Abstract
Freshly ejaculated spermatozoa are incapable or poorly capable of fertilizing an oocyte. The fertilization aptness of spermatozoa depends on the appropriate and time-dependent acquisition of hyperactivation, chemotaxis, capacitation, and the acrosome reaction, where calcium (Ca2+) is extensively involved in almost every step. A literature review showed that several ion channel proteins are likely responsible for regulation of the Ca2+ uptake in spermatozoa. Therefore, manipulation of the functions of channel proteins is closely related to Ca2+ influx, ultimately affecting male fertility. Recently, it has been shown that, together with different physiological stimuli, protein-protein interaction also modifies the Ca2+ influx mechanism in spermatozoa. Modern proteomic analyses have identified several sperm proteins, and, therefore, these findings might provide further insight into understanding the Ca2+ influx, protein functions, and regulation of fertility. The objective of this review was to synthesize the published findings on the Ca2+ influx mechanism in mammalian spermatozoa and its implications for the regulation of male fertility in the context of sperm proteins. Finally, Pathway Studio (9.0) was used to catalog the sperm proteins that regulate the Ca2+ influx signaling by using the information available from the PubMed database following a MedScan Reader (5.0) search.
1. Introduction
Spermatozoa are atypical cells with peculiar functionality: they are produced in one organism and released, and then they invade another organism and deliver their genetic material into a host cell to produce offspring by sexual reproduction. It is a well-known fact that only about 1 in 25,000 spermatozoa finally reaches the fallopian tube and gets the opportunity to fertilize an oocyte. In the mid-20th century, it had been claimed that mammalian spermatozoa are unable to fertilize an oocyte before achieving functional maturation, which occurs during their journey through the female reproductive tract for a finite period of time [1, 2]. This fundamental maturational process is chiefly regulated by numerous signaling cascades, and calcium (Ca2+) plays a dynamic role in this process, as an intracellular second messenger. Several studies have hypothesized that elevation of sperm intracellular Ca2+ ([Ca2+]i)/Ca2+ influx regulates motility, hyperactivation, chemotaxis, capacitation, and the acrosome reaction and facilitates the spermatozoa reaching and fertilizing of an oocyte [3–8]. Therefore, understanding the mechanism that regulates the Ca2+ influx in spermatozoa is a matter of utmost importance.
Previous studies have shown that the Ca2+ entry mechanisms are regulated via numerous Ca2+ permeable channel proteins in spermatozoa [6, 9, 10]. Therefore, the factors that regulate the functions of those channels will ultimately help us understand how male fertility is regulated. Recent applications of proteomic approaches such as two-dimensional polyacrylamide gel electrophoresis, mass spectrometry, and differential in-gel electrophoresis have yielded the identification of several sperm-specific proteins [11, 12]. These discoveries have provided new insight into protein functions and enabled us to recognize diverse sperm-specific processes in order to differentiate normal from abnormal spermatozoa [11]. Mature spermatozoa are widely known to be silent in both transcription and translation [11, 13, 14] or poorly capable of translation [15]; therefore, studies on individual sperm proteomes have described the importance of spermatozoal posttranslational modifications and their ability to induce physiological changes as a prerequisite for successful fertilization.
Torres-Flores et al. [16] have shown that human spermatozoa exposed to the phosphodiesterase inhibitor papaverine cause activation of protein kinase A (PKA) and stimulate the progesterone-induced Ca2+ influx via the cyclic adenosine monophosphate- (cAMP-) dependent pathway. Although these authors did not evaluate the relationship between in vitro fertility and Ca2+ influx, changes in intracellular pH and increased tyrosine phosphorylation ultimately provide a potential clue regarding sperm fertility competence. In another study, to evaluate hamster spermatozoa capacitation capability, comparative association was observed between pyruvate dehydrogenase A, Ca2+ influx, cAMP, and reactive oxygen species [17]. Additionally, Breitbart et al. [18] reported that polymerization of globular- (G-) actin to filamentous- (F-) actin occurs during capacitation. As capacitation and the acrosome reaction are Ca2+-mediated events [4, 5], one can, without considering further signaling cascade, assume that remodeling the actin structure might be linked with the regulation of Ca2+ influx in spermatozoa.
Recently, in our laboratory, we found that the manipulation of sperm proteins such as ubiquinol-cytochrome-c reductase core protein 2 (UQCRC2) [39], voltage-dependent anion channels proteins (VDACs) [4], and arginine vasopressin [5] could control the Ca2+ influx in spermatozoa and regulate capacitation, the acrosome reaction, and fertility. Therefore, design and construction of a similar study with most of the identified sperm proteins available from several protein databases might provide a more realistic insight into the Ca2+ influx, protein functions, and fertility. The present work reviews the latest information published by other laboratories as well as our research team on the aforementioned aspects of spermatozoa and their potential implications for diagnosis and prognosis of male fertility.
2. Mechanism of Ca2+ Influx in Mammalian Spermatozoa
The ultimate goal of fertilization of mammalian sperm is to fuse with and deliver their genetic materials into an oocyte [2, 40, 41]. For fertilization to occur completely, the spermatozoa must experience various obstacles both in vitro and in vivo [40, 41]. Ca2+ ions act as central signaling molecules; once they enter the spermatozoa, they exert allosteric regulatory effects on enzymes and many proteins [10, 21, 42]. Indeed, numerous elegant research findings have contributed significantly to our understanding of the molecular signaling of Ca2+ influx, especially through monitoring the activity of individual cells. However, most of the studies are discrete and often do not represent a cumulative idea. This section presents a compilation of some basic information regarding the Ca2+ entry mechanism into mammalian spermatozoa by recapitulating scientific evidence.
The literature reviewed shows that the primary source of Ca2+ for spermatozoa is the external environment: the fallopian tube in the female reproductive tract (in vivo) and culture media (in vitro) [8], and simultaneously increasing [Ca2+]i regulates the release of Ca2+ into the cell. Therefore, how Ca2+ crosses into cells through the sperm plasma membrane is a matter of paramount importance. In eukaryotic cells, the Ca2+ influx occurs through specific Ca2+ permeable ion channel proteins located on the plasma membrane [43, 44] such as classical voltage-gated (high and low) Ca2+ (Cavs), transient receptor potential (TRP), and cyclic nucleotide-gated (CNG) channels [9]. Recently, Ren and Xia have proposed four criteria to identify sperm ion channel proteins: detectability in sperm, preferably with knockout sperm as a negative control; ability to produce ion channel current detectable by patch-clamp recording; blocking of the channels that impairs normal sperm function; and mutations of gene encoding the ion channel proteins leading to sperm malfunctions [10].
The CatSper family of channels is the newest and only family of voltage-gated Ca2+ channels that meets most of the aforementioned criteria and essentially regulates Ca2+ entry into cells and is therefore crucial for sperm fertility [9, 45]. Four pore-forming CatSper channel proteins, CatSper 1–4, and at least two auxiliary subunits, CatSperβ and CatSperγ, have been identified in a wide range of animals, including humans and mice [46, 47]. Physiologically, CatSper members are permeable to Ca2+,whereas the CatSper knockdown sperm does not have the channel current that is detected in the principal piece of wild-type sperm [20, 48]. Most of the channel proteins, including CatSper members, have been identified in the principal piece of spermatozoa [20, 46, 47, 49] (Figure 1). Although the explanation of such subcellular localization is still debated, it might be because of interactions among the channel proteins and with the auxiliary subunits, although a further study is needed to resolve this issue. Collectively, these proteins play a key role in various cellular processes via regulation of the membrane potential and intracellular ionic balance. Carlson et al. [50] and Quill et al. [51] have conclusively proved that CatSper1 and CatSper2 null mice are sterile owing to their inability to generate the sperm-hyperactivated motility prerequisite for penetration of an oocyte extracellular matrix. In effect, the complete or partial absence of single or multiple Ca2+ channels is responsible for infertility or subfertility, although their underlying signaling cascade has not been properly studied.
Figure 1.
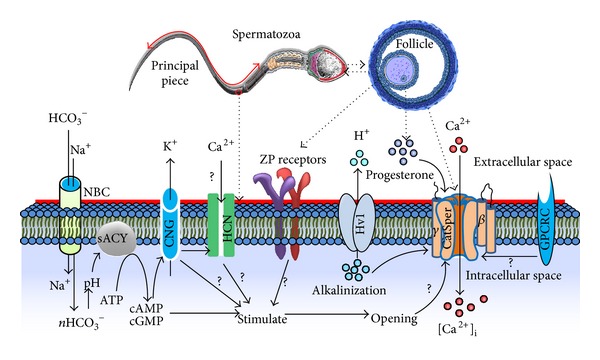
Possible signal transduction mechanisms of mammalian sperm Ca2+ influx through the Ca2+ permeable channel proteins. Previously published studies were used as references to summarize the list of channel proteins in spermatozoa. The channel proteins are localized mainly in the principle piece of spermatozoa. The follicular fluid and several factors in the fallopian tube (in vitro media) stimulate the receptors for spermatozoa Ca2+ influx. Ca2+ influx in spermatozoa is principally regulated by CatSper channels; however, the possible interaction between other channels that are responsible for controlling the opening of CatSper and allowing the Ca2+ into cells is indicated by arrow signs (red circle). The different channel proteins that are depicted in the diagram include the Na+-coupled HCO3 − transporter (NBC) family, soluble adenylyl cyclase (sACY), adenosine triphosphate (ATP), cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), cyclic nucleotide-gated ion channel (CNG), hyperpolarization-activated cyclic nucleotide-gated channel (HCN), zona pellucida (ZP), the voltage-gated proton channel (Hv1), glutamate receptor family class-C (GPCRC), and an unknown mechanism (?).
Previously, it has been reported that CatSper-dependent increases of [Ca2+]i in spermatozoa are induced by several psychological stimuli such as cyclic nucleotides (e.g., cAMP and cGMP) [29, 30, 52], soluble adenylyl cyclase [29, 52], zona pellucida glycoprotein [34, 35, 38], serum albumin [37, 38], secretion of cumulus oophorus [38], intracellular alkalization [3, 53], and pH [6, 21]. A recent study showed that endocrine disruptors such as p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE) promoted Ca2+ entry into spermatozoa by activating CatSper channels, even at a physiological concentration [36]. In addition, several other components are also known to play an important role in Ca2+ influx mechanisms in mammalian spermatozoa by regulating the opening of CatSper members, including the flagellar voltage-gated proton channel (Hv1) [21], Ca2+-ATPase pump [33], several cyclic nucleotide-gated ion channels (CNG) [27, 54], hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [27], and G-protein coupled receptors (GPCRs).
A hypothetical signaling cascade of Ca2+ influx pathways and interaction of several channel proteins is depicted in Figure 1. Although the functions of several ion channel proteins together with their concurrent relationship with numerous stimuli have been well studied [21, 27, 38], several fundamental questions remain unanswered; for example, how do these channels/stimuli regulate the Ca2+ influx during spermatozoa processes such as capacitation, the acrosome reaction, and fertilization? Do they work alone or together with other channel proteins to regulate Ca2+ influx? Moreover, which other parameters that remain undetected could have an effect on Ca2+ influx? Therefore, future research should focus on resolving these issues. Table 1 summarizes the proposed effect of Ca2+ ion channels and their physiological role that ultimately helps Ca2+ influx into mammalian spermatozoa.
Table 1.
Summary of published works on ion channels and physiological stimuli of mammalian spermatozoa that regulate the Ca2+ influx mechanism.
| Name of channel/stimuli | Localization on spermatozoa/availability | Role in Ca2+ influx | Role in sperm physiology | Effect of knocking down/absence | Reference |
|---|---|---|---|---|---|
| CatSper CatSper 1 CatSper 2 CatSper 3 CatSper 4 CatSperβ CatSperγ |
Principal piece | Regulates Ca2+ influx | Ca2+ uptake, hyperactivated motility | Sterile | Barratt and Publicover, [19]; Qi et al. [20] |
|
| |||||
| Hv1 | Principal piece | Intracellular pH, alkalization thus stimulate Ca2+ influx | Extrudes protons from flagella, alkalization | Fertile | Lishko et al. [21], Lishko et al. [22] |
|
| |||||
| I ATP | Midpiece | Selectively transports the Ca2+ | Ca2+ influx, alkalization | Fertile | Navarro et al. [23] |
|
| |||||
| TRPC | Principal piece, midpiece | Stimulates opening of CatSper | Ca2+ influx, cell depolarization | Fertile | Gees et al. [24], Castellano et al. [25] |
|
| |||||
| CNG | Sperm flagellum, head | Stimulates opening of CatSper via cAMP/cGMP | Ca2+ influx | Fertile | Biel and Michalakis [26] |
|
| |||||
| HCN | Flagellum | Depolarization and opening of CatSper | Ca2+ influx | Fertile | Wiesner et al. [27] |
|
| |||||
| SOC | Plasma membrane | ZP-induced Ca2+ influx | Sperm chemotactic | Subfertile | Yoshida et al. [28] |
|
| |||||
| sACY cAMP/cGMP | Intracellular space and cell membrane | Activates CatSper, CNG, and HCN to regulate Ca2+ influx | Ca2+ influx, alkalization | Sterile | Esposito et al. [29], Hess et al. [30] |
|
| |||||
| GPCR(s) | Principal piece, midpiece | ZP-induced Ca2+ influx increases in [Ca2+]i | Maintains fertilization | Subfertile | Fukami et al. [31] Fukami et al. [32] |
|
| |||||
| PLCδ | Acrosome | ZP induced increases in [Ca2+]i | Ca2+ influx | Subfertile | Fukami et al. [32] |
|
| |||||
| Ca2+-ATPase pump | Principal piece | Intracellular pH and alkalization | Ca2+ influx, capacitation | Motility loss results in infertility | Wennemuth et al. [33] |
|
| |||||
| ZP glycoproteins | Follicle | Induced Ca2+-dependent increase in [Ca2+]i | Hyperactivation, capacitation | Delayed capacitation | Florman [34], Florman et al. [35] |
|
| |||||
| Endocrine disruptor (p,p′-DDE) |
Female reproductive tract | Activates CatSper | Ca2+ influx | Motility loss, delayed capacitation | Tavares et al. [36] |
|
| |||||
| BSA | Extracellular space | Similar to ZP glycoprotein | In vitro capacitation | Motility loss, subfertility |
Xia and Ren [37] Bailey and Storey [38] |
|
| |||||
| Oviductal and follicular fluid | Extracellular space (in vivo) |
Ca2+-dependent increase in [Ca2+]i in sperm | Ca2+ influx | Motility loss delayed capacitation | Xia and Ren [37] |
Hv1: voltage-gated proton channel; I ATP : ATP-gated channel; TRPC: transient receptor potential channels; CNG: cyclic nucleotide-gated ion channel; HCN: hyperpolarization-activated cyclic nucleotide-gated channel; SOC: store-operated Ca2+ channel; cAMP: cyclic adenosine monophosphate; cGMP: cyclic guanosine monophosphate; sACY: soluble adenylyl cyclase; GPCR: glutamate receptor family class-C; PLCδ: phospholipase C zeta; ZP: zona pellucida; p,p′-DDE: p,p'-dichlorodiphenyldichloroethylene; BSA: bovine serum albumin.
3. Effect of Ca2+ Influx on Male Fertility
Ca2+ triggers multiple physiological events in spermatozoa, such as hyperactivation, chemotaxis, capacitation, and the acrosomal reaction, all of which are essential for successful fertilization. In mammalian spermatozoa, numerous Ca2+ permeable channel proteins control intracellular pH, and the pH-dependent Ca2+ influx is measured by the whole-cell patch clamp technique [9, 20]. A review of the literature showed that a potential functional interaction exists between the sperm proteins and Ca2+ permeable channel proteins, thus modulating the Ca2+ influx mechanism [4, 5, 39] and playing a vital role in adjusting male fertility. However, the mechanism by which Ca2+ triggers intracellular signaling to regulate physiological events in spermatozoa and the role of sperm proteins in adjustment of Ca2+ influx into cells remains unclear. This topic is emphasized below.
3.1. Ca2+ Influx, Sperm Hyperactivation, Chemotaxis, and Protein Functions
In general, mature spermatozoa are held immotile within the epididymis. However, they quickly begin to swim following release. This is known as activation of motility and is characterized by symmetrical flagellar beats [55, 56]. The terms sperm activation and hyperactivation have quite different meanings. The spermatozoa become hyperactivated when the amplitude of the flagellar bend increases and produces a highly asymmetrical beat. In vivo, hyperactivation of spermatozoa facilitates the release of sperm from oviductal storage and boosts them through mucus in the oviductal lumen and matrix of the cumulus oophorus during fertilization [7]. In contrast, chemotaxis is a form of sperm movement in which spermatozoa move toward a concentration gradient of a chemoattractant released from the oocyte [57, 58]. However, the molecular event that characterizes spermatozoa chemotaxis is only partially known [57].
There is strong evidence to support that sperm hyperactivation and chemotaxis are required for penetrating the zona pellucida [48, 57, 59, 60]. Incubation of spermatozoa with an extracellular Ca2+ source induces hyperactivation in mammalian spermatozoa [61, 62] and chemotaxis in starfish [57]. In addition, measuring cytoplasmic Ca2+ levels by using the fluorescent Ca2+ indicator indo-1 proved that spermatozoa hyperactivation is potentially regulated by Ca2+ influx. However, it is unknown whether Ca2+ influx independently induces hyperactivation/chemotaxis in mammalian spermatozoa. Ho and Suarez [56] proposed that sperm hyperactivation induced by Ca2+ influx is mainly pH-dependent because sperm require a pH of 7.9–8.5 for hyperactivation, whereas activation can occur at a pH < 7.0. The proposed model of Ca2+-induced hyperactivation is represented in Figure 2.
Figure 2.
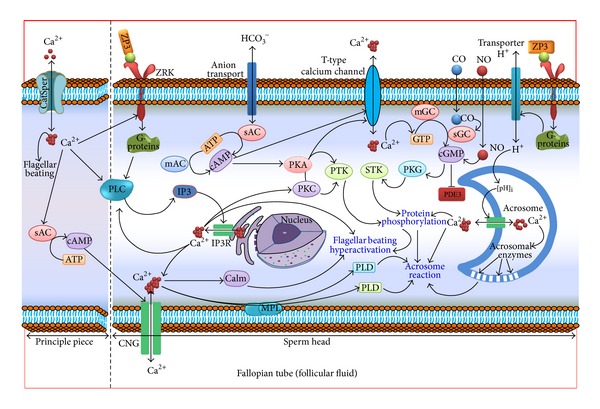
Schematic diagram showing the mechanism of Ca2+ regulated hyperactivation, capacitation, and the acrosome reaction of spermatozoa, which are three principal events of fertilization. Ca2+ together with ZP3 (zona pellucida glycoprotein-3) exhibits the most important role in sperm binding and acrosomal reaction. Ca2+ triggers the zona pellucida (ZP) receptors of cell membrane that activate G-proteins in the sperm head. Activated G-proteins stimulate the H+ transporter to increase intracellular pH, ultimately inducing the acrosomal reaction and hyperactivation by catalyzing the acrosomal enzymes [91]. Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are produced from adenosine triphosphate (ATP) owing to enzymatic catalysis by soluble adenylate cyclase (sAC) and guanylate cyclase (sGC), respectively, in mature spermatozoa. The bicarbonate ions activate the sAC; however, follicular fluid also stimulates the sAC through release of Ca2+ ions via the CatSper channel (principal piece). However, G-protein mediated signal transduction activates sAC and phospholipase-C (PLC) that ultimately causes tyrosine phosphorylation [51, 92], which is responsible for events such as capacitation and the acrosomal reaction. Likewise, extracellular signals such as nitric oxide (NO) and carbon monoxide (CO) stimulate membrane-bound GC (mGC) and sGC, respectively, to synthesize cGMP. Increases in cGMP level evoke a concomitant increase in cAMP by inhibiting its PDE3. However, the increased Ca2+ level can also directly catalyze cAMP [93, 94]. Activated sAC, sGC, and PLC stimulate the generation of the second messengers' inositol trisphosphate (IP3) like cAMP, cGMP. The IP3 binds to the IP3 receptor (IP3R) to increase [Ca2+]i via the release of the [Ca2+]i storage ions. Concurrently, the second messengers activate protein kinases (PKA, PKC, and PKG), in turn gating ions through the T-type calcium channels, cyclic-nucleotide gated ion channel (CNG), and so on, that together with the activation of protein tyrosine kinases (PTK) and serine/threonine protein kinase (STK) cause increased protein phosphorylation [93, 94]. Additionally, the CatSper Ca2+ activates calmodulin (Calm), phospholipase-A (PLA), and phospholipase-D (PLD) with increased generation of other second messengers during the acrosome reaction. Ca2+ influx together with increased protein phosphorylation brings about the capacitation response that is responsible for the waveform asymmetry of motility termed hyperactivation during fertilization. Both hyperactivation and the acrosomal reaction boost flagellar beating, ultimately resulting in the penetration of the outer egg coat and subsequent fertilization of the mature ovum [91–95].
It has recently been found by our laboratory that treatment of mouse spermatozoa with nutlin-3a, a small molecule antagonist of the mouse double minute 2 repressor, potentially downregulates the functions of the ubiquinol-cytochrome-c reductase complex component UQCRC2 and correlated with significantly reduced [Ca2+]i and sperm hyperactivation. This study provided insight that the Ca2+ influx in spermatozoa is partially regulated by UQCRC2 protein. Kwon et al. [4] reported that blocking VDAC with 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) significantly decreased sperm hyperactivation. A significant decrease in [Ca2+]i was observed in (−) DIDS conditions, while [pH]i significantly increased in (−) DIDS, regardless of Ca2+. Simultaneously, a significantly elevated [pH]i was observed in (+) Ca2+. This study provides strong evidence that the modulation of Ca2+ influx by VDACs is pH-dependent, which is consistent with the result of a previous study by Ho and Suarez [56]. Moreover, another study proposed that deamino [Cys 1, d-ArgS] vasopressin (dDAVP), an AVPR2 agonist, significantly decreased sperm motility and intracellular pH, but, interestingly, it increased [Ca2+]i by regulating the function of arginine vasopressin in mice spermatozoa. However, it remains to be clarified as to why spermatozoa motility is decreased even in increased [Ca2+]i conditions.
On the basis of the findings of the aforementioned studies, it is tempting to hypothesize that spermatozoa hyperactivation is mostly controlled by Ca2+ influx. However, potential interactions exist between protein functions. Therefore, Ca2+ influx, protein interaction, and hyperactivation might give numerous different annotations of upcoming research in this field. We have illustrated a schematic representation of different signaling pathways involving sperm proteins by using Pathway Studio. These proteins exhibit significant modifications to induce sperm hyperactivation and chemotaxis in spermatozoa by regulating Ca2+ influx (Figure 3).
Figure 3.
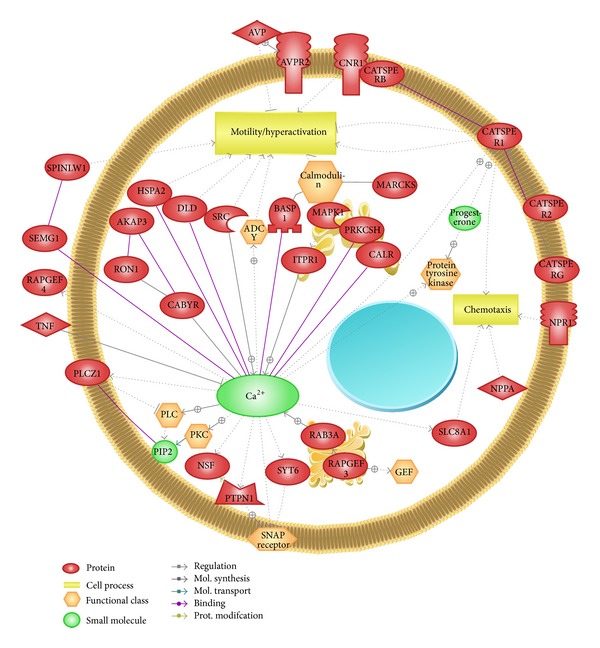
Schematic representation of interactions among ~35 proteins related to Ca2+ regulated spermatozoa hyperactivation and chemotaxis. The figure was produced by use of Pathway Studio (9.0) following the MedScan Reader (5.0) protein search from PubMed database [12].
3.2. Ca2+ Influx versus Capacitation, the Acrosomal Reaction, Fertilization, and Sperm Proteome
Mammalian fertilization is a species-specific episode that is accomplished by a complex set of molecular events. To fertilize an oocyte, multiple extreme changes occur in spermatozoa that begin from its formation in the testes of the male reproductive tract to its penetration and fusion with an egg in the female reproductive tract. Although spermatozoa are motile as well as morphologically normal after ejaculation, they are unable to fertilize an oocyte [59]. They gain the fertilization ability only after educating in the female reproductive tract [40], and the modifications that spermatozoa experience during this time are collectively known as “capacitation.” Only capacitated spermatozoa can undergo the acrosome reaction through binding to the egg zona pellucida, and they finally become capable of penetrating and fertilizing the egg [4, 18, 39].
The term “capacitation” was proposed by Austin in 1952 [1], although this concept was initially described by both Chang and Austin in 1951 [2, 41]. In fact, in vivo capacitation takes place in the female reproductive tract; however, it is also possible to capacitate spermatozoa in vitro by using particular media containing appropriate electrolytes and pH [2]. In an elegant review, Visconti summarized that the early stage of capacitation mainly comprises the bicarbonate-mediated activation of sperm motility, whereas the late stages include intracellular alkalinization, increase in protein tyrosine phosphorylation, and preparation for the acrosomal reaction [63]. These temporal differences in capacitation and the acrosome reaction require numerous mechanisms, and Ca2+ influx plays a significant role in the process [63, 64]. Fraser [65] reported that capacitation is a comparatively slow event that requires several hours to complete and is mainly regulated by a modest rise in [Ca2+]i, whereas the acrosome reaction is an exocytosis process that occurs very rapidly (within a minute) and is triggered by a large influx of [Ca2+]i [65, 66].
Although the biochemical phenomenon of Ca2+ regulated capacitation and the acrosome reaction have been known for the last two decades, the molecular basis of this process is still poorly understood. For capacitation, the cholesterol influx initially stimulates the elevation of [Ca2+]i and bicarbonate into the spermatozoa and finally activates PKA and tyrosine phosphorylation, respectively, via the production of the cAMP [66–68]. In addition, binding to the zona pellucida causes additional activation of cAMP/PKA and protein kinase C (PKC) [68–70]. Spermatozoa need [Ca2+]i influx to proceed further, and they are believed to be activated by PKC through the opening of the calcium channels. Interestingly, PKA together with a secondary messenger, inositol trisphosphate, activates calcium channels localized in the outer acrosomal membrane and increases the calcium concentration in the cytosol. Further increase of cytosolic Ca2+ influx occurs through a store-operated calcium entry mechanism in the plasma membrane, resulting in further depletion of Ca2+ in the acrosome [68, 69].
In support of the aforesaid studies, several recent studies on the same topic have also hypothesized that, after the morphological maturation of spermatozoa for sperm-oocyte fusion, [Ca2+]i decreases because acrosome-reacted spermatozoa release a substantial amount of Ca2+ from their inner cell layers [71, 72]. Ca2+-mediated capacitation and the acrosome reaction have been illustrated in Figure 2 for better understanding. However, for a more in-depth understanding, we recommend reading some excellent reviews on this topic [63, 67, 73–77].
A review of the literature showed that several sperm proteins potentially regulate the Ca2+-dependent capacitation and the acrosome reaction in mammalian spermatozoa [4, 5, 39]. However, how these proteins regulate the Ca2+ influx in spermatozoa is a matter that remains to be elucidated. Breitbart et al. [18] reported that formation of F-actin mostly depends on PKA, protein tyrosine phosphorylation, and phospholipase D activation during capacitation. Ca2+ is one of the principle regulators of capacitation, and it is therefore tempting to hypothesize that organizational modification of F-actin in spermatozoa together with interacting with other sperm proteins has potential influence on Ca2+ influx. A similar finding has been established more precisely by another study [78], where boar sperm capacitation was studied by combined application of computational and experimental approaches. These authors reported that the boar spermatozoa capacitation network contains several connecting cascades, whereas only three nodes bound to all the subcellular compartments are involved in spermatozoa postejaculatory signaling, such as [Ca2+]i, ATP, and actin polymerization. Removal of the actin polymerization node from this aforesaid network causes disorganization of the network topography and affects capacitation, and this has been confirmed by zona pellucida-induced capacitation and the acrosomal reaction in an in vitro demonstration [78].
In another study, Patrat et al. [79] showed that progesterone (P4) that is secreted by cumulus cells directly acts on the sperm plasma membrane and triggers the intracellular signals and enzymatic pathways involved in the acrosome reaction. P4 regulates the acrosome reaction and is mediated by a compulsory Ca2+ increase. This study found that P4 induced the activation of Gi/Go protein-coupled and protein tyrosine kinase receptors, and it affected capacitation and the acrosome reaction. In contrast, Ca2+ regulated exocytosis of spermatozoa requires active acrosomal proteins such as N-ethylmaleimide-sensitive factor (NSF) [66]. Additionally, the same research team showed that the ras-related protein Rab-3A (RAB3A) is also necessary for Ca2+-dependent exocytosis. Interestingly, Rab3A activation of acrosomal exocytosis requires active NSF. Therefore, protein-protein interaction might also play a potential role in regulating Ca2+ influx. All of these observations seem to be consistent with the idea that Ca2+ functions are regulated by sperm proteins during fertilization. However, the key question is how do these proteins modify Ca2+ influx in spermatozoa?
Recently, in our laboratory, we used mice spermatozoa to evaluate the interrelationship of proteins related to Ca2+ influx, including UQCRC2 [39], arginine vasopressin [5], and VDACs [4], and evaluate their effects on capacitation and the acrosome reaction. It is likely that a sustained phase of Ca2+ is required for fertilization and might be regulated by the complex interaction of numerous sperm proteins. Therefore, studies to identify proteins that might have the ability to induce such a change are worth undertaking. Application of Pathway Studio helped us represent over 40 proteins that are potentially implicated in Ca2+ mediated regulation of capacitation, the acrosome reaction, and male fertility (Figure 4).
Figure 4.
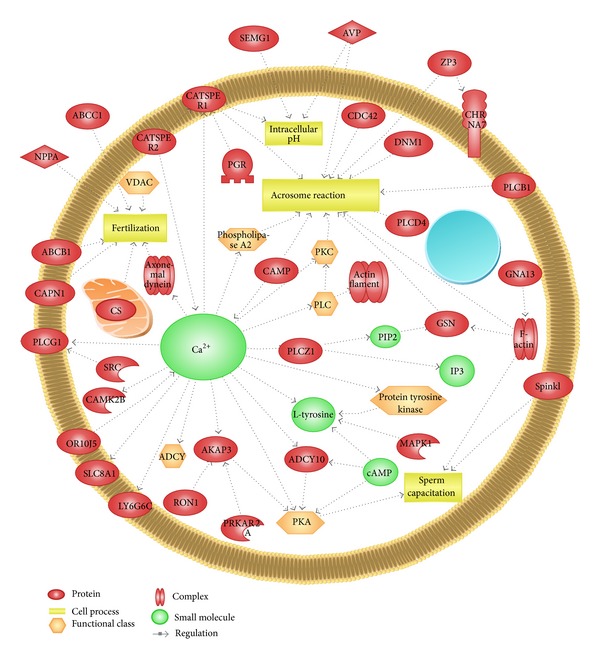
Schematic representation of interactions among ~40 proteins related to Ca2+ regulated spermatozoa capacitation, the acrosome reaction, and fertilization. The figure was produced by use of Pathway Studio (9.0) following the MedScan Reader (5.0) protein search from PubMed database [12].
3.3. Ca2+ Influx and Postfertilization Egg Activation in Context of Sperm Proteome
Ca2+ influx in spermatozoa is not only important for sperm maturation, but it is also equally important for activation and development of the oocyte. A study of egg activation by Ca2+ was conducted by Steinhardt and colleagues in 1974 and showed remarkable findings [80]. Steinhardt et al. [80] reported that administration of Ca2+ ionophores induced the early events of hamster egg activation. Thus far, it has been shown that the eggs of almost all species are activated by an increase in Ca2+ oscillation by spermatozoa during fertilization [81, 82]. However, how the spermatozoa trigger the oocyte Ca2+ oscillation remains to be elucidated. Several hypotheses have been proposed to describe these mechanisms [83–86].
It has been reported previously [83] that the spermatozoa introduce Ca2+ influx into oocytes by a specific protein called oscillogen in hamsters. Recent studies have shown that phospholipase C zeta (PLCζ), a novel sperm-specific agent, is responsible for induction of Ca2+ oscillation in eggs after sperm-egg membrane fusion [87–89]. According to this mechanism, the sperm protein PLCζ causes the release of [Ca2+]i in eggs and is mediated via inositol 1,4,5-trisphosphate (InsP3) receptors (hypothetical depiction in Figure 5). Even when the InsP3 or its derivatives are injected into unfertilized, mature eggs, oscillation occurs due to the unique feedback properties of the InsP3 receptors in mouse eggs [90]. However, it is still unknown whether there are any other factors/proteins available in spermatozoa that also have similar effects. We illustrated the relevant signaling and metabolic pathways by using sperm proteins to facilitate the understanding of the mechanisms behind Ca2+ mediated activation of oocytes (Figure 6).
Figure 5.
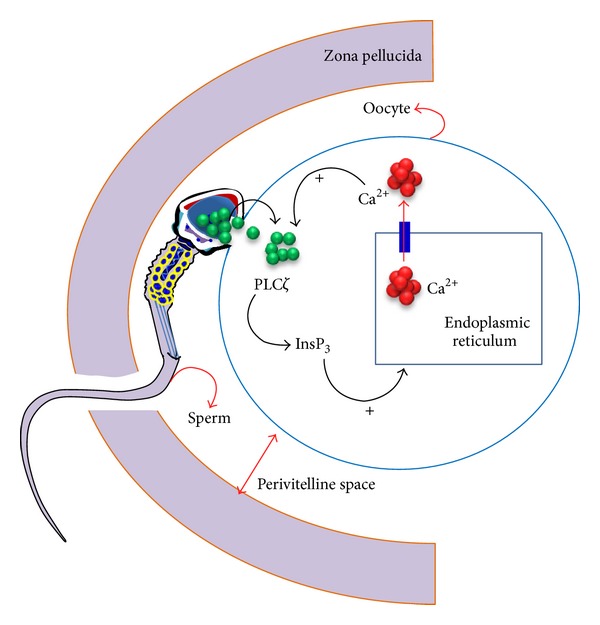
Schematic representation showing the Ca2+ influx mechanism in mammalian eggs stimulated by mature spermatozoa. Spermatozoa donate the phospholipase C isoform zeta (PLCζ) protein within a few minutes of sperm-egg fusion (represented by green color circle). Inositol 1,4,5-trisphosphate (InsP3) is produced due to the hydrolysis of PLCζ, which subsequently triggers the nsP3 receptor-mediated Ca2+ release (indicated by red color circle) from the endoplasmic reticulum of the oocyte. Simultaneously, the increased cytoplasmic Ca2+ leads to further PLCζ stimulation, leading to the positive feedback loop of Ca2+ and InsP3 rise. The hypothesis has been modified from Swann [96] and Swann et al. [97].
Figure 6.
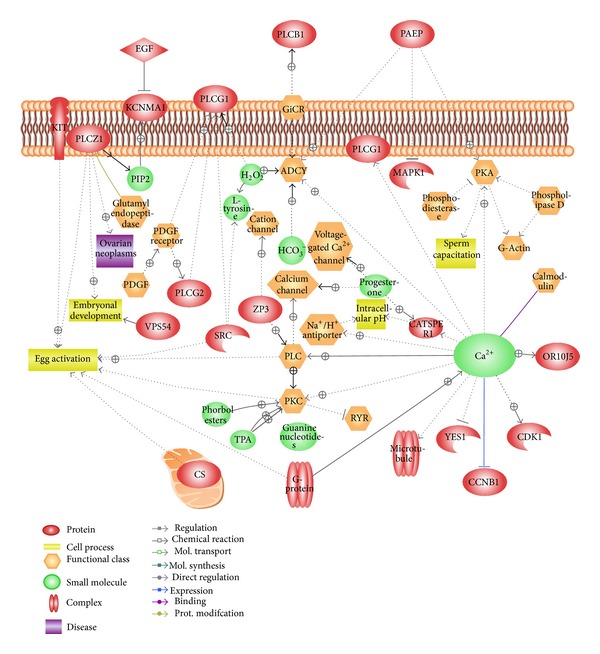
Schematic representation of interactions among ~30 proteins related to Ca2+ regulated egg activation and embryonic development. The figure was produced by the use of Pathway Studio (9.0) following the MedScan Reader (5.0) protein search from PubMed database [12].
4. Future Prospects
The maturational events of mammalian spermatozoa are strictly regulated through the well-coordinated Ca2+ influx. It is the central regulator of many key activities in spermatozoa, all of which are necessary for fertilization. However, our current understanding at the molecular level concerning Ca2+ signaling in the spermatozoa is insufficient. Therefore, a better understanding of such an event can provide a more complete comprehension of Ca2+ regulated sperm functions and fertility optimization.
A large number of Ca2+ permeable ion channel proteins have been identified [10, 43] that collectively regulate the Ca2+ influx mechanism in spermatozoa. Although the recent application of patch-clamp recordings of channel current significantly improves our understanding of the functions of these channel proteins, several basic aspects remain unsolved, such as identifying the functions of individual channels in spermatozoa and how these channels coordinate Ca2+ influx. Therefore, production of knockdown animals and using them as negative controls compared with their wild counterparts might provide more specific ideas about channel functions. CatSper is the one of the well-studied channel proteins [10] and the functions of different pore-forming CatSper channels (1–4) and auxiliary subunits (CatSperβ and CatSperγ) remain a matter to be elucidated.
A literature review found that the Ca2+ influx mechanism in spermatozoa is regulated by several physical stimuli, although the underlying mechanism is less clearly defined. Protein-protein interactions also potentially regulate the Ca2+ uptake mechanism in spermatozoa. Although recently applied proteomic approaches have identified several sperm-specific proteins, their functions in Ca2+ regulation and interaction with channel proteins are unclear. Therefore, future research should target this topic to provide a robust understanding of Ca2+ and male fertility in both humans and other animal species.
Acknowledgments
This study was financially supported by the Cooperative Research Program for Agriculture Science and Technology Development (Project no. PJ008415), Rural Development Administration, Republic of Korea. Md Saidur Rahman was supported through the “Chung-Ang University Young Scientist Scholarship (CAYSS),” Chung-Ang University, Republic of Korea.
Conflict of Interests
The authors have declared there is no conflict of interests that could be perceived as prejudicing the impartiality of this paper.
References
- 1.Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170(4321):p. 326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- 2.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168(4277):697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 3.Mannowetz N, Naidoo NM, Choo S-A, Smith JF, Lishko PV. Slo1 is the principal potassium channel of human spermatozoa. eLife. 2013;2 doi: 10.7554/eLife.01009.e01009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon W-S, Park Y-J, El-Sayed AM, Pang M-G. Voltage-dependent anion channels are a key factor of male fertility. Fertility and Sterility. 2013;99(2):354–361. doi: 10.1016/j.fertnstert.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Kwon W-S, Park Y-J, Kim Y-H, You Y-A, Kim IC, Pang M-G. Vasopressin effectively suppresses male fertility. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054192.e54192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. The Journal of Physiology. 2010;588(23):4667–4672. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez SS. Control of hyperactivation in sperm. Human Reproduction Update. 2008;14(6):647–657. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 8.Foresta C, Rossato M. Calcium influx pathways in human spermatozoa. Molecular Human Reproduction. 1997;3(1):1–4. doi: 10.1093/molehr/3.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439(7077):737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 10.Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology. 2010;25(3):165–175. doi: 10.1152/physiol.00049.2009. [DOI] [PubMed] [Google Scholar]
- 11.Rahman MS, Lee J-S, Kwon W-S, Pang M-G. Sperm proteomics: road to male fertility and contraception. International Journal of Endocrinology. 2013;2013:11 pages. doi: 10.1155/2013/360986.360986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park Y-J, Kwon W-S, Oh S-A, Pang M-G. Fertility-related proteomic profiling bull spermatozoa separated by percoll. Journal of Proteome Research. 2012;11(8):4162–4168. doi: 10.1021/pr300248s. [DOI] [PubMed] [Google Scholar]
- 13.Baker MA, Nixon B, Naumovski N, Aitken RJ. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Systems Biology in Reproductive Medicine. 2012;58(4):211–217. doi: 10.3109/19396368.2011.639844. [DOI] [PubMed] [Google Scholar]
- 14.Oliva R, de Mateo S, Estanyol JM. Sperm cell proteomics. Proteomics. 2009;9(4):1004–1017. doi: 10.1002/pmic.200800588. [DOI] [PubMed] [Google Scholar]
- 15.Gur Y, Breitbart H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes & Development. 2006;20(4):411–416. doi: 10.1101/gad.367606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres-Flores V, Hernández-Rueda YL, Neri-Vidaurri PD, et al. Activation of protein kinase A stimulates the progesterone-induced calcium influx in human sperm exposed to the phosphodiesterase inhibitor papaverine. Journal of Andrology. 2008;29(5):549–557. doi: 10.2164/jandrol.107.004614. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V, Kota V, Shivaji S. Hamster sperm capacitation: role of pyruvate dehydrogenase A and dihydrolipoamide dehydrogenase. Biology of Reproduction. 2008;79(2):190–199. doi: 10.1095/biolreprod.107.066704. [DOI] [PubMed] [Google Scholar]
- 18.Breitbart H, Cohen G, Rubinstein S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction. 2005;129(3):263–268. doi: 10.1530/rep.1.00269. [DOI] [PubMed] [Google Scholar]
- 19.Barratt CLR, Publicover SJ. Sperm are promiscuous and CatSper is to blame... The EMBO Journal. 2012;31(7):1624–1626. doi: 10.1038/emboj.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi H, Moran MM, Navarro B, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471(7338):387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 22.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140(3):327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 23.Navarro B, Miki K, Clapham DE. ATP-activated P2X2 current in mouse spermatozoa. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14342–14347. doi: 10.1073/pnas.1111695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harbor Perspectives in Biology. 2010;2(10) doi: 10.1101/cshperspect.a003962.a003962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellano LE, Treviño CL, Rodríguez D, et al. Transient receptor potential (TRPC) channels in human sperm: expression, cellular localization and involvement in the regulation of flagellar motility. FEBS Letters. 2003;541(1–3):69–74. doi: 10.1016/s0014-5793(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 26.Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handbook of Experimental Pharmacology. 2009;191:111–136. doi: 10.1007/978-3-540-68964-5_7. [DOI] [PubMed] [Google Scholar]
- 27.Wiesner B, Weiner J, Middendorff R, Hagen V, Kaupp UB, Weyand I. Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. The Journal of Cell Biology. 1998;142(2):473–484. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida M, Ishikawa M, Izumi H, de Santis R, Morisawa M. Store-operated calcium channel regulates the chemotactic behavior of ascidian sperm. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):149–154. doi: 10.1073/pnas.0135565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito G, Jaiswal BS, Xie F, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess KC, Jones BH, Marquez B, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Developmental Cell. 2005;9(2):249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukami K, Nakao K, Inoue T, et al. Requirement of phospholipase Cδ4 for the zona pellucida-induced acrosome reaction. Science. 2001;292(5518):920–923. doi: 10.1126/science.1059042. [DOI] [PubMed] [Google Scholar]
- 32.Fukami K, Yoshida M, Inoue T, et al. Phospholipase Cδ4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. The Journal of Cell Biology. 2003;161(1):79–88. doi: 10.1083/jcb.200210057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wennemuth G, Babcock DF, Hille B. Calcium clearance mechanisms of mouse sperm. The Journal of General Physiology. 2003;122(1):115–128. doi: 10.1085/jgp.200308839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Florman HM. Sequential focal and global elevations of sperm intracellular Ca2+ are initiated by the zona pellucida during acrosomal exocytosis. Developmental Biology. 1994;165(1):152–164. doi: 10.1006/dbio.1994.1242. [DOI] [PubMed] [Google Scholar]
- 35.Florman HM, Tombes RM, First NL, Babcock DF. An adhesion-associated agonist from the zona pellucida activates G protein-promoted elevations of internal Ca2+ and pH that mediate mammalian sperm acrosomal exocytosis. Developmental Biology. 1989;135(1):133–146. doi: 10.1016/0012-1606(89)90164-4. [DOI] [PubMed] [Google Scholar]
- 36.Tavares RS, Mansell S, Barratt CLR, Wilson SM, Publicover SJ, Ramalho-Santos J. p, p′-DDE activates CatSper and compromises human sperm function at environmentally relevant concentrations. Human Reproduction. 2013;28(12):3167–3177. doi: 10.1093/humrep/det372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reproductive Biology and Endocrinology. 2009;7, article 119 doi: 10.1186/1477-7827-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey JL, Storey BT. Calcium influx into mouse spermatozoa activated by solubilized mouse zona pellucida, monitored with the calcium fluorescent indicator, fluo-3. Inhibition of the influx by three inhibitors of the zona pellucida induced acrosome reaction: tyrphostin A48, pertussis toxin, and 3-quinuclidinyl benzilate. Molecular Reproduction and Development. 1994;39(3):297–308. doi: 10.1002/mrd.1080390307. [DOI] [PubMed] [Google Scholar]
- 39.Shukla KK, Kwon W-S, Rahman MS, Park Y-J, You Y-A, Pang M-G. Nutlin-3a decreases male fertility via UQCRC2. PLoS ONE. 2013;9(8) doi: 10.1371/journal.pone.0076959.e76959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York, NY, USA: Raven Press; 1994. pp. 189–317. [Google Scholar]
- 41.Austin CR. Observations on the penetration of the sperm in the mammalian egg. Australian Journal of Scientific Research B: Biological Sciences. 1951;4(4):581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- 42.Strünker T, Goodwin N, Brenker C, et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471(7338):382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 43.Darszon A, Acevedo JJ, Galindo BE, et al. Sperm channel diversity and functional multiplicity. Reproduction. 2006;131(6):977–988. doi: 10.1530/rep.1.00612. [DOI] [PubMed] [Google Scholar]
- 44.Pietrobon D, di Virgilio F, Pozzan T. Structural and functional aspects of calcium homeostasis in eukaryotic cells. European Journal of Biochemistry. 1990;193(3):599–622. doi: 10.1111/j.1432-1033.1990.tb19378.x. [DOI] [PubMed] [Google Scholar]
- 45.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427(6972):360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Xia J, Cho K-H, Clapham DE, Ren D. CatSperβ, a novel transmembrane protein in the CatSper channel complex. The Journal of Biological Chemistry. 2007;282(26):18945–18952. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Liu J, Cho K-H, Ren D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biology of Reproduction. 2009;81(3):539–544. doi: 10.1095/biolreprod.109.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren D, Navarro B, Perez G, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413(6856):603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nature Cell Biology. 2003;5(12):1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 50.Carlson AE, Westenbroek RE, Quill T, et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):14869–14874. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Cann MJ, Litvin TN, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289(5479):625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 53.Clapham DE. Sperm BerserKers. eLife. 2013;2 doi: 10.7554/eLife.01469.e01469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weyand I, Godde M, Frings S, et al. Cloning and functional expression of acyclic-nucleotide-gated channel from mammalian sperm. Nature. 1994;368(6474):859–863. doi: 10.1038/368859a0. [DOI] [PubMed] [Google Scholar]
- 55.Suarez SS, Dai X. Hyperactivation enhances mouse sperm capacity for penetrating viscoelastic media. Biology of Reproduction. 1992;46(4):686–691. doi: 10.1095/biolreprod46.4.686. [DOI] [PubMed] [Google Scholar]
- 56.Ho H-C, Suarez SS. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction. 2001;122(4):519–526. doi: 10.1530/rep.0.1220519. [DOI] [PubMed] [Google Scholar]
- 57.Böhmer M, Van Q, Weyand I, et al. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. The EMBO Journal. 2005;24(15):2741–2752. doi: 10.1038/sj.emboj.7600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Anzi B, Chandler DE. A sperm chemoattractant is released from xenopus egg jelly during spawning. Developmental Biology. 1998;198(2):366–375. [PubMed] [Google Scholar]
- 59.Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Human Reproduction Update. 2006;12(1):23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 60.Suarez SS, Varosi SM, Dai X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(10):4660–4664. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marquez B, Suarez SS. Soluble adenylyl cyclase is required for activation of sperm but does not have a direct effect on hyperactivation. Reproduction, Fertility and Development. 2008;20(2):247–252. doi: 10.1071/rd07146. [DOI] [PubMed] [Google Scholar]
- 62.Xia J, Reigada D, Mitchell CH, Ren D. CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biology of Reproduction. 2007;77(3):551–559. doi: 10.1095/biolreprod.107.061358. [DOI] [PubMed] [Google Scholar]
- 63.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(3):667–668. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. Journal of Reproductive Immunology. 2002;53(1-2):133–150. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- 65.Fraser LR. Sperm capacitation and the acrosome reaction. Human Reproduction. 1998;13(1):9–19. doi: 10.1093/humrep/13.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 66.Michaut M, Tomes CN, de Blas G, Yunes R, Mayorga LS. Calcium-triggered acrosomal exocytosis in human spermatozoa requires the coordinated activation of Rab3A and N-ethylmaleimide-sensitive factor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(18):9996–10001. doi: 10.1073/pnas.180206197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breitbart H. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Molecular and Cellular Endocrinology. 2002;187(1-2):139–144. doi: 10.1016/s0303-7207(01)00704-3. [DOI] [PubMed] [Google Scholar]
- 68.Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cellular and Molecular Biology. 2003;49(3):321–327. [PubMed] [Google Scholar]
- 69.Breitbart H, Naor Z. Protein kinases in mammalian sperm capacitation and the acrosome reaction. Reviews of Reproduction. 1999;4(3):151–159. doi: 10.1530/ror.0.0040151. [DOI] [PubMed] [Google Scholar]
- 70.Breitbart H, Spungin B. The biochemistry of the acrosome reaction. Molecular Human Reproduction. 1997;3(3):195–202. doi: 10.1093/molehr/3.3.195. [DOI] [PubMed] [Google Scholar]
- 71.Florman HM, Jungnickel MK, Sutton KA. Shedding Light on Sperm pHertility. Cell. 2010;140(3):310–312. doi: 10.1016/j.cell.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 72.Etkovitz N, Rubinstein S, Daniel L, Breitbart H. Role of PI3-kinase and PI4-kinase in actin polymerization during bovine sperm capacitation. Biology of Reproduction. 2007;77(2):263–273. doi: 10.1095/biolreprod.106.056705. [DOI] [PubMed] [Google Scholar]
- 73.Visconti PE, Florman HM. Mechanisms of sperm-egg interactions: between sugars and broken bonds. Science Signaling. 2010;3(142, article pe35) doi: 10.1126/scisignal.3142pe35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abou-haila A, Tulsiani DRP. Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Archives of Biochemistry and Biophysics. 2009;485(1):72–81. doi: 10.1016/j.abb.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Naz RK, Rajesh PB. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reproductive Biology and Endocrinology. 2004;2, article 75 doi: 10.1186/1477-7827-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tulsiani DRP, Abou-Haila A, Loeser CR, Pereira BMJ. The biological and functional significance of the sperm acrosome and acrosomal enzymes in mammalian fertilization. Experimental Cell Research. 1998;240(2):151–164. doi: 10.1006/excr.1998.3943. [DOI] [PubMed] [Google Scholar]
- 77.Zaneveld LJD, de Jonge CJ, Anderson RA, Mack SR. Human sperm capacitation and the acrosome reaction. Human Reproduction. 1991;6(9):1265–1274. doi: 10.1093/oxfordjournals.humrep.a137524. [DOI] [PubMed] [Google Scholar]
- 78.Bernabò N, Berardinelli P, Mauro A, et al. The role of actin in capacitation-related signaling: an in silico and in vitro study. BMC Systems Biology. 2011;5, article 47 doi: 10.1186/1752-0509-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patrat C, Auer J, Fauque P, Leandri RL, Jouannet P, Serres C. Zona pellucida from fertilised human oocytes induces a voltage-dependent calcium influx and the acrosome reaction in spermatozoa, but cannot be penetrated by sperm. BMC Developmental Biology. 2006;6, article 59 doi: 10.1186/1471-213X-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinhardt RA, Epel D, Carroli EJ, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- 81.Theodoridou M, Nomikos M, Parthimos D, et al. Chimeras of sperm PLCζ reveal disparate protein domain functions in the generation of intracellular Ca2+ oscillations in mammalian eggs at fertilization. Molecular Human Reproduction. 2013;19(12):852–864. doi: 10.1093/molehr/gat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzalez-Garcia JR, Machaty Z, Lai FA, Swann K. The dynamics of PKC-induced phosphorylation triggered by Ca2+ oscillations in mouse eggs. Journal of Cellular Physiology. 2013;228(1):110–119. doi: 10.1002/jcp.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitaker MJ, Steinhardt RA. Ionic regulation of egg activation. Quarterly Reviews of Biophysics. 1982;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- 84.Jaffe LF. Sources of calcium in egg activation: a review and hypothesis. Developmental Biology. 1983;99(2):265–276. doi: 10.1016/0012-1606(83)90276-2. [DOI] [PubMed] [Google Scholar]
- 85.Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Developmental Biology. 1993;158(1):62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- 86.Swann K, Ozil J-P. Dynamics of the calcium signal that triggers mammalian egg activation. International Review of Cytology. 1994;152:183–222. doi: 10.1016/s0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- 87.Swann K, Lai FA. PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell Calcium. 2013;53(1):55–62. doi: 10.1016/j.ceca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Yu A, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K. PLCζ causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P2 . Molecular Biology of the Cell. 2012;23(2):371–380. doi: 10.1091/mbc.E11-08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phillips SV, Yu Y, Rossbach A, et al. Divergent effect of mammalian PLCζ in generating Ca2+ oscillations in somatic cells compared with eggs. Biochemical Journal. 2011;438(3):545–553. doi: 10.1042/BJ20101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swann K, Yu Y. The dynamics of calcium oscillations that activate mammalian eggs. The International Journal of Developmental Biology. 2008;52(5-6):585–594. doi: 10.1387/ijdb.072530ks. [DOI] [PubMed] [Google Scholar]
- 91.Jeon B-G, Moon J-S, Kim K-C, Lee H-J, Choe S-Y, Rho G-J. Follicular fluid enhances sperm attraction and its motility in human. Journal of Assisted Reproduction and Genetics. 2001;18(8):407–412. doi: 10.1023/A:1016674302652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ho H-C, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Developmental Biology. 2002;250(1):208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- 93.Luconi M, Forti G, Baldi E. Pathophysiology of sperm motility. Frontiers in Bioscience. 2006;11(2):1433–1447. doi: 10.2741/1894. [DOI] [PubMed] [Google Scholar]
- 94.Revelli A, Ghigo D, Moffa F, Massobrio M, Tur-Kaspa I. Guanylate cyclase activity and sperm function. Endocrine Reviews. 2002;23(4):484–494. doi: 10.1210/er.2001-0020. [DOI] [PubMed] [Google Scholar]
- 95.Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiological Reviews. 2004;84(1):137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 96.Swann K. Soluble sperm factors and Ca2+ release in eggs at fertilization. Reviews of Reproduction. 1996;1(1):33–39. doi: 10.1530/ror.0.0010033. [DOI] [PubMed] [Google Scholar]
- 97.Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCζ . Reproduction. 2004;127(4):431–439. doi: 10.1530/rep.1.00169. [DOI] [PubMed] [Google Scholar]


