Abstract
Objectives
The aim of this study was to characterize cardiac reactivity measures, heart rate (HR) and heart rate variability (HRV), following acute intravenous (IV) alcohol administration and their association with subjective responses in social drinkers.
Methods
24 subjects (11 females) received IV alcohol infusions to attain and clamp the breath alcohol concentration (BrAC) at 50 mg% or placebo in separate sessions. Serial 5-min cardiac recordings at baseline and during the infusion were analyzed to obtain frequency and time-domain cardiac measures. Self-reported subjective perceptions were also obtained at the same time-points.
Results
HR showed significant decreases from baseline, while the HRV measure pNN50 showed steady increases during the ascending phase of alcohol infusion. HR was inversely correlated with pNN50 across time and treatment. There was a significant association of HR with subjective feelings of high, intoxication, feelings and liking of drug effects across time during the ascending phase.
Conclusions
Acute IV alcohol resulted in decreases in HR and increases in HRV consistent with autonomic parasympathetic activation. The association of these changes with subjective responses suggests that cardiac reactivity may serve as a physiological marker of subjective alcohol effects. This study broadens the understanding of acute cardiovascular effects of alcohol and clinically significant cardiac conditions like arrhythmia and cardiomyopathy associated with chronic alcohol drinking.
Keywords: Alcohol Clamp, Intravenous Infusion, Heart Rate, Heart Rate Variability, Subjective Perceptions
INTRODUCTION
Cardiac physiology is well-established to be influenced by alcohol with studies demonstrating effects of acute alcohol exposure on heart rate (HR), cardiac output, and blood pressure. In healthy individuals, alcohol ingestion has acute cardiovascular and autonomic effects that depend on the time elapsed after alcohol intake (Bau et al., 2011). These autonomic effects may include parasympathetic (vagal) impulses acting on the muscarinic receptors to generate bradycardia and sympathetic nerve impulses acting on the beta-adrenergic receptors (with the withdrawal of parasympathetic impulses) to induce tachycardia (Fauci et al., 2008). Alcohol can enhance these autonomic effects acting on the sympathetic system elevating HR (Ray et al., 2006), and influencing elevated parasympathetic vagal tone following acute intake that may lead to intoxication, bradycardia and hypotension (Eliaser and Giansiracusa, 1956; Trappe 2010). Some studies involving healthy populations suggest that acute alcohol effects support vagal mediation (Newlin et al., 1990), and smaller HR increases, indicating reduction of sympathetic influences at higher doses of alcohol, have been reported (Levenson et al., 1980). There are a limited number of reports of acute alcohol-induced decreases in HR (Friedman, 1984; Tsutsui et al., 1992; Trejbal and Mitro, 2008), with one study suggesting that alcohol’s negative ionotropic effect may be masked by autonomic nervous system (ANS) regulation (Child et al., 1979).
Alcohol also appears to have long-term effects on cardiac function. Chronic alcoholics demonstrate cardiac dysfunction, indicated by high HR and lowered heart rate variability (HRV) with moderate alcoholic doses (Metgaard and Somnier, 1981; Weise et at., 1986). Altered cardiac reactivity to alcohol-related cues has also been shown in alcoholics. High-craving alcoholics showed an immediate HR deceleration following exposure to masked and non-consciously accessible alcohol pictures (Hauser et al., 2003). More recently, Garland et al. (2012) have demonstrated a relationship between cue-elicited heart rate variability and relapse in alcoholics. Another recent study indicated that lower resting heart rate variability is associated with higher craving in alcohol dependent outpatients (Quintana et al., 2013a).
Almost all the studies examining cardiac responses to alcohol have been conducted using oral drinking paradigms, with responses measured across a range of alcohol concentrations (Romanowicz et al., 2011), and many have attempted to evaluate acute psychological and physiological responses (for example, intoxication, craving, cue-reactivity) to the patterns of exposure (Brunelle et al., 2004; Brunelle et al., 2007). Studies have shown significant changes in heart rate following acute oral alcohol along the ascending limb of the BrAC-time curve (Conrod et al., 1997). Studies have also shown associations between this heart-rate increase and subjective perceptions of stimulation (Brunelle et al., 2007), suggesting that sensitivity to alcohol-induced heart rate stimulation during the ascending limb of the blood alcohol curve may be a useful and informative marker for understanding susceptibility to alcoholism (Conrod et al., 2001).
Cardiac physiology has very rapid and complex endogenous and exogenous regulatory mechanism (Sizarov et al., 2011), which can present challenges in precise characterization of instantaneous psycho-physiological responses. In addition, the substantial variability in alcohol absorption kinetics following oral administration has limited the scope of accurate description of the immediate cardiac response to alcohol exposure, as well as the effect of the phase (ascending or descending) of the blood alcohol concentration vs. time curve. Thus, the objective of our study was to examine the effects of acute intravenous (IV) alcohol administration on measures of cardiac reactivity – heart rate and heart rate variability – in social drinkers. We hypothesized that acute IV alcohol administration would result in changes in heart rate and heart-rate variability in social drinkers, and that the effect of alcohol on HR and HRV would be associated with subjective responses to alcohol. We used the IV alcohol clamp method to achieve and maintain a precise target breath alcohol concentration, thus minimizing the substantial inter-individual variability in alcohol exposure following oral administration (Ramchandani et al., 1999; Ramchandani et al., 2006). This study was conducted in male and female social drinkers in two age groups – younger (21–25 years) and older (55–65 years), which provided an opportunity to examine the influence of sex and age on alcohol-induced changes in heart rate and heart rate variability. This was particularly relevant since studies have demonstrated significant effects of age and sex on baseline measures of HR and HRV in healthy adults (Agelink et al., 2001; Koskinen et al., 2009).
METHODS
Study Population
Data was collected as a part of a larger study examining the effects of age and sex on alcohol metabolism and response, and was approved by the Combined Neuroscience Institutional Review Board at the NIH. Participants were enrolled following written informed consent. The study sample consisted of a total of 24 (13 male and 11 female) non-smoking participants, aged 21–65 years, in good health as determined by a screening evaluation consisting of medical and psychiatric history and physical exam conducted by the study physician, electrocardiogram, and lab tests. Females of child-bearing potential had a negative urine pregnancy (human chorionic gonadotropin hormone, HCG) test prior to each study session. Older females were postmenopausal for at least one year prior to participation in the study.
Participants were excluded from the study, based on the results of the screening evaluation, if they had a current or prior history of cardiovascular, respiratory, gastrointestinal, hepatic, renal, endocrine, or reproductive disorders; current history of Axis-I psychiatric illness; current or prior history of any alcohol or drug dependence or abuse; positive urine drug screen for illicit drugs; self-reported abstention from alcohol; pregnancy or intention to become pregnant; menstrual cycle irregularities; use of oral contraceptive pills in female subjects; or use of prescription or over-the-counter (OTC) medications known to interact with alcohol within two weeks of the study. Table 1 lists morphometric information on the subjects, stratified into 4 sub-groups based on age and gender, as well as measures of recent drinking obtained during the weeks prior to the study sessions, including total standard drinks per week, number of drinking days per week, number of drinks per drinking day, and interval (in days) between most recent alcohol use prior to each study session.
Table 1.
Demographic characterization and recent drinking history of study sample. Data shown are Mean ± Standard Deviation.
| Measures | Younger Females (n=5) | Younger Males (n=9) | Older Females (n=6) | Older Males (n=4) | p-values for group differences1 |
|---|---|---|---|---|---|
| Age [years] | 22.8 ± 1.3 | 22.9 ± 0.9 | 59.5 ± 3.93 | 61 ± 1.83 | NS |
| Height [cm] | 169.2 ± 7.3 | 178.5 ± 6.9 | 163.9 ± 5.1 | 182.0 ± 4.9 | Sex: p<0.001 |
| Weight [kg] | 69.0 ± 10.7 | 78.1 ± 10.1 | 69.0 ± 6.0 | 95.9 ± 13.0 | Sex: p<0.001 |
| Body Mass Index | 20.4 ± 2.8 | 22.0 ± 2.3 | 21.0 ± 1.6 | 26.4 ± 4.0 | Sex: p=0.005 Age: p=0.034 |
| Recent Drinking History (average per week prior to study sessions) | |||||
| Total Drinks per Week | 1.9 ± 4.2 | 3.5 ± 3.4 | 2.8 ± 3.7 | 3.9 ± 5.4 | NS |
| Drinking Days per Week | 0.6 ± 1.9 | 1.1 ± 1.6 | 2.2 ± 1.7 | 2.5 ± 2.5 | Age: p=0.016 |
| Drinks per Drinking Day | 1.3 ± 3.2 | 2.7 ± 2.6 | 1.1 ± 2.8 | 1.1 ± 4.1 | NS |
| Interval (days) between most recent alcohol use and study sessions2 | 6.7 ± 2.8 | 5.3 ± 2.3 | 4.3 ± 2.4 | 4.3 ± 3.5 | Age: p=0.039 |
Main effects only. No significant interactions noted.
no difference in interval of non-drinking days prior to study sessions between alcohol and placebo sessions.
Study Design
This was a two-session, randomized, placebo-controlled, single-blind study. Participants received, in separate sessions in counter-balanced order, infusions of alcohol or saline (placebo) to achieve and maintain target breath alcohol concentrations (BrAC) of 50 mg% or 0 mg% (for placebo sessions). This target BrAC level is associated with the consumption of 2–3 alcoholic beverages in a social setting by social drinkers. The typical interval between study sessions was seven days, with a range of three to thirty days to accommodate individual and clinic schedules as well as to allow all younger women to be tested in the follicular phase of their menstrual cycles.
Procedures
During each session, participants arrived at the NIH Clinical Center at approximately 7:00AM, having fasted since midnight prior to the study session. A breathalyzer test was performed to ensure zero alcohol concentrations using the handheld breathalyzer Alcotest 7410 plus (Draeger Safety Inc., Co), and a urine beta-hCG test was performed on the female subjects to ensure that they were not pregnant at the start of each study session. Participants received a light breakfast (~300 Kcal) approximately 1 hour prior to the infusion in an attempt to standardize the effects of food on alcohol pharmacokinetics during the study (Ramchandani et al., 2001). An in-dwelling intravenous catheter was inserted into the ante-cubital vein of the non-dominant arm using sterile technique; this catheter was used for alcohol or placebo infusion and blood sampling.
Subjects received infusions of either 6% v/v alcohol or 0.9% normal saline administered in counter-balanced order between sessions. The infusion was based on a rate-profile physiologically-based pharmacokinetic model for alcohol (Ramchandani et al., 1999) using individualized estimates of the model parameters, which were based on the participant’s height, weight, age, gender. The profile consists of an exponentially increasing infusion rate from the start of the infusion until the target breath alcohol concentration (BrAC) of 50 mg% was reached at 15 min, followed by an exponentially decreasing infusion rate, which tapered to a constant steady-state value to maintain (or “clamp”) the BrAC at the target value for a predetermined duration of 165 min. For the placebo session, participants received an infusion of 0.9% saline using the identical infusion rate profile as that for the alcohol session to ensure identical fluid exposure. Serial BrAC measurements were obtained using the breathalyzer approximately every 5 min during both sessions, to ensure that the BrACs were within 5 mg% of the target and to enable minor adjustments to the infusion rates to overcome errors in parameter estimation and experimental variability (Ramchandani et al., 1999; Ramchandani and O’Connor, 2006). At the end of 3 hours, the infusion was terminated. BrAC measurements were obtained every 15–30 min until BrAC fell below 20 mg% after which the participant was provided with a meal and discharged.
Dependent Measures
HR was recorded using the MiniLogger Series ML-2000 system (Mini Mitter, Bend OR). This telemetric device uses a remote chest transponder attached to the chest with the help of electrodes, which relays each interbeat interval (N-N interval in Hertz (Hz)) back to it wirelessly. The electrodes were placed following insertion of the IV catheter, and HR recorded continuously, starting at least 15 min prior to the start of the infusion and ending approximately 15-min after the end of the infusion. The MiniLogger system has been documented to have very high accuracy (± 1 beat), resolution (1 beat per min), range (0–250 beats per min), and interbeat interval monitoring accuracy (± 1 millisecond or 1000 Hz) (MiniLogger Instrument Manual), as well as reliability (intra-class correlation coefficients ranging from 0.84–0.93) (Troutman et al., 1999). At the end of the session, the data were downloaded to a PC, cleaned to remove artifacts using the ML-2000 software, and assembled into a spreadsheet for further analysis.
Subjective response to alcohol was measured using self-report questionnaires including the Drug Effects Questionnaire (DEQ) (Jameson et al., 1989; de Wit and McCraken, 1990) and a visual analog scale measuring feelings of high and intoxication. The measures were obtained at baseline and serially during the infusion at 0, 5, 15, 30, 60, 90, 120, 135, 150 and 165 min following the start of infusion for 5 min at each of the time-points.
Data Analysis
The raw data for each session were processed, using in-house software developed in Matlab (Mathworks Inc., Natick, MA) to extract HR measures. A 5-min epoch just prior to the start of the infusion was processed to obtain baseline measures. Following the start of the infusion (time= 0 min), 5-min epochs were processed at the following intervals to obtain the dependent measures: 0–5, 5–10, 15–20, 30–35, 60–65, 90–95, 105–110, 120–125, 135–140, 150–155, 165–170, 175–180 min. Given that electrical artifacts may be present in any HRV data (Berntson and Stowell, 1998), each epoch was filtered using the processing software to automatically remove electromagnetic field interference (values > 4000 Hz), and then visually inspected for respiratory or other artifacts. A few epochs (7% and 17% of the epochs for the alcohol and placebo sessions, respectively) had to be excluded from analyses due to a large proportion of artifacts or electromagnetic field interference, or technical errors in data collection or download from the instrument.
The primary measures obtained at each time-point were mean heart rate (MHR), measured as the average NN interval in each epoch, and SDNN, the standard deviation of the NN interval in each epoch. Additional cardiac measures, as defined by the Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology (Malik, 1996) included: (1) NN50 (the number of successive NN intervals that differ by more than 50 ms), (2) pNN50 (NN50 count divided by the total number of all NN intervals), (3) RMSSD (root mean square of mean squared difference of successive NN intervals), (4) Total Power (TP) measured as the variation in HRV of all NN intervals across the frequency range (0.04 – 0.4 Hz), and (5) LF/HF ratio (low frequency [total spectral power of all the NN intervals between 0.04 and 0.15 Hz]/high frequency [total power of all NN intervals between 0.15 and 0.4 Hz]).
Change in HR frequency and variability measures and subjective response were evaluated, in separate analyses, across treatment groups (alcohol vs. placebo) and time using repeated-measures analysis of variance (RM-ANOVA). Sex and age and measures of recent drinking history were included as covariates in the analysis. Data were analyzed using mixed effects models (proc mixed) in SAS (version 9.3, SAS Institute Inc., Cary, NC). Association between HR measures and subjective response was examined using regression analysis. Since the main effect of alcohol on HR measures were seen during the ascending limb (first 15 min of infusion), the association analysis of HR measures and subjective responses were limited to the ascending limb of the BrAC-time curve. Regression analyses were conducted using SPSS version 19.0 (SPSS Inc., Chicago, IL).
RESULTS
HR and HRV: Time and Treatment Effects
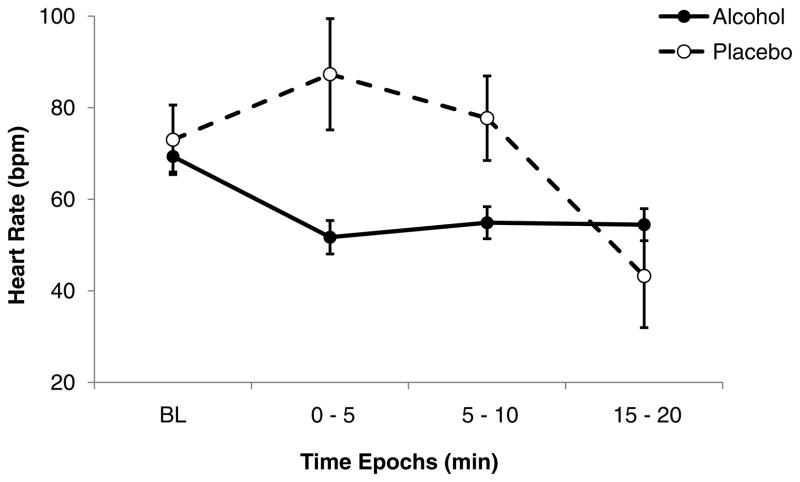
The time course of mean HR (MHR) showed a reduction from baseline values during the first 15 min of the infusion, (i.e., during the ascending phase of the BrAC-time curve) for both alcohol and placebo sessions, with no consistent changes during the clamp phase of the infusion. Given this pattern of response, we focused on changes during the ascending limb of the BrAC-time curve, and comparison of MHR by time between sessions (Figure 1) indicated a significant treatment x time interaction (F (3, 30) = 4.36, p = 0.012), with post-hoc tests indicating significant differences between alcohol and placebo sessions at the 0–5 min (p = 0.0009) and 5–10 min (p = 0.039) epochs. There was also a significant age group X treatment X time interaction (F (3, 30) = 4.88, p = 0.007), with post-hoc tests indicating greater alcohol-induced decrease in heart rate at the 0–5 min and 5–10 min epochs in older compared to the younger subjects.
Figure 1.
Time course of mean (with SE bars) heart rate during the ascending phase of the infusion profile. Closed symbols: alcohol session; open symbols: placebo session. There was a significant treatment x time interaction (F (3, 30) = 4.36, p = 0.012), with post-hoc tests indicating significant differences between alcohol and placebo sessions at the 0–5 min (p = 0.0009) and 5–10 min (p = 0.039) epochs.
Heart rate variability, as measured by SDNN, showed a small initial decrease in the alcohol session and an initial increase in the placebo session, with values in both sessions returning to nearly baseline values by 15 min. These effects did not reach statistical significance, although there was a significant time X age group interaction (F (3, 50) = 4.54, p = 0.0069), suggesting that there was a greater time-related effect in older subjects compared to the younger subjects. There were no consistent effects seen for the RMSSD.
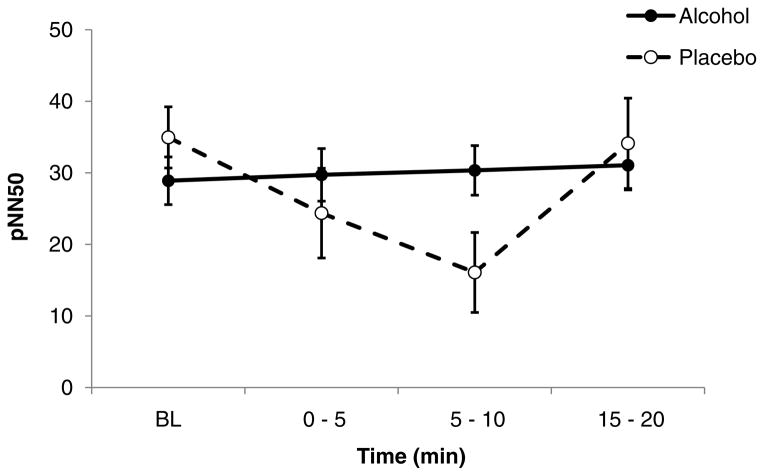
Another heart rate variability measure pNN50, showed a significant main effect of time (F (3, 60) = 3.03, p = 0.036), and a treatment X time interaction during the ascending limb (F (3, 23) = 3.58, p = 0.029). As illustrated in figure 2, the pNN50 showed a decrease during the ascending limb of the placebo session that was suppressed during the alcohol session, particularly at the 5–10 min epoch (p = 0.035). There was significant interactions of treatment X sex (F (1, 20) = 6.25, p = 0.021), with females showing higher values than males. The remaining HR and HRV measures, LF/HF ratio, RMSSD and NN50 did not show consistent treatment or time effects in this sample.
Figure 2.
Time course of mean (with SE bars) pNN50 during the ascending phase of the infusion profile. Closed symbols: alcohol session; open symbols: placebo session. There was a significant treatment x time interaction (F (3, 23) = 3.58, p = 0.029), with post-hoc tests indicating significant differences between alcohol and placebo sessions at the 5–10 min (p = 0.035) epoch.
Recent drinking history measures, including the interval between the most recent alcohol use and each study session, had no effect on the dependent measures or treatment and time-related effects. As anticipated, there was a significant association between the frequency and time domain measures of HR and HRV. During alcohol session, MHR showed inverse correlations with pNN50 (r = −0.27, p = 0.034) and RMSSD (r = −0.30, p = 0.012); while RMSSD was strongly correlated with pNN50 (r = 0.73, p < 0.0001). During placebo session, MHR was inversely correlated with pNN50 (r = −0.50, p = 0.004) and LF/HF was inversely associated with NN50 (r = −0.52, p = 0.018).
Association of HR with Subjective Response Measures
Examination of changes in subjective response measures showed monotonic increases in subjective response with time during the ascending phase of the alcohol session, and negligible changes during the placebo session. There were significant associations of subjective measures of high, intoxication (measured with the SHAS) with time and HR, indicating that time-related changes in heart rate were predictive of subjective measures of high and intoxication following alcohol. There were significant time X treatment interactions for subjective high (SHIH) (p < 0.001) and intoxication (SITX) (p < 0.001), as well as DEQ measures of feeling drug effects (DFEEL) (p = 0.002) and feeling high (DHIH) (p = 0.007). Given this pattern of responses, subsequent analysis of the association between HR measures and subjective responses focused on changes during the alcohol session only.
Table 2 shows the results of the regression of subjective response measures as a function of time and mean HR along with age group and sex. There were significant bivariate correlations between MHR and subjective high (r = 0.51, p < 0.05) and between HR and subjective intoxication (r = 0.57, p = 0.01). Age had a significant effect on subjective measure of feeling high following alcohol. These subjective response analyses with placebo were not significant.
Table 2.
Results of regression analysis of subjective measures with heart rate, age group, sex, and time. Data shown are coefficient of association (R2) for each subjective measure, along with standardized coeffieients (B) and p-values for each predictor. SHAS: Subjective High Assessment Scale; DEQ: Drug Effects Questionnaire; NS: Not significant.
| Independent Variable | SHAS High | SHAS Intoxicated | DEQ Feel Drug Effects | DEQ Like Drug Effects | DEQ Like More Drug | DEQ Feel High |
|---|---|---|---|---|---|---|
| R2=0.232 | R2=0.271 | R2=0.277 | R2=0.166 | R2=0.136 | R2=0.168 | |
| Age | B=−0.138 p=0.036 |
NS | B=−0.143 p=0.070 |
B=−0.179 p=0.076 |
B=−0.350 p=0.042 |
NS |
| MHR | B=0.178 p =0.018 |
B=0.185 p=0.012 |
B=0.245 p=0.007 |
B=0.264 p=0.022 |
NS | B=0.217 p=0.029 |
| Time | B=868 p=0.000 |
B=0.869 p=0.000 |
B=1.046 p=0.000 |
B=0.716 p=0.020 |
B=1.070 p=0.040 |
B=1.030 p=0.000 |
| Sex | NS | NS | NS | NS | NS | NS |
A similar pattern was seen for measures of feeling drug effects, liking drug effects and feeling high from the DEQ. Significant associations were seen with DEQ measures and time and MHR; indicating that time-related changes in heart rate were predictive of subjective measures of feeling and liking drug effects following alcohol. The correlation coefficients of MHR with DEQ measures were moderate to high (Feeling drug effects: r = 0.59, Liking drug effects: r = 0.51) at the significance level of 0.05.
DISCUSSION
The focus of the current study was to examine the effect of acute IV infusion of alcohol on cardiac reactivity, and the association of these changes with subjective response to alcohol in healthy individuals. The primary findings of this study were that acute IV alcohol resulted in a decrease in heart rate, and an associated increase in heart rate variability during the ascending phase of the IV infusion of alcohol compared with saline. These cardiac reactivity changes were associated with increases in subjective measures of high, intoxication, feeling drug effects, and liking drug effects. The pattern of heart rate changes following alcohol are consistent with an autonomic parasympathetic effect of alcohol resulting in a depression of cardiac contractility. Under placebo conditions, there was no significant relationship between MHR and subjective measures.
Previous studies have demonstrated both increases and decreases in heart rate, sometimes accompanied by increases in heart rate variability, that appear to vary with route of administration, dose, time following administration, and phase (ascending vs. descending) of the BrAC-time curve. Some studies have shown reductions in heart rate following alcohol compared to placebo in humans (Newlin et al., 2007, Levenson et al., 1980, Kupari et al., 1983, Weise et al., 1986), and animal models (Stratton et al., 1981). In these studies, the changes have been attributed to an alcohol-induced increase in parasympathetic control of heart rate regulation, or an alcohol-induced decrease in sympathetic tone. There are other studies that have demonstrated increases in heart rate following alcohol exposure (Conrod et al., 2001; Brunelle et al., 2004; Ray et. al, 2006; Bau et al., 2011). In these studies, the increased heart rate was attributed to an increase sympathetic arousal following oral alcohol administration. In our study, alcohol was administered systemically and resulted in a decrease in heart rate that may be a result of increased parasympathetic drive or decreased sympathetic tone (Weise et al., 1986, Polanczyk et al., 1998).
The current study also demonstrated an increase in heart rate variability, as measured by pNN50, following IV alcohol relative to placebo. pNN50 represents the parasympathetic change during respiratory changes (Calvert, 1998; Sztajzel, 2004), and increased pNN50 has been found to be associated with a decrease in vagus nerve activity (Malliani et al., 1994). Decreased vagal tone, as measured by spectral analysis of HRV data, has also been reported following oral alcohol ingestion (Levanon et al., 2002). The increase in the pNN50 values during the ascending phase of the alcohol infusion compared to placebo supports the hypothesis that the pharmacological effects of alcohol may be a result of increased parasympathetic influences. Not unexpectedly, the decrease in HR was inversely correlated with increase in the heart rate variability measure pNN50. This may be explained by the fact that a lower heart rate would result in elongation of the NN interval and a reduced number of NN events, and consequently a lower proportion of NN intervals that differed by more than 50 ms during the measured interval.
The effect of IV alcohol on HRV measures in this study contrast the findings of previous studies that have demonstrated decreases in measures of HRV following oral alcohol administration (Koskinen et al., 1994; Vaschillo et al., 2008; also see review by Romanowicz et al., 2011). Indeed, most studies have reported reductions in HRV following acute alcohol administration, although all but one of these studies used oral alcohol administration. A previous study using IV alcohol did not find changes in HRV, although the participants in that study were males with coronary heart disease that received IV alcohol to a much higher target blood alcohol level of 120 mg% (Rossinen et al., 1999). The review by Romanowicz (2011) also emphasized the wide variance in alcohol effects on HRV as a function of route of administration, dose, resulting breath alcohol levels, as well as inter-individual variation in response. It is possible that the difference between the findings of the current study and previous studies is due to the use of the IV route of administration in the current study, which provides a rapid controlled increase in systemic exposure to the target level (50 mg%), which may have activated parasympathetic mechanisms resulting in increased HRV. Additionally, the findings of this study are consistent with a recent study demonstrating increased HRV in moderate drinkers compared to abstainers (Quintana et al., 2013b). Another recent review indicates that use of alcohol approximating 1 to 2 standard drinks is associated with increased HRV compared with abstention or less frequent use (Karypak, 2013). This review also indicates that heavier use of alcohol may be associated with decreased HRV, suggesting a J-shaped relationship between alcohol use and HRV changes.
Results also indicated the influence of age and sex on the cardiac reactivity induced by alcohol in this study. There was a greater alcohol-induced decrease in heart rate during the ascending phase in the older subjects, as well as a greater time-related effect of alcohol on SDNN in the older subjects. While age-related influences on the cardiovascular and other pharmacodynamic effects of acute alcohol administration have not been well-studied, the current findings are consistent with other reports of greater sensitivity to alcohol and other psychopharmacological agents in older individuals (Kalant, 1998, Bowie and Slattum, 2007).
The changes in heart rate and heart rate variability following alcohol were associated with increased subjective responses to alcohol on measures of high, intoxication, feeling drug effects, and liking drug effects. The changes in cardiac reactivity during the ascending phase of the BrAC-time curve, when subjective perceptions of alcohol’s effects are also increasing in intensity, suggest a relationship between these cardiac and behavioral changes. While causality cannot be determined from these data, they do imply that they may be related. Similar associations between cardiac responses and subjective responses to alcohol have been previously reported, however in these studies, the increased subjective response was associated with increases in heart rate seen following oral alcohol administration (Brunelle et al., 2007; Conrod et al., 1997; Ray et al., 2006). On the other hand, our finding of decreased heart rate associated with increased subjective response to alcohol is consistent with previous reports (Newlin et al., 1990; Levenson et al., 1980). These studies have suggested that the decrease in heart rate following alcohol may reflect a stress-dampening or tension-reduction effect of alcohol that could lead to the subjective perceptions of high and intoxication following alcohol (Greeley and Oei, 1999). Many studies have implied that the stimulant-like effects are predominantly seen in the early phases of alcohol exposure, usually when BrAC levels are rising (Friedman et al., 1980; Mello, 1983; Conrod et al., 2001; also see recent review by Hendler et al., 2013), and some of these effects may be accompanied by a decreased in stress-responsiveness, including a lowering of heart-rate following alcohol administration. There are also studies demonstrating that cues associated with alcohol can result in alterations in heart rate and heart rate variability (Hauser et al., 2003; Ingjaldsson et al., 2003; Garland et al., 2012), suggesting that the tachycardia and decreased heart rate variability seen in oral alcohol studies may be due, in part, to the olfactory, visual and gustatory cues associated with drinking alcohol. The current study used IV alcohol which is devoid of these cues, thereby reflecting a more systemic response to alcohol compared to that seen following oral alcohol, which may explain the differences between this study and prior studies that used oral alcohol administration.
Changes in cardiac reactivity following acute alcohol exposure may be associated with chronic effects of prolonged alcohol use. Heart rate and heart rate variability changes have demonstrated greater efficacy over quiescent cardiac reactivity in reflecting autonomic responses and predicting subsequent physiological outcomes (Billman and Kukielka, 2006; Thayer and Lane, 2000). Indeed, recent reviews have indicated the potential utility of alcohol-induced change in heart-rate variability as a biomarker of alcoholism (Romanowicz et al., 2011; Karypak et al., 2013). Decreased HRV has been associated with increased cardiovascular morbidity and mortality (Liao et al., 1997; La Rovere et al., 2003), while increased HRV may been associated with better physical and mental wellbeing and health outcomes, as recently reviewed by Kemp and Quintana (2013). In further support of this, moderate alcohol consumption is associated with increased HRV (Quintana et al., 2013a) and confers a protective effect in comparison to abstinence, while heavy alcohol consumption and alcohol dependence are associated with decreased HRV and poorer outcomes (Quintana et al., 2013c; Karypak et al., 2013). However, further studies may be necessary to better understand the acute cardiac effects of alcohol, and how they relate to the mechanisms underlying the cardioprotective effects of low to moderate alcohol consumption. This study also demonstrates that the IV alcohol clamp provides a well-controlled experimental platform with minimal pharmacokinetic variability to study the acute effects of alcohol on measures of cardiac reactivity and contractility in populations at risk for clinically significant cardiac conditions such as cardiomyopathy or arrhythmias.
The HR and HRV paradigms and the associations with the subjective measures may serve as a measure of the positive reinforcing properties of acute alcohol exposure. This investigation can also help broaden the understanding of the relationship between acute cardiovascular effects and clinically significant cardiac medical conditions like cardiomyopathy and arrhythmia.
Acknowledgments
This study was supported by the NIAAA Division of Intramural Clinical and Biological Research (1Z01 AA000466). The authors gratefully acknowledge the NIH Clinical Center Alcohol Clinic and Day Hospital staff for clinical support, research staff (Elizabeth Edenberg, Mike Hoefer, Nina Saxena, Shilpa Kumar, Seth Eappen, Julnar Issa) for data collection support, and the study volunteers for their participation in the study.
References
- Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, Ziegler D. Standardized tests of heart rate variability: normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clinical autonomic research: Official journal of the Clinical Autonomic Research Society. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- Bau PFD, Moraes RS, Bau CHD, Ferlin EL, Rosito GA, Fuchs FD. Acute ingestion of alcohol and cardiac autonomic modulation in healthy volunteers. Alcohol. 2011;45:123–129. doi: 10.1016/j.alcohol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Stowell JR. ECG artifacts and heart period variability: Don’t miss a beat! Psychophysiology. 1998;35:127–132. [PubMed] [Google Scholar]
- Billman GE, Kukielka M. Effects of endurance exercise training on heart rate variability and susceptibility to sudden cardiac death: Protection is not due to enhanced cardiac vagal regulation. J App Physio. 2006;100:896–906. doi: 10.1152/japplphysiol.01328.2005. [DOI] [PubMed] [Google Scholar]
- Bowie MW, Slattum PW. Pharmacodynamics in the elderly: A review. Am J Geriatr Pharmacother. 2007;5:263–303. doi: 10.1016/j.amjopharm.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Assaad JM, Barrett SP, Ávila C, Conrod PJ, Tremblay RE, Pihl RO. Heightened heart rate response to alcohol intoxication is associated with a reward-seeking personality profile. Alcohol Clin Exp Res. 2004;28:394–401. doi: 10.1097/01.alc.0000117859.23567.2e. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Barrett SP, Pihl RO. Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Hum Psychopharmacol. 2007;22:437–443. doi: 10.1002/hup.866. [DOI] [PubMed] [Google Scholar]
- Calvert CA. Heart rate variability. Vet Clin N Am Small Anim Pract. 1998;28:1409–1427. doi: 10.1016/s0195-5616(98)50129-5. [DOI] [PubMed] [Google Scholar]
- Child JS, Kovick RB, Levisman JA, Pearce ML. Cardiac effects of acute ethanol ingestion unmasked by autonomic blockade. Circulation. 1979;59:120–125. doi: 10.1161/01.cir.59.1.120. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Biphasic effects of Alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcohol Clin Exp Res. 1997;21(1):140–149. [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacol. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- de Wit H, McCracken SG. Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res. 1990;14:63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Eliaser M, Jr, Giansiracusa FJ. The heart and alcohol. Cali med. 1956;84:234. [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 17 McGraw Hills; 2008. [Google Scholar]
- Friedman HJ, Carpenter JA, Lester D, Randall CL. Effect of alpha-methyl-p-tyrosine on dose dependent mouse strain differences in locomotor activity after ethanol. J Stud Alcohol. 1980;41:1–7. doi: 10.15288/jsa.1980.41.1. [DOI] [PubMed] [Google Scholar]
- Friedman HS. Cardiovascular effects of alcohol with particular reference to the heart. Alcohol (Fayetteville, NY) 1984;1:333–339. doi: 10.1016/0741-8329(84)90057-0. [DOI] [PubMed] [Google Scholar]
- Garland EL, Franken IHA, Howard MO. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology. 2012;222:17–26. doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeley J, Oei T. Alcohol and tension reduction. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. 2. New York: Guilford Press; 1999. pp. 203–248. [Google Scholar]
- Ingjaldsson JT, Thayer JF, Laberg JC. Craving for alcohol and pre-attentive processing of alcohol stimuli. Intl J Psychophysiol. 2003;49:29–39. doi: 10.1016/s0167-8760(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Behavioral Neurobiology of Alcohol Addiction. Springer; Berlin Heidelberg: 2013. Stimulant and sedative effects of alcohol; pp. 489–509. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Jameson JL, Johanson CE, de Wit H. The use of choice procedures for assessing the reinforcing properties of drugs in humans. Testing for abuse liability of drugs in humans. In: Fischman MW, Mello NK, editors. Testing for abuse liability of drugs in humans. US Government Printing Office; Washington, D.C: 1989. pp. 123–146. NIDA Research Monograph No. 92. [Google Scholar]
- Kalant H. Pharmacological interactions of aging and alcohol. In: Gomberg ESL, Hegedus AM, Zucker RA, editors. Alcohol problems and aging. Bethesda, MD: NIH; 1998. NIAAA Research monograph No. 33. NIH Pub. No. 98–4163. [Google Scholar]
- Karpyak VM, Romanowicz M, Schmidt JE, Lewis KA, Bostwick JM. Characteristics of Heart Rate Variability in Alcohol-Dependent Subjects and Nondependent Chronic Alcohol Users. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12270. available online. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS. The Relationship Between Mental and Physical Health: Insights from the Study of Heart Rate Variability. Int J Psychophysiology. 2013 doi: 10.1016/j.ijpsycho.2013.06.018. Available online: doi: http://dx.doi.org/10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed]
- Koskinen P, Virolainen J, Kupari M. Acute alcohol intake decreases short-term heart rate variability in healthy subjects. Clinical Science. 1994;87(Pt 2):225–230. doi: 10.1042/cs0870225. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Kähönen M, Jula A, Laitinen T, Keltikangas-Järvinen L, Viikari J, et al. Short-term heart rate variability in healthy young adults. Autonomic Neuroscience: Basic and Clinical. 2009;145(1–2):81–88. doi: 10.1016/j.autneu.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Kupari M, Eriksson CJP, Heikkila J, Ylikahri R. Alcohol and the heart. Intense hemodynamic changes associated with alcohol flush in Orientals. Acta Medica Scandinavica. 1983;213:91–98. [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- Levanon D, Goss B, Chen JDZ. Inhibitory effect of white wine on gastric myoelectrical activity and the role of vagal tone. Digestive Diseases and Sciences. 2002;47:2500–2505. doi: 10.1023/a:1020560026051. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Sher KJ, Grossman LM, Newman J, Newlin DB. Alcohol and stress response dampening: pharmacological effects, expectancy, and tension reduction. J Ab Psych. 1980;89:528–538. doi: 10.1037//0021-843x.89.4.528. [DOI] [PubMed] [Google Scholar]
- Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, et al. Cardiac autonomic function and incident coronary heart disease: A population-based case-cohort study. American Journal of Epidemiology. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- Malik M, Camm AJ, Bigger JT, Jr, Breithardt G, Cerutti S, Cohen RJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Euro Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F. Methods for assessment of sympatho-vagal balance: Power spectral analysis. In: Levy MN, Schwartz PJ, editors. Vagal control of the heart: Experimental basis and clinical implications. Armonk, NY: Futura; 1994. pp. 433–454. [Google Scholar]
- Mello N. A behavioral analysis of the reinforcing properties of alcohol and other drugs in man. In: Kissin B, Begleiter H, editors. The biology of alcoholism. Vol. 7. Plenum Press; New York: 1983. [Google Scholar]
- Metgaard B, Somnier F. Cardiac neuropathy in chronic alcoholics. Clinic Neuro Neurosurg. 1981;83:219–224. doi: 10.1016/0303-8467(81)90044-5. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Levenson RW. Pre-ejection period: Measuring beta-adrenergic influences upon the heart. Psychophysiology. 2007;16:546–552. doi: 10.1111/j.1469-8986.1979.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Byrne EA, Porges SW. Vagal Mediation of the effect of alcohol on Heart Rate. Alcohol Clin Exp Res. 1990;14:1403–1421. doi: 10.1111/j.1530-0277.1990.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Polanczyk CA, Rohde LEP, Mores RS, Ferlin EL, Leite C, Ribeiro JP. Sympathetic nervous system representation in time and frequency domain indices of heart rate variability. Eur J Appl Physiol. 1998;79:69–73. doi: 10.1007/s004210050475. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, McGregor IS, Hickie IB, Kemp AH. Heart rate variability predicts alcohol craving in alcohol dependent outpatients: Further evidence for HRV as a psychophysiological marker of self-regulation. Drug Alcohol Depend. 2013a;132:395–398. doi: 10.1016/j.drugalcdep.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, McGregor IS, Hickie IB, Kemp AH. Moderate alcohol intake is related to increased heart rate variability in young adults: Implications for health and well-being. Psychophysiology. 2013b doi: 10.1111/psyp.12134. Available online. [DOI] [PubMed] [Google Scholar]
- Quintana DS, McGregor IS, Guastella AJ, Malhi GS, Kemp AH. A meta-analysis on the impact of alcohol dependence on short-term resting-state heart rate variability: Implications for cardiovascular risk. Alcohol Clin Exp Res. 2013c;37(Suppl 1):E23–E29. doi: 10.1111/j.1530-0277.2012.01913.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Kwo PY, Li TK. Effect of food and food composition on alcohol elimination rates in healthy men and women. J Clin Pharmacol. 2001;41:1345–1350. doi: 10.1177/00912700122012814. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S. Studying alcohol elimination using the alcohol clamp method. Alcohol Res Health. 2006;29:286–290. [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Neumark Y, Zimmermann US, Morzorati SL, de Wit H. The alcohol clamp: Applications, challenges, and new directions - an RSA 2004 symposium summary. Alcohol Clin Exp Res. 2006;30:155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Ray L, McGeary J, Marshall E, Hutchison KE. Risk factors for alcohol misuse: Examining heart rate reactivity to alcohol, alcohol sensitivity, and personality constructs. Add Beh. 2006;31:1959–1973. doi: 10.1016/j.addbeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Romanowicz M, Schmidt JE, Bostwick JM, Mrazek DA, Karpyak VM. Changes in heart rate variability associated with acute alcohol consumption: Current knowledge and implications for practice and research. Alcohol Clin Exp Res. 2011;35:1092–1105. doi: 10.1111/j.1530-0277.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- Rossinen J, Sinisalo J, Partanen J, Nieminen MS, Viitasalo M. Effects of acute alcohol infusion on duration and dispersion of QT interval in male patients with coronary artery disease and in healthy controls. Clinical Cardiology. 1999;22:591–594. doi: 10.1002/clc.4960220910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizarov A, Ya J, De Boer BA, Lamers WH, Christoffels VM, Moorman AFM. Formation of the building plan of the human heart: Morphogenesis, growth, and differentiation. Circulation. 2011;123:1125–1135. doi: 10.1161/CIRCULATIONAHA.110.980607. [DOI] [PubMed] [Google Scholar]
- Stratton HT, Dormer KJ, Zeiner AH. The cardiovascular effects of ethanol and acetaldehyde in exercising dogs. Alcohol Clin Exp Res. 1981;5:56–63. doi: 10.1111/j.1530-0277.1981.tb04865.x. [DOI] [PubMed] [Google Scholar]
- Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Dis. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Trappe HJ. Life-threatening brady- and tachyarrhythmias. Internist. 2010;51:975–986. doi: 10.1007/s00108-009-2539-z. [DOI] [PubMed] [Google Scholar]
- Trejbal K, Mitro P. ECG changes in alcoholic intoxication. Vnitr Lek. 2008;54:410–414. [PubMed] [Google Scholar]
- Troutman SR, Allor KM, Hartmann DC, Pivarnik JM. MINI-LOGGER® reliability and validity for estimating energy expenditure and heart rate in adolescents. Research Quarterly for Exercise and Sport. 1999;70(1):70–74. doi: 10.1080/02701367.1999.10607732. [DOI] [PubMed] [Google Scholar]
- Tsutsui M, Matsuguchi T, Tsutsui H, Yoshida T, Yoshihara S, Yamamoto K, et al. Alcohol-induced sinus bradycardia and hypotension in patients with syncope. Jap Heart J. 1992;33(6):875–879. doi: 10.1536/ihj.33.875. [DOI] [PubMed] [Google Scholar]
- Van De Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation. 1997;95:1449–1454. doi: 10.1161/01.cir.95.6.1449. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, Ray S. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1-hz stimulation. Psychophysiology. 2008;45:847–858. doi: 10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise F, Krell D, Brinkhoff N. Acute alcohol ingestion reduces heart rate variability. Drug Alc Dep. 1986;17:89–91. doi: 10.1016/0376-8716(86)90040-2. [DOI] [PubMed] [Google Scholar]
- Weise F, Muller D, Krell D. Heart rate variability in withdrawing alcoholic patients. Drug & Alc Dep. 1985;16(1):85–88. doi: 10.1016/0376-8716(85)90085-7. [DOI] [PubMed] [Google Scholar]