Abstract
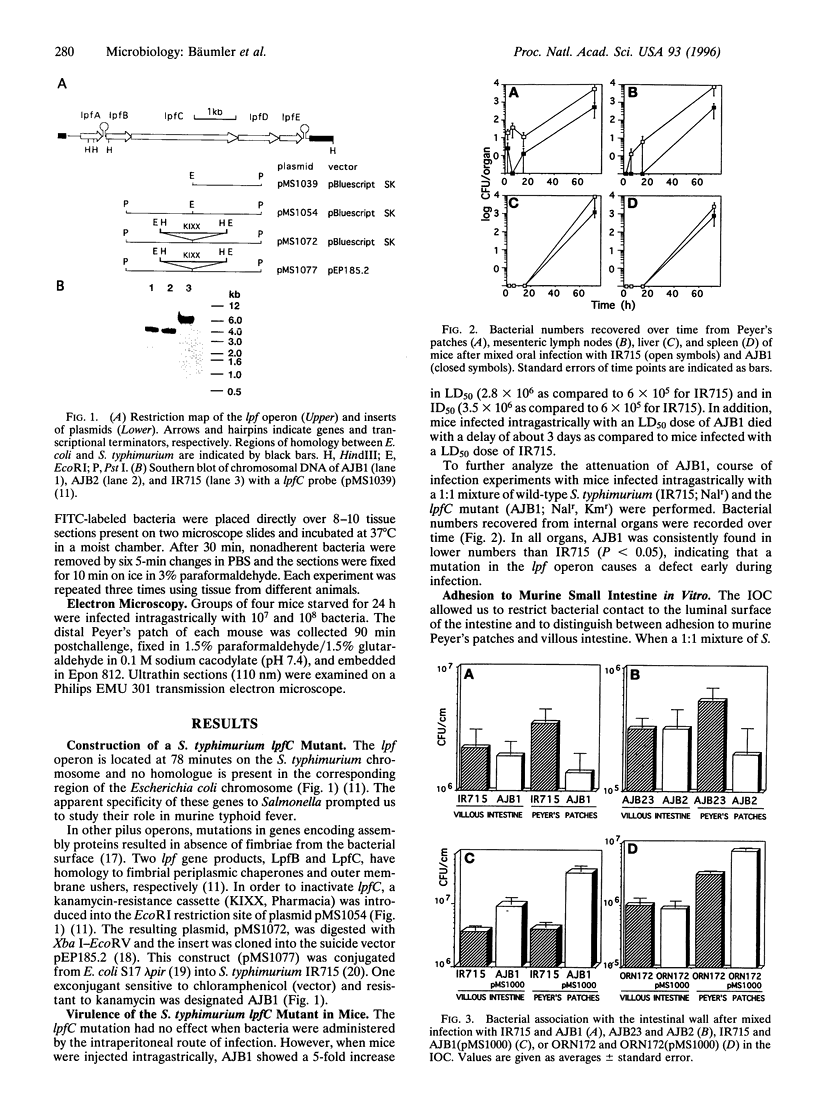
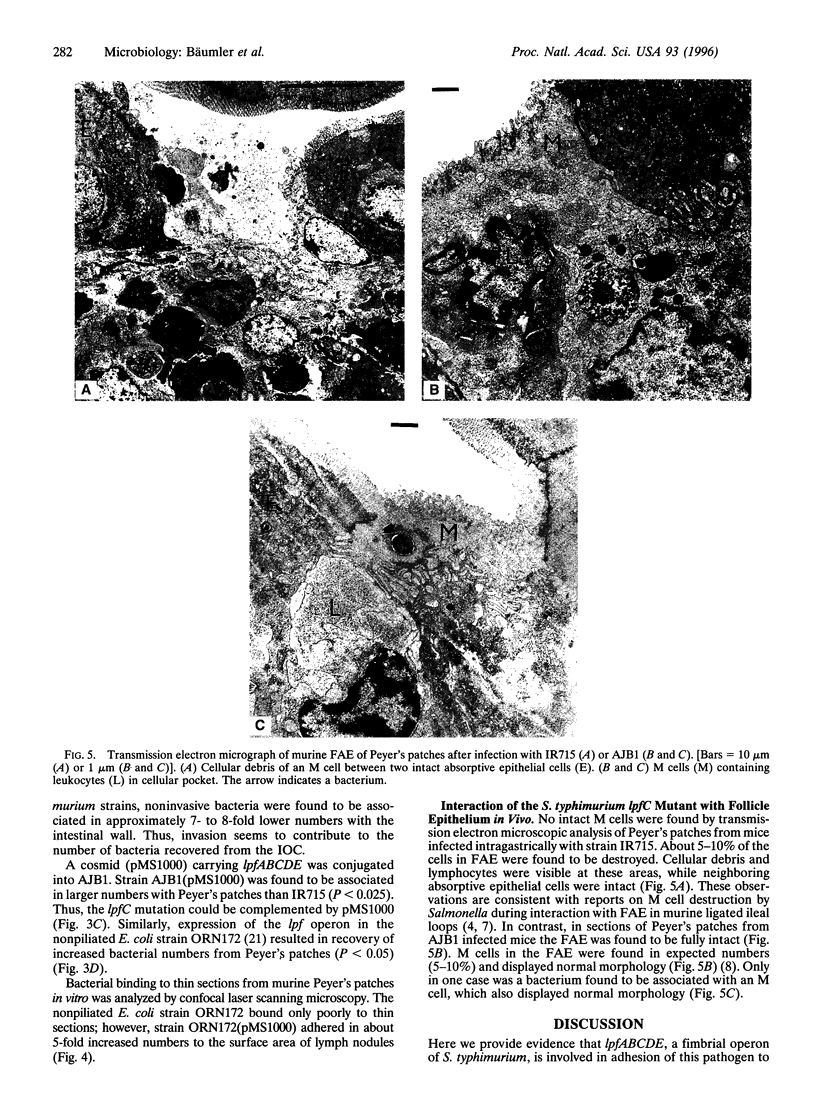
We investigated the role of the Salmonella typhimurium fimbrial operon formed by the genes lpfABCDE in infection of mice. A mutant in lpfC, the gene encoding the fimbrial outer membrane usher, had an approximately 5-fold increased 50% lethal dose when administered orally to mice. When mice were infected with a mixture of the lpfC mutant and isogenic wild-type S. typhimurium, the lpfC mutant was recovered in lower numbers from Peyer's patches, mesenteric lymph nodes, liver, and spleen. In an organ culture model using murine intestinal loops, lpfC mutants were shown to be associated in lower numbers than wild-type bacteria with Peyer's patches but not with villous intestine. The defect of the lpfC mutant in adhesion to Peyer's patches could be complemented by introducing lpfABCDE on a cosmid. Similarly, heterologous expression of the Salmonella lpf operon in Escherichia coli resulted in an increased adhesion to histological thin sections of Peyer's patch lymph follicles. Electron microscopic analysis of histological sections taken from Peyer's patches after intragastric infection of mice showed that, in contrast to the S. typhimurium wild type, the isogenic lpfC mutant did not destroy M cells of the follicle-associated epithelium. These data show that the Salmonella lpf operon is involved in adhesion to murine Peyer's patches.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Bäumler A. J., Heffron F. Identification and sequence analysis of lpfABCDE, a putative fimbrial operon of Salmonella typhimurium. J Bacteriol. 1995 Apr;177(8):2087–2097. doi: 10.1128/jb.177.8.2087-2097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Jepson M. A., Simmons N. L., Hirst B. H. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res Microbiol. 1994 Sep;145(7):543–552. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Collazo C. M., Zierler M. K., Galán J. E. Functional analysis of the Salmonella typhimurium invasion genes invl and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995 Jan;15(1):25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Kaper J. B. Enteropathogenic Escherichia coli. Infect Immun. 1992 Oct;60(10):3953–3961. doi: 10.1128/iai.60.10.3953-3961.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Ochman H. Cognate gene clusters govern invasion of host epithelial cells by Salmonella typhimurium and Shigella flexneri. EMBO J. 1993 Oct;12(10):3779–3787. doi: 10.1002/j.1460-2075.1993.tb06056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski C., Kutschka U., Schmoranzer H. P., Naumann M., Stallmach A., Hahn H., Menge H., Riecken E. O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989 Mar;57(3):673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann A. W., Schmidt G., Rowley D. Intestinal colonization and virulence of Salmonella in mice. Infect Immun. 1978 Dec;22(3):763–770. doi: 10.1128/iai.22.3.763-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Ghori N., Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994 Jul 1;180(1):15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder S. A., Badger J. L., Bryant G. O., Pepe J. C., Miller V. L. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene. 1993 Dec 22;136(1-2):271–275. doi: 10.1016/0378-1119(93)90478-l. [DOI] [PubMed] [Google Scholar]
- Kohbata S., Yokoyama H., Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer's patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol. 1986;30(12):1225–1237. doi: 10.1111/j.1348-0421.1986.tb03055.x. [DOI] [PubMed] [Google Scholar]
- Kuehn M. J., Jacob-Dubuisson F., Dodson K., Slonim L., Striker R., Hultgren S. J. Genetic, biochemical, and structural studies of biogenesis of adhesive pili in bacteria. Methods Enzymol. 1994;236:282–306. doi: 10.1016/0076-6879(94)36022-7. [DOI] [PubMed] [Google Scholar]
- Mathan M. M., Mathan V. I. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S314–S318. doi: 10.1093/clinids/13.supplement_4.s314. [DOI] [PubMed] [Google Scholar]
- Que J. U., Hentges D. J. Effect of streptomycin administration on colonization resistance to Salmonella typhimurium in mice. Infect Immun. 1985 Apr;48(1):169–174. doi: 10.1128/iai.48.1.169-174.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozdzinski E., Tuomanen E. Interactions of bacteria with leukocyte integrins. Methods Enzymol. 1994;236:333–345. doi: 10.1016/0076-6879(94)36025-1. [DOI] [PubMed] [Google Scholar]
- Sory M. P., Cornelis G. R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994 Nov;14(3):583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Stojiljkovic I., Bäumler A. J., Heffron F. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol. 1995 Mar;177(5):1357–1366. doi: 10.1128/jb.177.5.1357-1366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Trier J. S. Structure and function of intestinal M cells. Gastroenterol Clin North Am. 1991 Sep;20(3):531–547. [PubMed] [Google Scholar]
- Wassef J. S., Keren D. F., Mailloux J. L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989 Mar;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall L. D., Russell P. W., Harris S. L., Orndorff P. E. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5 promoter. J Bacteriol. 1993 May;175(9):2770–2778. doi: 10.1128/jb.175.9.2770-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]






