Abstract
Background
The purpose of this study was to evaluate amifostine for protection from cisplatin-induced serious hearing loss in patients with average-risk medulloblastoma by extending a previous analysis to a much larger sample size. In addition, this study aimed to assess amifostine with serious hearing loss in patients with high-risk medulloblastoma treated with cisplatin.
Methods
Newly diagnosed medulloblastoma patients (n = 379; ages 3–21 years), enrolled on one of 2 sequential St. Jude clinical protocols that included 4 courses of 75 mg/m2 cisplatin, were compared for hearing loss by whether or not they received 600 mg/m2 of amifostine immediately before and 3 hours into each cisplatin infusion. Amifostine administration was not randomized. The last audiological evaluation between 5.5 and 24.5 months following protocol treatment initiation was graded using the Chang Ototoxicity Scale. A grade of ≥2b (loss requiring a hearing aid or deafness) was considered a serious event.
Results
Among average-risk patients (n = 263), amifostine was associated with protection from serious hearing loss (adjusted OR, 0.30; 95% CI, 0.14–0.64). For high-risk patients (n = 116), however, there was not sufficient evidence to conclude that amifostine prevented serious hearing loss (OR, 0.89; 95% CI, 0.31–2.54).
Conclusions
Although patients in this study were not randomly assigned to amifostine treatment, we found evidence in favor of amifostine administration for protection against cisplatin-induced serious hearing loss in average-risk but not in high-risk, medulloblastoma patients.
Keywords: audiology, brain neoplasms, late effects, ototoxicity, platinum drugs
Cisplatin is a platinum-based chemotherapeutic agent used in frontline treatment regimens for a variety of brain and other solid tumors of childhood including average-risk and high-risk medulloblastoma.1,2 Unfortunately, cisplatin is a potent ototoxin. Cisplatin and other platinum-based chemotherapeutic agents cause cochlear (sensory) hair cell destruction, initially at the base of the cochlea where high frequency sounds are processed, and then progressing to the lower frequency sounds (and speech ranges) with increasing cumulative doses.3,4 Cisplatin treatment results in a high proportion of patients with permanent bilateral sensorineural hearing loss, with young children being more susceptible than older children.5 Approximately 50% of childhood medulloblastoma occurs before age 5 years and 80% before age 10 years;6 thus, cisplatin-induced hearing loss is often experienced during critical stages of speech and language acquisition and development.3,7 Considering that the combined effects of other treatments, namely surgery and craniospinal irradiation, result in neurocognitive deficits, it follows that the added insult of sensorineural hearing loss can be a significant detriment to the long term academic and social well-being of the surviving child.7
Currently, no established treatments or procedures exist to prevent platinum-induced hearing loss in children or adults.3,8,9 Amifostine, a prodrug metabolized in humans to WR-1065,10 is a thiol-reducing agent and potent free-radical scavenger with demonstrated otoprotective properties against cisplatin in experiments using hamsters11 and guinea pigs.12 Evaluation of amifostine as a cisplatin otoprotectant in childhood cancer treatment has been limited to small studies with results suggesting no positive effect.13–16 In contrast, we previously reported protection against cisplatin-induced ototoxicity from amifostine in 62 average-risk, newly diagnosed medulloblastoma participants treated in 2 consecutive multi-institutional medulloblastoma clinical trials (SJMB96 and SJMB03), compared with 35 medulloblastoma participants treated with the same cisplatin dosing schedule on SJMB96 who did not receive amifostine.17 Among the non-amifostine-treated participants, 37% had serious ototoxicity (hearing loss requiring hearing aids or resulting in deafness), while only 14.5% of the amifostine-treated participants experienced serious ototoxicity (P = .005).17 We present here an extended analysis of amifostine in average-risk medulloblastoma patients using a much larger patient base now available (n = 263) and examine for the first time the potential benefit of amifostine in children being treated for high-risk medulloblastoma (n = 116).
Materials and Methods
Patients
Patients with average-risk or high-risk newly diagnosed medulloblastoma were enrolled on one of 2 successive clinical trial protocols, SJMB96 or SJMB03, at St. Jude Children's Research Hospital or at one of 9 collaborating institutions. The SJMB96 protocol was amended towards the end of the recruitment period to include amifostine administration for protection against ototoxicity, although no randomization was employed. Both study protocols were approved by the Human Subjects Institutional Review Boards at St. Jude Children's Research Hospital and each of the participating institutions. Eligibility criteria and treatment regimens were previously described.17,18 Eligibility for inclusion in this analysis included diagnosis of medulloblastoma between September 1996 and March 2012, age at diagnosis of at least 3 years, and at least one audiological examination between 5.5 and 24.5 months after protocol treatment initiation. Patients with nontransient hearing loss in at least one ear at baseline were excluded, as were patients who did not receive cisplatin.
Treatment Protocol
After resection, participants on SJMB96 and SJMB03 were classified as having average-risk medulloblastoma (≤1.5 cm2 residual tumor and no metastatic disease) or high-risk medulloblastoma (>1.5 cm2 residual disease and/or metastatic disease localized to the neuraxis) according to a modified Chang staging system.19 Participants with high-risk medulloblastoma were treated using craniospinal irradiation (M0-1, 36 Gy; M2-3, 36–39.6 Gy) and supplemental (“boost”) irradiation to the tumor bed using conformal treatment methods (total dose 55.8 Gy). When appropriate, local sites of metastasis received supplemental irradiation (total dose 50.4–54 Gy). Participants with average-risk medulloblastoma received 23.4 Gy craniospinal irradiation and supplemental irradiation to the posterior fossa (cumulative dose 36 Gy) and tumor bed (total dose 55.8 Gy; 2 cm margin). On the SJMB03 protocol, supplemental irradiation of the posterior fossa was eliminated for average-risk participants, and the clinical target volume to the tumor bed was reduced from 2.0 cm (SJMB96 protocol) to 1.0 cm for all participants. Following radiation therapy, at ∼12 weeks posttreatment initiation, all participants (both protocols) received 4 cycles of cyclophosphamide, vincristine, cisplatin, and stem cell or bone marrow rescue. Each cycle was 28 days in duration, and the cisplatin dose level was 75 mg/m2, to a cumulative prescribed dose of 300 mg/m2.
Amifostine Administration
Based on the pharmacokinetic disposition of amifostine and its active metabolite WR1065, we chose to administer amifostine at a dosage of 600 mg/m2 as a 1 minute intravenous infusion immediately preceding and again 3 hours into each of the 4 courses of cisplatin infusion. The supportive care guidelines followed for amifostine administration have been previously described and included prehydration, withholding hypertension medication 24 hours before treatment and performing blood pressure control procedures, and close monitoring of and corrective measures for calcium level.7
Audiological Methods
Audiological evaluations, with method dependent upon participant age, cognition, development, and cooperation, included conventional pure-tone audiometry, conditioned play audiometry, visual reinforcement audiometry, speech audiometry, tympanometry, distortion product otoacoustic emissions (DPOAEs), auditory brainstem response (ABR), and/or auditory steady-state response (ASSR). Otoscopy and tympanometry were performed on each participant to assess the integrity of the outer and middle ear spaces. Air conduction thresholds were measured in a sound-treated booth at 0.25, 0.50, 1, 2, 3, 4, 6, and 8 kHz to determine hearing sensitivity in decibels (dBs) hearing level. Bone conduction thresholds were obtained at 0.25, 0.50, 1, 2, 3, 4 kHz in dBs hearing level, as needed to determine the nature of the hearing impairment. Although conventional audiometry remains the standard for ototoxicity monitoring, more objective diagnostic measurements were required for a small number of participants. Click and tone-burst ABR, ASSR, and/or DPOAEs were performed on participants who were unable to respond to conventional audiometric techniques (eg, young age or developmental delay). ABR and ASSR evaluations were performed to estimate peripheral hearing sensitivity at frequencies 0.50, 1, 2, 4, and 8 kHz. DPOAEs were evaluated to determine cochlear outer hair cell function at frequencies 1–8 kHz. Of the total number of evaluable participants, 18 (5%) received an ABR/ASSR evaluation at baseline with subsequent conventional audiometric evaluations; 7 (2%) had DPOAEs performed for the baseline evaluation with subsequent conventional audiometric evaluations, and 5 (1%) received an ABR/ASSR evaluation at baseline and throughout treatment. Audiological procedures were completed by a certified audiologist at each of the 10 collaborative study sites.
Every audiological evaluation was reviewed and assigned an ototoxicity grade by a clinical research audiologist (JB) at St. Jude Children's Research Hospital. The prospective ototoxicity monitoring protocol consisted of an evaluation at the following time points: baseline (occurred within 2 weeks of initiation of radiation therapy), before each of the 4 high-dose cisplatin chemotherapy cycles, and at 3, 6, 9, 18, and 24 months after completion of treatment. In our previous amifostine evaluation,17 the ototoxicity grading criteria were based on a Children's Cancer Group method with grade 3 defined as >25 dB hearing loss at 2000 Hz and grade 4 as ≥40 dB loss at 2000 Hz.20 In our present study, ototoxicity grade for each audiological evaluation in each ear was based on the more contemporary Chang Ototoxicity Scale (Table 1).21 Similar to our previous study, serious hearing loss for this analysis was defined as deafness or loss requiring a hearing aid, which corresponds to Chang grades 2b, 3, or 4.22 For asymmetrical hearing loss, the child's worse ear grade was used for the analysis.
Table 1.
Chang ototoxicity grading scale*
| Grade 0 | ≤20 dB at 1, 2, and 4 kHz |
| Grade 1a | ≥40 dB at any frequency of 6–12 kHz |
| Grade 1b | >20 and < 40 dB at 4 kHz |
| Grade 2a | ≥40 dB at 4 kHz and above |
| Grade 2b | >20 and < 40 dB at any freq below 4 kHz |
| Grade 3 | ≥40 dB at 2 or 3 kHz and above |
| Grade 4 | ≥40 dB at 1 kHz and above |
*Sensorineural hearing threshold (dB hearing level) bone conduction or air conduction with normal tympanogram. See reference #21 for details on the grading scale.
Abbreviation: dB, decibel.
Statistical Approach
Chi-square exact tests were used to examine the association between the distribution of Chang grade and amifostine treatment status. Univariable and multivariable logistic regression methods were used to study associations between dichotomized ototoxicity outcomes and a predetermined set of covariates. In building multivariable logistic regression models, a backwards selection approach was employed where goodness of fit across candidate models was compared via Akaike and Bayesian information criteria. All P values were based on 2-sided tests and were not adjusted for multiplicity.
Results
Patient Participation
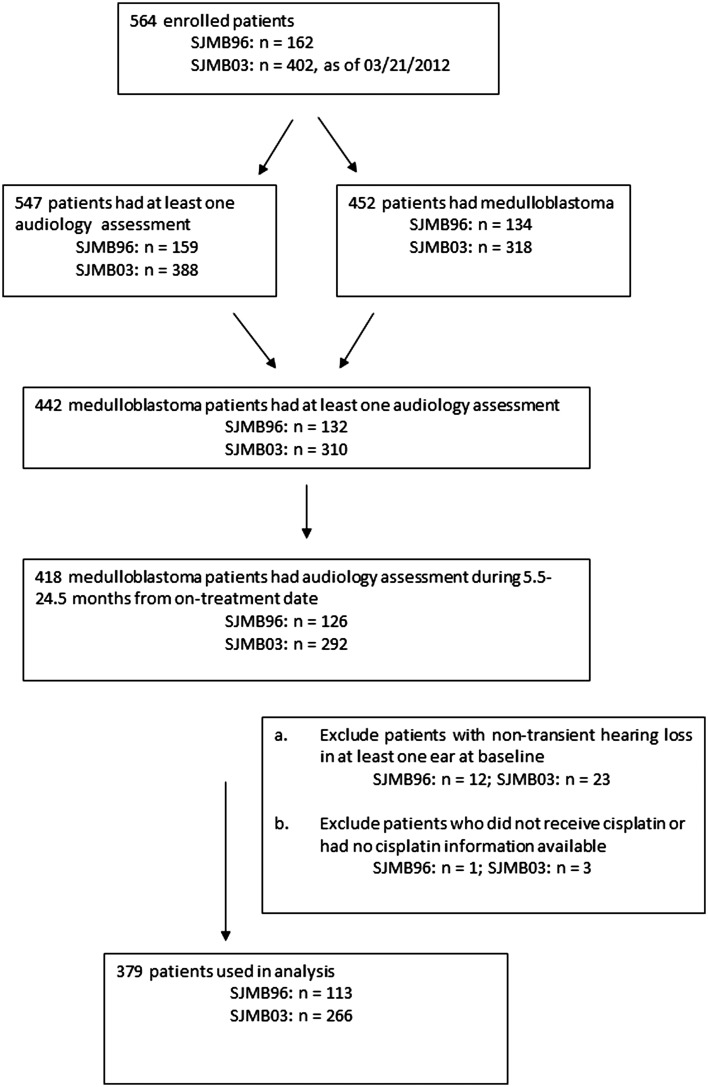
Of 452 participants with medulloblastoma who were enrolled in SJMB96 or SJMB03 through March 2012, 418 had at least one audiology examination during the eligible time period (5.5 to 24.5 months postprotocol treatment initiation, [ie, 1.5–21.5 months post initiation of cisplatin treatment]). Of these, 35 were not eligible because they had nontransient hearing loss at baseline in at least one ear, and 4 more were excluded because they did not receive cisplatin or had no cisplatin information available. Thus, 379 participants were included in the analysis (Fig. 1).
Fig. 1.
Flow diagram of study participation.
Participant Characteristics by Amifostine Status
Table 2 provides selected characteristics of participating patients by amifostine treatment status. Distributions of sex, race, and disease risk level were similar between the 2 groups, as was median cumulative cisplatin dose (∼300 mg/m2 in both groups). Median months from treatment initiation to last hearing evaluation was 19.5 in the amifostine-treated group, compared with 18.9 in the nontreated group. Of the 51 participants who did not receive amifostine and thus served as the referent (control) group for the analysis, 34 (67%) were classified as average risk and 17 as high risk. For those who received amifostine, 229 (70%) had average-risk tumors, and 99 had high-risk tumors.
Table 2.
Characteristics of 379 medulloblastoma participants by amifostine treatment status
| Variable | Amifostine = No (n = 51) | Amifostine = Yes (n = 328) |
|---|---|---|
| Age at study enrollment (years) [median (min, max]) | 7.3 (3.2, 17.2) | 8.3 (3.1, 21.6) |
| Sex | ||
| Female (%) | 18 (35.3%) | 118 (36.0%) |
| Male (%) | 33 (64.7%) | 210 (64.0%) |
| Race | ||
| Non-white (%) | 13 (25.5%) | 73 (22.3%) |
| White | 38 (74.5%) | 255 (77.7%) |
| Institution | ||
| St. Jude | 27 (52.9%) | 165 (50.3%) |
| Collaborative institution | 24 (47.1%) | 163 (49.7%) |
| Cumulative cisplatin dosage (mg/m2) [median (min, max)] | 301.0 (76.8, 329.4) | 299.8 (74.5, 312.2) |
| Time from treatment initiation to latest audiogram (months) [median (min, max]) | 18.9 (6.3, 24.3) | 19.5 (5.6, 24.5) |
| Study protocol | ||
| SJMB03 | 3 (5.9%) | 263 (80.2%) |
| SJMB96 | 48 (94.1%) | 65 (19.8%) |
| Disease risk category | ||
| Average | 34 (66.7%) | 229 (69.8%) |
| High | 17 (33.3%) | 99 (30.2%) |
| Chang Grade | ||
| 0 | 9 (17.7%) | 118 (36.0%) |
| 1a | 9 (17.7%) | 60 (18.3%) |
| 1b | 4 (7.8%) | 24 (7.3%) |
| 2a | 2 (3.9%) | 22 (6.7%) |
| 2b | 5 (9.8%) | 19 (5.8%) |
| 3 | 18 (35.3%) | 77 (23.5%) |
| 4 | 4 (7.8%) | 8 (2.4%) |
Abbreviation: St Jude, St. Jude Children's Research Hospital.
Hearing Loss by Amifostine Treatment Status
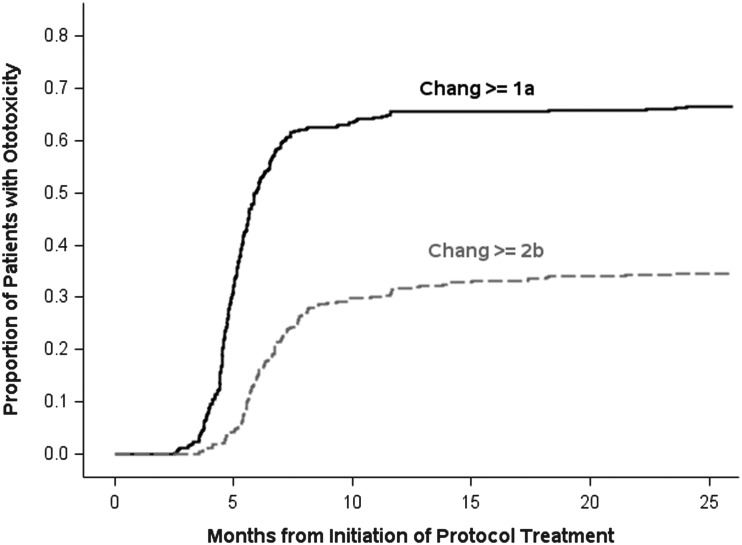
Some degree of hearing loss (Chang grade ≥1a) was evident in 67% of participants. Fig. 2 provides cumulative incidence curves, plotted as proportions, from treatment initiation to first audiologic evaluation with evidence of (i) any hearing loss and (ii) serious hearing loss (Chang grade ≥2b). The curves illustrate that hearing loss occurred shortly after cisplatin initiation and plateaued by 9 months. As shown in Table 3, the distribution by Chang grade, not accounting for disease risk category, showed a clear tendency toward worse hearing level among the 51 participants who did not receive amifostine, relative to the 328 participants who received amifostine treatment (P = .05, chi-square exact test). Serious hearing loss was evident in 53% of participants who did not receive amifostine compared with 32% of participants who were treated with amifostine (P = .004; univariable logistic regression model). As noted in Table 3 based on univariable models, younger age at diagnosis, male sex, and high-risk disease were also found to be associated with significant hearing loss. Being treated on SJMB03 and larger cumulative doses of cisplatin were associated with lower risk of significant ototoxicity. As per protocol, cisplatin treatment was discontinued for participants who experienced significant hearing loss, which explains the latter result. Based on a multivariable model, the odds ratio for the effect of amifostine on Chang grade ≥2b (vs <2b) adjusted for disease risk category, age at diagnosis, and sex, was 0.43 (95% CI, 0.23–0.80), suggesting a strong reduction in risk for serious hearing loss among participants treated with amifostine in this study (detailed results of this model are in Supplementary Table 1). Note that because the craniospinal radiation dose was determined by disease risk and there was very little deviation from the prescribed dose, radiation dose was accounted for in these analyses by way of disease risk. We chose to incorporate disease risk rather than craniospinal radiation dose into our models for ease of interpretation.
Fig. 2.
Cumulative proportion of ototoxicity versus time from protocol treatment initiation.
Table 3.
Univariable and multivariable logistic regression results for risk of serious hearing loss versus. demographic and clinical covariates
| Variable | Univariable Logistic Regression Model |
Multivariable Logistic Regression Model |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Error | Odds Ratio (95% CI) | P value | Estimate | Standard Error | Odds Ratio (95% CI) | P value | |
| Age at diagnosis | −0.099 | 0.032 | 0.91 (0.85–0.96) | .002 | −0.089 | 0.033 | 0.92 (0.86– 0.98) | .007 |
| Race (white) | 0.184 | 0.263 | 1.20 (0.72–2.01) | .48 | ||||
| Sex (male) | 0.578 | 0.235 | 1.78 (1.13–2.82) | .01 | 0.581 | 0.245 | 1.79 (1.11–2.89) | .02 |
| Cumulative cisplatin dose | -4.272 | 2.710 | 0.014 (<0.001–2.83) | .12 | ||||
| Study (SJMB03) | −0.917 | 0.232 | 0.40 (0.25–0.63) | <.001 | ||||
| Risk (high) | 0.744 | 0.230 | 2.10 (1.34–3.30) | .001 | −0.261 | 0.609 | * | .67 |
| Amifostine (yes) | −0.885 | 0.305 | 0.41 (0.23–0.75) | .004 | −1.206 | 0.383 | * | .002 |
| Risk by amifostine interaction* | 1.087 | 0.661 | — | .10 | ||||
| Amifostine vs no amifostine for average-risk disease | 0.30 (0.14–0.64) | |||||||
| Amifostine vs no amifostine for high-risk disease | 0.89 (0.31–2.54) | |||||||
*Since the multivariable model contains a risk by amifostine interaction term, the odds ratio for amifostine versus no amofostine needs to be calculated by risk level , and odds ratios associated with the main effects (ie, risk alone or amofostine alone) are not meaningful.
Hearing Loss by Disease Risk and Amifostine Treatment Status
Table 4 provides data on the distribution of hearing levels stratified by disease risk category and amifostine treatment status. Overall, a larger proportion of participants with high-risk medulloblastoma experienced some degree of hearing loss (76%) than did the average-risk participants (62%, P = .01, chi-square exact test), and the distribution was weighted toward more severe loss in the high-risk participants. For average-risk participants, hearing level was significantly worse among those not treated with amifostine compared with participants who received amifostine treatment (P = .01, chi-square exact test). In contrast, the overall distribution by Chang grade did not differ statistically in the high-risk disease group by amifostine treatment status (P = .93). The moderated effect of amifostine by disease risk on Change grade ≥2b hearing loss is further illustrated in Table 3 and in Supplementary Fig. 1. The right half of Table 3 summarizes the results of the multivariable logistic regression analysis. After adjustment for age at diagnosis and sex, and incorporating disease risk-amifostine interaction, amifostine appeared to provide a strong protective benefit against cisplatin-induced serious hearing loss in average-risk participants (adjusted OR, 0.30 [95% CI, 0.14–0.64]), but not for high-risk participants (adjusted OR, 0.89 [95% CI, 0.31–2.54]).
Table 4.
Chang ototoxicity scale grade level by disease risk category and amifostine treatment status
| Chang grade | Average Risk |
High Risk |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amifostine: No (n = 34) |
Amifostine: Yes (n = 229) |
Overall (n = 263) |
Amifostine: No (n = 17) |
Amifostine: Yes (n = 99) |
Overall (n = 116) |
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| 0 | 4 | 11.8 | 95 | 41.5 | 99 | 37.6 | 5 | 29.4 | 23 | 23.2 | 28 | 24.1 |
| 1a | 7 | 20.6 | 46 | 20.1 | 53 | 20.2 | 2 | 11.8 | 14 | 14.1 | 16 | 13.8 |
| 1b | 3 | 8.8 | 14 | 6.1 | 17 | 6.5 | 1 | 5.9 | 10 | 10.1 | 11 | 9.5 |
| 2a | 2 | 5.9 | 15 | 6.6 | 17 | 6.5 | 0 | 0 | 7 | 7.1 | 7 | 6.0 |
| 2b | 4 | 11.8 | 12 | 5.2 | 16 | 6.1 | 1 | 5.9 | 7 | 7.1 | 8 | 6.9 |
| 3 | 11 | 32.4 | 43 | 18.8 | 54 | 20.5 | 7 | 41.2 | 34 | 34.3 | 41 | 35.3 |
| 4 | 3 | 8.8 | 4 | 1.8 | 7 | 2.7 | 1 | 5.9 | 4 | 4.0 | 5 | 4.3 |
Discussion
In this study of 379 newly diagnosed childhood medulloblastoma participants, we confirmed an earlier result from our multi-institutional study that amifostine, administered at 600 mg/m2 both immediately before and again 3 hours into each of four 75 mg/m2 cisplatin infusions provides protection from cisplatin-induced sensorineural hearing loss. Here we extend the previous finding using a much larger sample size and show that the otoprotective effect of amifostine in average-risk participants (adjusted OR, 0.30 [95% CI, 0.14–0.64]) was not present in high-risk participants (adjusted OR, 0.89 [95%CI, 0.31–2.54). Given that the cisplatin and amifostine dosing schedules were identical between the high-risk and average-risk participants in both study protocols, one or more other factors appeared to differentially influence the ototoxicity outcome. One could surmise that a shared genetic trait influencing disease pathology, radiation effect, and response to amifostine is a conceivable explanation,23 albeit unlikely, or simply that the added radiation boost to the posterior fossa in the high-risk participants is causally related to the difference. However, the prevalence of Chang grade ≥2b hearing loss among children not treated with amifostine was the same for high-risk and average-risk participants (53% for each), and there was no evidence of an association between disease risk and level of Chang grade (P = .68, Cochran-Armitage trend test), so the latter explanation seems doubtful. It is also possible that the finding in the high-risk participants is falsely negative due to sparse data. Only 17 participants in the high-risk group were not treated with amifostine, and 99 were treated with amifostine, so we had limited statistical power to detect a significant protective effect in that subgroup.
Amifostine was previously evaluated for protection against cisplatin-induced hearing loss in children in 2 randomized clinical trials, as reviewed in detail by van As et al.24 Gallegos-Castorena et al.14 randomized 28 children with osteosarcoma to no amifostine (n = 13) or amifostine (n = 15; 740 mg/m2) prior to cisplatin infusions of 150 mg/m2. None of the participants in either the amifostine group or the control group had grade 3 or 4 ototoxicity, and there was no statistical difference in the overall distribution of ototoxicity grade between the 2 groups, although the sample size was quite small. Katzenstein et al.,15 in a Children's Oncology Group randomized trial of 82 evaluable participants with hepatoblastoma, compared amifostine (740 mg/m2 15 min before each cycle of 100 mg/m2 cisplatin or 3 mg/m2 cisplatin if younger than 1 year; n = 37) with no otoprotective agent (n = 45) in an unplanned interim analysis unrelated to ototoxicity. In both treatment arms, the incidence of significant hearing loss (> 40 dB at 3–5 kHz or worse) was 38%. In a nonrandomized study, Marina et al.16 evaluated amifostine in 25 pediatric germ cell tumors. That study administered intravenous amifostine at 825 mg/m2 over 15 minutes, 30 minutes before cisplatin infusion of 40 mg/m2 per day for 5 consecutive days for each cycle. They reported that 18 of 24 (75%) evaluable participants had grade 2–4 hearing loss using the Brock grading scale,25 similar in proportion to a historical comparison group from a previous extragonadal childhood germ cell study (41/55 with grade 2–4, 75%). Finally, Fisher et al.13 administered amifostine at 1000 mg/m2 prior to and 4 hours into each cisplatin infusion of 70 mg/m2 in 11 children undergoing treatment for medulloblastoma or supratentorial PNET. In audiological evaluations conducted at 1–3 years post treatment, 4 of the 11 participants had hearing loss severe enough to require hearing aids and 3 others had grade 2 hearing loss. Although clearly limited by sample size and study design issues,24 none of these studies provided evidence in support of amifostine for cisplatin-induced ototoxicity among pediatric solid tumor patients.
Sodium thiosulfate, an alternative to amifostine, is currently being tested in clinical trials for otoprotection in solid tumor treatment through the Children's Oncology Group ACCL0431 protocol24,26 and for hepatoblastoma treatment in the International Society of Pediatric Oncology SIOPEL-6 protocol.24 Results have not been published from either of these trials to date. Sodium thiosulfate, like amifostine, is a thiol-reducing agent with strong antioxidant properties3 and is reported to be easier to administer than amifostine and have less potential for clinically significant adverse effects.26 Of the 263 participants in SJMB03 who received amifostine in our study, 25 (9.5%) experienced grade 3 or 4 toxicity secondary to amifostine: 3 with a grade 3 allergic reaction; 19 with grade 3 hypotension; and 2 with grade 4 hypotension.
Considerations for interpreting our results include the fact that we did not randomize participants to amifostine or no amifostine treatment, and the non-amifostine-treated group used for comparison purposes was largely accrued in the early stages of the SJMB96 study. We also did not have systematic data available on cochlear radiation doses, although, as we previously reported,17 no difference in mean cochlear radiation dose was observed in a subgroup analysis of 56 participants that compared those with grade 3 ototoxicity to those with < grade 3 (mean cochlear dose of 49 Gy for each group). In addition, the time frame for audiological follow-up in our study was defined by the latest examination that occurred between 5.5–24.5 months following protocol treatment initiation, potentially failing to capture delayed onset or further deterioration of hearing loss. Although our data demonstrated that hearing loss stabilized at 9 months following initiation of protocol treatment, continued decline in hearing sensitivity years after therapy has been documented in patients treated with cisplatin27,28 and cranial radiation.29,30
The strengths of this research include the prospective and comprehensive audiological evaluations conducted and the standardized protocol used throughout the study period for administration of cisplatin, for effective supportive care for amifostine, and for administration of amifostine. In addition, the results of our published pharmacokinetic study of amifostine and WR1065 in this patient population further lead credence to the selection of the amifostine dosage and schedule for this patient population.10 We also used a standardized coding method, the Chang ototoxicity grading scale,21 to assess hearing level for all participants, which has notable advantages over the NCI CTCAE v3.0 method and other earlier ototoxicity scales3,20 including the scale used in our previous analysis.
In summary, in this large study of medulloblastoma patients treated with 300 mg/m2 cumulative dose cisplatin, amifostine provided clinically meaningful benefit for reducing serious cisplatin-induced hearing loss in participants treated for average-risk disease but not for participants treated for high-risk disease. As such, the new St. Jude medulloblastoma protocol (SJMB12), which opened at St. Jude Children's Research Hospital in June 2013 and will include 19 collaborative sites across North America, Australia, and New Zealand, incorporates amifostine for patients with average-risk, but not high-risk, medulloblastoma using the dosing schedule described herein.
Supplementary Material
Funding
Funding from the National Cancer Institute Cancer Center CORE grant CA 21765, the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer (MACC), and the America Lebanese Syrian Associated Charities (ALSAC).
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Orgel E, Jain S, Ji L, et al. Hearing loss among survivors of childhood brain tumors treated with an irradiation-sparing approach. Pediatr Blood Cancer. 2012;58:953–958. doi: 10.1002/pbc.23275. [DOI] [PubMed] [Google Scholar]
- 2.Lafay-Cousin L, Purdy E, Huang A, et al. Early cisplatin induced ototoxicity profile may predict the need for hearing support in children with medulloblastoma. Pediatr Blood Cancer. 2013;60:287–292. doi: 10.1002/pbc.24307. [DOI] [PubMed] [Google Scholar]
- 3.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston Ototoxicity Scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Der Hulst RJAM, Dreschler WA, Urbanus NAM. High frequency audiometry in prospective clinical research of ototoxicity due to platinum derivatives. Ann Otol Rhinol and Laryngol. 1998;97:133–137. doi: 10.1177/000348948809700208. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Gurney JG, Severson RK, Davis S, et al. Incidence of cancer in children in the United-States - sex-specific, race-specific, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186–2195. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Gilmer-Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 8.Blakley BW, Cohen JI, Doolittle ND, et al. Strategies for prevention of toxicity caused by platinum-based chemotherapy: review and summary of the annual meeting of the Blood-Brain Barrier Disruption Program, Gleneden Beach, Oregon, March 10, 2001. Laryngoscope. 2002;112:1997–2001. doi: 10.1097/00005537-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Rybak LP, Mukherjea D, Jajoo S, et al. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219:177–186. doi: 10.1620/tjem.219.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKibbin T, Panetta JC, Fouladi M, et al. Clinical pharmacokinetics of amifostine and WR1065 in pediatric patients with medulloblastoma. Clin Cancer Res. 2010;16:1049–1057. doi: 10.1158/1078-0432.CCR-09-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church MW, Blakley BW, Burgio DL, et al. WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: dose-dependent effects. J Assoc Res Otolaryngol. 2004;5:227–237. doi: 10.1007/s10162-004-4011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain AE, Blakley BW, Nicolas M, et al. Assessment of the protective effects of amifostine against cisplatin-induced toxicity. J Otolaryngol. 2003;32:294–297. doi: 10.2310/7070.2003.11264. [DOI] [PubMed] [Google Scholar]
- 13.Fisher MJ, Lange BJ, Needle MN, et al. Amifostine for children with medulloblastoma treated with cisplatin-based chemotherapy. Pediatr Blood Cancer. 2004;43:780–784. doi: 10.1002/pbc.20132. [DOI] [PubMed] [Google Scholar]
- 14.Gallegos-Castorena S, Martínez-Avalos A, Mohar-Betancourt A, et al. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatr Hem Oncol. 2007;24:403–408. doi: 10.1080/08880010701451244. [DOI] [PubMed] [Google Scholar]
- 15.Katzenstein HM, Chang KW, Krailo M, et al. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma. Cancer. 2009;115:5828–5835. doi: 10.1002/cncr.24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marina N, Chang KW, Malogolowkin M, et al. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ-cell tumors: a Children's Oncology Group study. Cancer. 2005;104:841–847. doi: 10.1002/cncr.21218. [DOI] [PubMed] [Google Scholar]
- 17.Fouladi M, Chintagumpala M, Ashley D, et al. Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma. J Clin Oncol. 2008;26:3749–3755. doi: 10.1200/JCO.2007.14.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 19.Chang CH, Housepian EM, Herbert C., Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 20.Gurney JG, Bass JK. New International Society of Pediatric Oncology Boston Ototoxicity Grading Scale for pediatric oncology: still room for improvement. J Clin Oncol. 2012;30:2303–2306. doi: 10.1200/JCO.2011.41.3187. [DOI] [PubMed] [Google Scholar]
- 21.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 22.Chang KW. Clinically accurate assessment and grading of ototoxicity. Laryngoscope. 2011;121:2649–2657. doi: 10.1002/lary.22376. [DOI] [PubMed] [Google Scholar]
- 23.Rednam S, Scheurer ME, Adesina A, et al. Glutathione S-transferase P1 single nucleotide polymorphism predicts permanent ototoxicity in children with medulloblastoma. Pediatr Blood Cancer. 2013;60:593–598. doi: 10.1002/pbc.24366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van As JW, van den Berg H, van Dalen EC. Medical interventions for the prevention of platinum-induced hearing loss in children with cancer. Cochrane Database Syst Rev. 2012;5:1–39. doi: 10.1002/14651858.CD009219.pub2. CD009219. [DOI] [PubMed] [Google Scholar]
- 25.Brock PR, Bellman SC, Yeomans EC, et al. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 26.Freyer DR, Sung L, Reaman GH. Prevention of hearing loss in children receiving cisplatin chemotherapy. J Clin Oncol. 2009;27:317–318. doi: 10.1200/JCO.2008.20.1160. [DOI] [PubMed] [Google Scholar]
- 27.Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 28.Einarsson EJ, Petersen H, Wiebe T, et al. Long term hearing degeneration after platinum-based chemotherapy in childhood. Int J Audiology. 2010;49:765–771. doi: 10.3109/14992027.2010.485595. [DOI] [PubMed] [Google Scholar]
- 29.Grau C, Overgaard J. Postirradiation sensorineural hearing loss: a common but ignored late radiation complication. Int J Radiat Oncol Biol Phys. 1996;36:515–517. doi: 10.1016/s0360-3016(96)00346-x. [DOI] [PubMed] [Google Scholar]
- 30.Ho WK, Wei WI, Kwong DL, et al. Long-term sensorineural hearing deficit following radiotherapy in patients suffering from nasopharyngeal carcinoma: a prospective study. Head Neck. 1999;21:547–553. doi: 10.1002/(sici)1097-0347(199909)21:6<547::aid-hed8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.