. Modified directly observed therapy for antiretroviral therapy: a primer from the field. Public Health Rep. 2007;122:472–481. doi: 10.1177/003335490712200408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. [Accessed October 10, 2008];Partners In Health 2008 Annual Report: Keeping Our Promises, Pushing the Boundaries, Ensuring a Sustainable Future. 2008 Available at: http://www.pih.org/inforesources/annual/PIH2008_annualreport.pdf.
- 53.Altice FL, Maru DS, Bruce RD, et al. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnsten J, Berg K, Li X, et al. Directly Observed Antiretroviral Therapy Provided in Methadone Clinics: A Randomized Controlled Trial. Presented at: 4th International Conference on HIV Treatment Adherence; 2009; Miami, Florida. [Google Scholar]
- 55.Babudieri S, Aceti A, D’Offizi GP, et al. Directly observed therapy to treat HIV infection in prisoners. JAMA. 2000;284:179–180. doi: 10.1001/jama.284.2.179. [DOI] [PubMed] [Google Scholar]
- 56.Foisy MM, Akai PS. Pharmaceutical care for HIV patients on directly observed therapy. Ann Pharmacother. 2004;38:550–556. doi: 10.1345/aph.1D444. [DOI] [PubMed] [Google Scholar]
- 57.Goggin K, Gerkovich M, Wright J, et al. Review of a randomized controlled community trial utilizing mDOT. Presented at: 2nd International Conference on HIV Treatment Adherence; March 8–10, 2006; Jersey City, NJ. 2006. [Google Scholar]
- 58.Horta A, Mendez J, Gonzalez E, et al. Directly administered antiretroviral therapy (DAART) and methadone for the treatment of HIV-infected patients. Presented at: 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 2007; Sydney, Australia. p. Abstract WEPEB102. [Google Scholar]
- 59.Idoko JA, Agbaji O, Agaba P, et al. Direct observation therapy-highly active antiretroviral therapy in a resource-limited setting: the use of community treatment support can be effective. Int J STD AIDS. 2007;18:760–763. doi: 10.1258/095646207782212252. [DOI] [PubMed] [Google Scholar]
- 60.Lucas GM, Mullen BA, Weidle PJ, et al. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42:1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- 61.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell CG, Freels S, Creticos CM, et al. Preliminary findings of an intervention integrating modified directly observed therapy and risk reduction counseling. AIDS Care. 2007;19:561–564. doi: 10.1080/09540120601040813. [DOI] [PubMed] [Google Scholar]
- 63.Myung P, Pugatch D, Brady MF, et al. Directly observed highly active antiretroviral therapy for HIV-infected children in Cambodia. Am J Public Health. 2007;97:974–977. doi: 10.2105/AJPH.2005.083253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parsons GN, Siberry GK, Parsons JK, et al. Multidisciplinary, inpatient directly observed therapy for HIV-1-infected children and adolescents failing HAART: A retrospective study. AIDS Patient Care STDS. 2006;20:275–284. doi: 10.1089/apc.2006.20.275. [DOI] [PubMed] [Google Scholar]
- 65.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007;46:238–244. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tinoco I, Giron-Gonzalez JA, Gonzalez-Gonzalez MT, et al. Efficacy of directly observed treatment of HIV infection: experience in AIDS welfare homes. Eur J Clin Microbiol Infect Dis. 2004;23:331–335. doi: 10.1007/s10096-003-1099-8. [DOI] [PubMed] [Google Scholar]
- 67.Tossonian H, Raffa J, Grebely J, et al. The impact of ongoing illicit drug use on virologic suppression in HIV-infected injection drug users receiving HAART. Presented at: XVII International AIDS Conference; 2008; Mexico City, Mexico. [Google Scholar]
- 68.Tyndall MW, McNally M, Lai C, et al. Directly observed therapy programmes for anti-retroviral treatment amongst injection drug users in Vancouver: access, adherence and outcomes. Int J Drug policy. 2007;18:281–287. doi: 10.1016/j.drugpo.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Wohl AR, Garland WH, Valencia R, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42:1619–1627. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 70.Simoni JM, Amico KR, Pearson CR, et al. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Current Infect Dis Rep. 2008;10:515–521. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ford N, Nachega JB, Engel ME, et al. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374:2064–2071. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- 72.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 74.Malta M, Strathdee SA, Magnanini MM, et al. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction (Abingdon, England) 2008;103:1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 75.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 76.Altice FL, Springer SA. DAART for human immunodeficiency virus-infected patients: studying subjects not at risk for nonadherence and use of untested interventions. Arch Intern Med. 2010;170:109–110. doi: 10.1001/archinternmed.2009.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartlett C, Doyal L, Ebrahim S, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess. 2005;9:iii–iv. ix–x, 1–152. doi: 10.3310/hta9380. [DOI] [PubMed] [Google Scholar]
- 78.Britton A, McKee M, Black N, et al. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy. 1999;4:112–121. doi: 10.1177/135581969900400210. [DOI] [PubMed] [Google Scholar]
- 79.Ellington L, Wahab S, Sahami Martin S, et al. Factors that influence Spanish- and English-speaking participants’ decision to enroll in cancer randomized clinical trials. Psychooncology. 2006;15:273–284. doi: 10.1002/pon.943. [DOI] [PubMed] [Google Scholar]
- 80.Peterson ED, Lytle BL, Biswas MS, et al. Willingness to participate in cardiac trials. Am J Geriatr Cardiol. 2004;13:11–15. doi: 10.1111/j.1076-7460.2004.01709.x. [DOI] [PubMed] [Google Scholar]
- 81.Gross R, Tierney C, Andrade A, et al. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169:1224–1232. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herbison P. Problems with meta-analysis. N Z Med J. 1999;112:38–41. [PubMed] [Google Scholar]
- 83.Juni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 84.Bangsberg D, Hammer G, Reynolds M, et al. Adherence Case Management (ACM) Does Not Sustain the Effect of Modified Directly Observed Therapy (MDOT) in HIV-Positive Homeless and Marginally Housed (H/M) Individuals (or does it?). Presented at: 4th International Conference on HIV Treatment Adherence; 2009; Miami, FL. [Google Scholar]
- 85.Maru DS, Bruce RD, Walton M, et al. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;50:176–181. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nachega J, Goliath R, Efron A, et al. Randomized trial of trained patient-nominated treatment supporters providing partial directly observed ART in South Aftican adults initiating HIV therapy. Presented at: 16th Conference on Retroviruses and Opportunistic Diseases; 2009; Montreal, Canada. [Google Scholar]
- 87.Sarna A, Luchters S, Geibel S, et al. Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2008;48:611–619. doi: 10.1097/QAI.0b013e3181806bf1. [DOI] [PubMed] [Google Scholar]
- 88.Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 89.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 90.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 91.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: Consensus Statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279:943–948. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 92.Watkins K Office HDR. Human Development Report 2007/2008: Fighting Climate Change: Human Solidarity in a Divided World. New York: United Nations Development Programme UN; 2007. [Google Scholar]
- 93.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 94.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 96.Team RDC. R: A language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 97.Franke M, Socci A, Kaigamba F, et al. Early self-reported adherence among patients receiving clinic-based and community-based care in rural Rwanda. Presented at: XVII International AIDS Conference; August 3–8, 2008; Mexico City, Mexico. 2008. [Google Scholar]
- 98.Grodensky C, Golin C, Sunli A, et al. Effect on antiretroviral adherence of directly observed therapy (DOT) versus keep-on-my-person medication (KOM) among HIV-infected prisoners: The DOT-KOM Study. Presented at: 4th International Conference on HIV Treatment Adherence; 2009; Miami, FL. [Google Scholar]
- 99.Taiwo BO, Idoko JA, Otoh I, et al. Patient-selected treatment partner is associated with superior virologic response to first-line HAART in Jos, Nigeria. Presented at: 3rd International Conference on HIV Treatment Adherence; 2008; Jersey City, NJ. p. Abstract 167. [Google Scholar]
- 100.Munoz M, Finnegan K, Zeladita J, et al. Community-based DOT-HAART accompaniment in an urban resource-poor setting. AIDS Behav. 2009 doi: 10.1007/s10461-009-9559-5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lanzafame M, Trevenzoli M, Cattelan AM, et al. Directly observed therapy in HIV therapy: a realistic perspective? J Acquir Immune Defic Syndr. 2000;25:200–201. doi: 10.1097/00042560-200010010-00018. [DOI] [PubMed] [Google Scholar]
- 102.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. [Google Scholar]
- 103.Puigventos F, Riera M, Delibes C, et al. Adherence to antiretroviral drug therapy. A systematic review. Med Clin (Barc) 2002;119:130–137. doi: 10.1016/s0025-7753(02)73341-1. in Spanish. [DOI] [PubMed] [Google Scholar]
- 104.Bradley-Ewing A, Thomson D, Pinkston M, et al. A qualitative examination of the indirect effects of modified directly observed therapy on health behaviors other than adherence. AIDS Patient Care STDS. 2008;22:663–668. doi: 10.1089/apc.2007.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitty JA, Macalino G, Taylor L, et al. Directly observed therapy (DOT) for individuals with HIV: successes and challenges. MedGenMed. 2003;5:30. [PubMed] [Google Scholar]
- 106.Smith-Rohrberg D, Mezger J, Walton M, et al. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S48–S53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- 107.Ford N, Nachega JB, Engel ME, et al. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374:2064–2071. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- 108.Glass TR, De Geest S, Weber R, et al. Correlates of self-reported non-adherence to antiretroviral therapy in HIV-infected patients: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2006;41:385–392. doi: 10.1097/01.qai.0000186371.95301.52. [DOI] [PubMed] [Google Scholar]
- 109.Horberg M, Silverberg M, Hurley L, et al. Influence of prior antiretro-viral experience on adherence and responses to new highly active anti-retroviral therapy regimens. AIDS Patient Care STDS. 2008;22:301–312. doi: 10.1089/apc.2007.0101. [DOI] [PubMed] [Google Scholar]
- 110.Kohli R, Lo Y, Howard AA, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:864–872. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 111.Behforouz HL, Farmer PE, Mukherjee JS. From directly observed therapy to accompagnateurs: enhancing AIDS treatment outcomes in Haiti and in Boston. Clin Infect Dis. 2004;38(Suppl 5):S429–S436. doi: 10.1086/421408. [DOI] [PubMed] [Google Scholar]
- 112.Smith-Rohrberg D, Altice FL. Randomized, controlled trials of directly administered antiretroviral therapy for HIV-infected patients: questions about study population and analytical approach. Clin Infect Dis. 2006;43:1221–1222. doi: 10.1086/508357. Author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 113.Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18:262–270. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 114.Wood E, Kerr T, Tyndall MW, et al. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22:1247–1256. doi: 10.1097/QAD.0b013e3282fbd1ed. [DOI] [PubMed] [Google Scholar]
- 115.Flanigan TP, Taylor LE, Mitty JA. Use of community-based, directly observed therapy for HIV infection: lessons learned for treatment of hepatitis C virus infection. Clin Infect Dis. 2005;40(Suppl 5):S346–S348. doi: 10.1086/427451. [DOI] [PubMed] [Google Scholar]
- 116.Behforouz HL, Kalmus A, Scherz CS, et al. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J Acquir Immune Defic Syndr. 2004;36:642–645. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.